Abstract
Genetic defects such as copy number variations (CNVs) in non-coding regions containing conserved non-coding elements (CNEs) outside the transcription unit of their target gene, can underlie genetic disease. An example of this is the short stature homeobox (SHOX) gene, regulated by seven CNEs located downstream and upstream of SHOX, with proven enhancer capacity in chicken limbs. CNVs of the downstream CNEs have been reported in many idiopathic short stature (ISS) cases, however, only recently have a few CNVs of the upstream enhancers been identified. Here, we set out to provide insight into: (i) the cis-regulatory role of these upstream CNEs in human cells, (ii) the prevalence of upstream CNVs in ISS and (iii) the chromatin architecture of the SHOX cis-regulatory landscape in chicken and human cells. Firstly, luciferase assays in human U2OS cells and 4C-seq both in chicken limb buds and human U2OS cells, demonstrated cis-regulatory enhancer capacities of the upstream CNEs. Secondly, CNVs of these upstream CNEs were found in three of 501 ISS patients. Finally, our 4C-seq interaction map of the SHOX region reveals a cis-regulatory domain spanning more than 1 Mb and harbouring putative new cis-regulatory elements.
Similar content being viewed by others
Introduction
The short stature homeobox-containing gene (SHOX; MIM *312865) is located in the pseudoautosomal region (PAR1) on the short arms of the X- and Y-chromosomes1. SHOX is a member of the paired-like homeobox-containing protein family of transcription factors and plays a crucial role in bone development and growth1,2,3. A strict regulation of its spatiotemporal expression is therefore of utmost importance. As for most developmental genes, the minimal promoter region of SHOX is not sufficient but requires the assistance of cis-regulatory elements such as enhancers4. To date, seven evolutionarily conserved non-coding elements (abbreviated as CNEs, ECRs or ECS) located downstream (CNE4, CNE5, ECR1 and ECS4/CNE9) and upstream (CNE-5, CNE-3 and CNE-2) of SHOX, have been shown to act as enhancers5,6,7,8. As SHOX does not have an orthologue in rodents but is highly conserved in chicken3 and as Shox expression was shown in chicken limb buds at developmental stage HH266, limb buds of chicken embryos have been used to confirm the enhancer potential of all CNEs, except for ECR16,7. In addition, chromosome conformation capture (3C) in chicken embryo limbs demonstrated that the downstream ECR1 and CNE9 interact with the Shox promoter8. Interestingly, the regulatory capacity of CNE4, CNE5 and CNE9 was also investigated in whole zebrafish embryos resulting in the identification of additional tissues under the regulatory control of these CNEs and the identification of smaller, more deeply conserved sub-sequences within these CNEs9. Cotney et al. recently performed H3K27ac ChIP-seq profiling in human embryonic limb buds from day 33 (E33) through to E4710. This revealed a genome-wide distribution of the active enhancer-associated histone marks H3K27ac in human limbs of different developmental stages. Despite all this data, functional studies specifically investigating the cis-regulatory activity of the upstream CNEs in relevant human systems are lacking as only the enhancer potential of the four downstream CNEs was substantiated in human U2OS osteosarcoma cells.
Heterozygous defects of SHOX or its enhancers explain ~60–80% of cases with Leri-Weill dyschondrosteosis (LWD, MIM 127300) and ~2–5% of cases with idiopathic short stature (ISS, MIM 300582), which is defined as a height below −2 standard deviations (SDS) in the absence of known causes11. Molecular defects leading to both phenotypes include intragenic SHOX mutations, complete or partial SHOX deletions and duplications and deletions and duplications of the downstream enhancer region1,12,13,14,15,16,17,18,19,20,21. Interestingly, only one deletion and duplication of the upstream enhancer region, have been described in ISS patients21,22.
Here, our aim was to provide insight into: (i) the cis-regulatory role of these upstream CNEs in relevant human cells, (ii) the prevalence of copy number variations (CNVs) of upstream CNEs in ISS and (iii) the chromatin architecture of the SHOX cis-regulatory landscape in chicken and human cells. Luciferase assays of the upstream CNEs revealed enhancer activity in human U2OS cells. In addition, these CNEs overlap with H3K27ac ChIP-seq marks previously generated in human embryonic limb buds. Furthermore, 4C-seq in chicken embryo limb buds and in U2OS cells uncovered interactions of the upstream CNEs with the Shox promoter. CNVs of these upstream CNEs were found in only three of 501 patients with unexplained ISS.
Results
Luciferase assays of upstream CNEs in human U2OS osteosarcoma cells
In ovo enhancer assays in chicken limb buds previously demonstrated that the upstream CNEs possess enhancer activity7. However, enhancer assays in relevant human cells have not yet been performed. Hence, luciferase assays of the upstream enhancers, CNE-5, CNE-3 and CNE-2, were carried out in human U2OS cells. For this purpose, the CNEs were cloned upstream of the SHOX promoter in the pGL3SHOXprom vector8, mimicking their chromosomal localization. The clones were co-transfected with a Renilla luciferase control vector into U2OS cells. The four downstream SHOX enhancer regions CNE4, CNE5, ECR1 and ECS4/CNE9 were used as positive controls whilst the pGL3SHOXprom vector with no enhancer sequence was used as the negative control. Increased luciferase activity was observed for all three upstream CNEs and positive controls (Fig. 1). The three upstream CNEs were also cloned downstream of the luciferase gene, to test out their localization dependency. All three showed enhancer activity (Fig. 1), demonstrating that the upstream CNEs act as enhancers in a localization independent manner in human U20S cells.
Enhancer activity of three upstream CNEs demonstrated by in vitro luciferase assays in human U2OS cells.
Luciferase reporter activity of U2OS cells transfected with the pGL3SHOXprom enhancer reporter plasmids and the Renilla luciferase control plasmid. The pGL3SHOXprom without any enhancer inserted was used as an empty vector, to normalize luciferase activity. Fold increase values were obtained by normalizing the relative luciferase units of each sample, first, with respect to the Renilla luciferase activity and second, to that transfected with the empty reporter plasmid. All values represent the mean and standard deviation of two biological replicates with each sample assayed in triplicate. ECS5, localized outside of the deleted region and which has previously been shown not to have enhancer activity, was utilized as a negative control5. The downstream SHOX enhancer regions CNE4, CNE5, ECR1 and CNE9/ECS4 were utilized as positive controls6,8. Increased luciferase activity was observed for all three upstream SHOX enhancer constructs, CNE-5, CNE-3 and CNE-2 constructs and the positive controls.
Exploration of the SHOX region in H3K27ac ChIP-seq marks in human embryonic limb buds10 showed the presence of active marks in all three upstream CNEs (CNE-5, CNE-3 and CNE-2), substantiating their enhancer activity in the human developing limb (Fig. 2).
H3K27ac distribution of the SHOX locus in human embryonic limbs from embryonic day 33 (E33) through E47.
Overview of the human SHOX locus (chrX:350,000–628,000; UCSC, Human Genome Browser, hg19), showing the distribution of enhancer-associated H3K27ac histone marks at different human embryonic limb bud stages, produced by Cotney et al. (2013) and retrieved from GEO dataset GSE42413. Four embryonic stages are represented: E33, E41, E44 and E47. Upstream enhancers CNE-5, CNE-3 and CNE-2 are highlighted with red bars. The two SHOX isoforms are represented in blue at the top. A conservation track is represented at the bottom.
Copy number profiling of upstream CNEs in cohort with unexplained ISS
CNV analysis of upstream CNEs
The study cohort consisted of 501 ISS patients. Deletions and duplications of SHOX and the downstream enhancer region were excluded prior to this study18 and intragenic mutations of SHOX were excluded in 56 of the 501 patients by Sanger sequencing of the coding regions. In the remaining 445 patients, coding mutations were excluded by a customized targeted amplicon-based next-generation sequencing approach23.
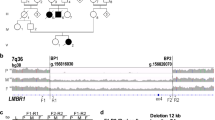
Overall, 501 patients with unexplained ISS underwent CNV analysis of the upstream enhancer region (CNE-5, CNE-3, CNE-2). A CNV of at least two of the upstream enhancers (two deletions, one duplication) was detected in three unrelated index cases (patients A, B and C) (Fig. 3). These CNVs were confirmed and delineated using customized high-resolution arrayCGH (Supplementary Fig. S1). An overview of these novel and previously reported upstream CNVs21,22 is given in Fig. 4. A summary of the sex, age, anthropometric and clinical details of the probands with these upstream CNVs and their family members is shown in Table 1.
Copy number profiling of upstream CNEs (CNE-5, CNE-3 and CNE-2) revealing two upstream deletions and one duplication.
Each panel shows the results of the CNV analysis using qPCR. The qPCR-derived copy number results are presented as relative quantities in a bar chart. Normal copy numbers are represented in grey, deletions are shown in red and duplications in blue. Error bars are added to allow interpretation of the assay’s precision.
Overview of all reported upstream CNVs in the SHOX region.
Overview of the SHOX region (hg19: chrX:151,000-628,000; UCSC, Human Genome Browser, hg19) with custom tracks showing the deletions (red bars) and duplications (blue bars) identified in this and previous studies21,22. A custom track representing the upstream enhancers (CNE-5, CNE-3 and CNE-2) is included, the RefSeq Genes track is shown at the top.
In patient A, we found a deletion of ~91 kb encompassing CNE-3 and CNE-2 (Fig. 3). Patient A is a 9-year-old Belgian girl referred for ISS (115.8 cm, −2.3 height standard deviation score, SDS), with a reduced arm span:height ratio which is indicative of shortening of upper limbs and suggestive of LWD. Her parents’ heights were both within normal limits. The deletion was shown to be paternal (Supplementary Fig. S2).
In patient B, a deletion of ~139 kb was observed containing CNE-5 and CNE-3 (Fig. 3). Patient B is a 13-year-old boy of Roma origin referred for ISS (130.5 cm, −2.5 SDS). His father’s height is 170 cm (1.03 SDS) and his mother’s height is 152 cm (−1.96 SDS). Paternal inheritance of the deletion was excluded but no maternal material was available to substantiate a maternal origin (Supplementary Fig. S2).
A duplication of ~256 kb containing all three upstream CNEs was identified in patient C (Fig. 3). Patient C is a 17-year-old Belgian girl with disproportionate short stature (153 cm, −2.3 SDS). Her father´s height is 163 cm (−2.6 SDS) and her mother’s height is 156 cm (−1.8 SDS). The duplication was shown to be paternal (Supplementary Fig. S2).
Structural variations of the SHOX region in open source and local databases
No upstream CNVs were identified in a control population of 340 healthy individuals with heights within the normal range21,22. To further evaluate the prevalence and associated phenotypic effects of the upstream CNVs, we searched for deletions and duplications of these regions in the Database of Genomic Variants (DGV)24, Database of Chromosomal Imbalance and Phenotype in Humans Using Ensembl Resources (Decipher)25 and our local arrayCGH database. Four duplications of the upstream region were present in DGV26,27,28 while 19 duplications and one deletion were reported in Decipher. However, short stature was only listed as part of the phenotypic spectrum for one of the duplications in Decipher. This duplication was predicted to be benign as the patient had three other CNVs, one of which was classified as probably pathogenic. Similarly, the majority of the Decipher listed CNVs encompassing the upstream enhancers are classified as likely benign and occur in combination with other CNVs25 (Supplementary Table S3).
In our local array CGH database, containing genomic profiles from over 3000 patients, one deletion and two duplications of the upstream enhancers were found in patients with syndromic phenotypes. As two of these patients display short stature in combination with a variety of symptoms, the short stature may be part of a syndrome and caused by a different genetic defect (Supplementary Table S3).
Chromatin interaction map (4C-seq) in chicken limb buds and in human U2OS cells
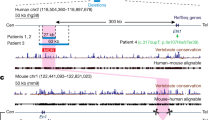
Several genomic alterations associated with SHOX-deficient phenotypes have been found within regulatory domains both upstream and downstream of the SHOX coding region15,16,20,21,22. Subsequent functional studies identified seven CNEs with enhancer properties in these regions and 3C analysis showed the interaction of downstream elements with the Shox promoter5,6,7,8, suggesting a complex regulation of SHOX. To further map the cis-regulatory landscape of Shox, we used 4C-seq, a chromosome conformation capture technique that has been used to identify cis-regulatory elements associated with Hox genes29,30,31. Using the Shox promoter as an anchor point, 4C-seq in chicken limb buds revealed widespread contacts between Shox and multiple upstream and downstream regions. The region extended almost 800 kb in the chicken genome, a region syntenic with approximately 1 Mb of human genomic DNA (Fig. 5A). All three known upstream enhancers (CNE-5, CNE-3 and CNE-2) contacted with the Shox promoter. Similar results were generated using 4C-seq in human U2OS cells (Fig. 5B). Interestingly, integration of the previously generated H3K27ac ChIP-seq marks in the SHOX locus with the 4C-seq peaks revealed numerous other active regions, upstream and downstream of the SHOX transcription unit (Fig. 5C). These findings suggest a complex regulation of SHOX expression by multiple cis-regulatory elements located upstream and downstream of the coding region within a large genomic region extending beyond 1 Mb (approximate boundaries chrX:284,600-1,355,600, hg19).
4C-seq chromatin interaction map of the cis-regulatory region surrounding SHOX.
(A) 4C-seq data of the chicken Shox locus (chr1:133,215,000-134,400,000; UCSC, galGal3) showing that the Shox promoter (blue bar, viewpoint) interacts with 800 kb of adjacent genomic regions that likely contain Shox cis-regulatory regions, a region syntenic with about 1 Mb of human genomic DNA (shown in panel (B)). Red bar marks the position of the upstream CNEs. The 4C-seq data indicate that the Shox regulatory landscape extends both upstream and downstream of the currently known, functionally validated enhancers. (B) 4C-seq data of the human SHOX locus (chrX:215,733–1,561,244; UCSC, hg19) showing that the SHOX promoter (blue bar, viewpoint) interacts with the upstream enhancers (red bars). The RefSeq Genes are shown a the top and the conservation track is included at the bottom of panel (B). (C) Distribution of enhancer-associated H3K27ac histone marks at different human embryonic limb bud stages, produced by Cotney et al. (2013).
Discussion
CNVs of cis-regulatory elements of strict spatiotemporally regulated transcription factor genes can often underlie human developmental disease, as is the case for SHOX, which is regulated by at least seven enhancers located downstream and upstream of SHOX5,6,7,8. Indeed, enhancer activity of the upstream CNEs (CNE-5, CNE-3, CNE-2) has been previously demonstrated by in ovo enhancer assays in chicken limb buds, however, specific functional data in relevant human cells are lacking. We therefore performed luciferase assays in human U2OS cells, showing increased luciferase activity for all three upstream CNEs in a localization-independent manner, confirming their enhancer activity in human cells. In addition, the upstream CNEs overlap with previously identified active enhancer-associated histone H3K27ac marks in human limbs of different developmental stages10. Cis-regulatory elements can only fulfil their function if the chromatin is in an open euchromatic conformation and if they can communicate with the promoter of a target gene4. The most favoured model to explain such communication is the looping model where transcription factors bound at the enhancer make direct contact with the promoter and/or with factors bound at the promoter, while the intervening DNA loops out32. This model forms the basis for the 3C/4C methods, which allow the identification of interactions between genomic fragments33. To test the interactions of the upstream enhancers, we performed 4C-seq in limb buds of chicken embryos and in human U2OS cells. Both experiments demonstrated interactions for all three upstream CNEs and the Shox promoter, emphasizing their cis-regulatory role in both chicken and human cells.
SHOX deficiency can result from a variety of molecular defects including intragenic SHOX mutations, complete and partial CNVs of SHOX, and CNVs of its enhancers located in the downstream PAR1 region1,12,13,14,15,16,17,18,19,20,21. The first CNVs of the upstream enhancer, a deletion and a duplication, were only recently identified in ISS patients21,22. As the upstream region has only been included relatively recently in the most frequently used diagnostic tool for CNV analysis of SHOX and the PAR1 region (i.e. multiplex ligation-dependent probe amplification, MLPA), CNVs of the upstream enhancers may have been missed and therefore their contribution to the pathogenesis of SHOX-deficient phenotypes might have been underestimated. Here, CNV analysis of the upstream CNEs in 501 cases with unexplained ISS revealed two upstream enhancer deletions and one duplication. Thus, the frequency of upstream CNVs in our ISS cohort is only ~0.6% while an even lower frequency was reported by Benito-Sanz et al.22. These low numbers are in contrast with the high frequencies observed for downstream CNVs. This might have several explanations: (i) Differences in the genomic content between the upstream and downstream region. Indeed, Durand et al. showed that the overall content of interspersed repeats of the downstream region is considerably higher than that of the upstream region thus increasing the probability of deletions and duplications7. (ii) Lack of screening studies of the upstream CNEs in large ISS cohorts as only this and the one reported by Benito-Sanz et al.22 have been undertaken to date. (iii) Smaller phenotypic effects of CNVs of upstream CNEs compared to those of downstream CNVs, which could be due to different spatiotemporal regulation, may lead to milder phenotypes that are not necessarily ascertained at genetic and endocrinology clinics22. In addition this could explain incomplete penetrance, as assumed for the upstream CNV detected in family A and for the previously reported upstream duplication21. Interestingly, incomplete penetrance was also observed in several families with the recurrent ~47.5 kb downstream deletion8, suggesting that CNVs of the upstream or downstream CNEs can act as rare functional variants that require (an) additional risk factor(s) to express their phenotypes. This is also supported by the relatively high number of CNVs encompassing one or more upstream CNEs found in DGV and Decipher.
Our data underscore that the clinical significance of upstream CNVs, especially duplications, is often ambiguous, hampering genetic counselling. The orientation and location of the duplicated segment, may cause overexpression of its target gene or interfere with the chromatin conformation, thereby impairing cis-regulation during development and causing disease34.
In ~60–80% of LWD and ~2–5% of ISS cases a SHOX-related genetic defect can be identified, leaving a large proportion of LWD and ISS cases molecularly unexplained. Besides the involvement of variants in other loci, genetic defects in novel, as yet unidentified cis-regulatory elements of SHOX may explain a proportion of these unexplained LWD and ISS cases. In order to delineate the cis-regulatory domain more precisely, we performed 4C-seq in different cells. Interestingly, the chromatin interaction maps delineate the cis-regulatory landscape of SHOX to a region that extends beyond 1 Mb in humans (approximate boundaries: chrX:284,600–1,355,600, hg19), which is larger than previously anticipated based on the genomic positions of the previously functionally studied CNEs (approximate boundaries: chrX:398,357–835,567, hg19). These data are consistent with the multiple enhancer-associated H3K27ac marks in human limbs distributed along the whole region and active at different developmental stages10. The boundaries proposed here appear to be larger than the topological domain (chrX:350,001–1,035,000) determined by Hi-C in GM12878 lymphoblastoid cells, (Supplementary Fig. S4)35. These differences can be explained by the presence of several assembly gaps in the SHOX region for which consequently no Hi-C data can be generated. Taking this into account, the regulatory domains mapped both by Hi-C and 4C-seq do have similar boundaries. This also illustrates that the spatial organisation of the genome in topological domains is stable across different cell types and is highly conserved throughout evolution36.
Integration of the 4C-seq interaction map generated here and H3K27ac histone marks will be instrumental for the identification of novel cis-regulatory elements. In this context, it is interesting to mention two recently reported deletions ~300 kb downstream of SHOX in LWD, not including any of the four known downstream enhancers37,38. These deletions harbour a high 4C-seq interaction peak and several H3K27ac marks possibly pointing to a novel SHOX enhancer (Supplementary Fig. S5). Further in vitro and in vivo assays are required however to substantiate this.
In conclusion, in vitro luciferase assays of upstream CNEs in human U2OS cells and active histone marks in human developing limbs support an enhancer role for the upstream CNEs in human cells. Chromosome conformation capture profiling (4C-seq) in embryonic chicken limb buds and human U2OS cells show cis-regulatory interactions of the upstream CNEs with the SHOX promoter. In addition, our study revealed a low frequency of upstream CNVs in a sizeable ISS cohort. Finally, the 4C-seq interaction and H3K27ac ChIP-seq profiles revealed that the cis-regulatory domain of SHOX extends beyond 1 Mb surrounding SHOX. Thus, novel cis-regulatory elements may be located upstream and downstream of SHOX, of which variations might contribute to SHOX-deficient phenotypes.
Materials and Methods
Luciferase assays
The enhancer reporter constructs contained the upstream enhancers, CNE-5, CNE-3 and CNE-2 upstream of the human SHOX promoter (−432 to +5 bp, NM_000451). The sequences including the upstream enhancers were amplified using the following primers: 5′- CAAACACGGAACAGCACACT -3′ and 5′- CCTGGGACAGACACGACC -3′ for CNE-5, 5′- CGAGGTGGATCAAAGTG -3′ and 5′- TGCTCTGCCATATCCTCAATC -3′ for CNE-3 and 5′- ACATGACAGCCGGGCCTCTG -3′ and 5′- GCGAGCCATAAAACAAGCTG -3′ for CNE-2 and cloned into the pGL3SHOXprom vector, upstream of the promoter (8), thus mimicking its chromosomal localization. The enhancers were also cloned downstream of the luciferase gene to test out localization dependency. The enzymes utilized were KpnI and NheI for cloning the enhancers upstream of the SHOX promoter and BamHI and SalI for cloning downstream of the luciferase gene. Luciferase assays were undertaken in human U2OS osteosarcoma cells, as previously reported8. The downstream SHOX enhancers, CNE4, CNE5, ECR1 and ECS4/CNE96,8 were also cloned and employed as positive controls while ECS55 and the empty pGL3SHOXprom vector (i.e. no enhancer) were used as negative controls. The data shown is the mean and standard deviations of three replicates of each transfection and two biological replicates, thus a total of six points for each enhancer.
Patient cohort
A total of 501 probands with ISS were referred to the Center for Medical Genetics in the Ghent University Hospital for genetic testing of the SHOX region. An informed consent was obtained from all subjects. Revisiting of clinical records was performed in cases in whom an upstream CNV was found. Whenever possible, clinical data included: sex, age, birth details, height standard deviation score (SDS) according to national standards, physical examination of extremities, X-rays of the lower arm and family histories including parental heights SDS (Table 1). The study was conducted in accordance with the tenets of Helsinki and was approved by the Ghent University Hospital Ethics Committee (approval 2004/094).
Pre-screening of SHOX and the neighbouring PAR1 region
Prior to this study, all cases were genotyped using our previously described PAR1 qPCR-based copy number analysis test18. As coding mutations of SHOX were excluded in only 56 of these cases by Sanger sequencing, the remainder of patients underwent in-house developed targeted next-generation sequencing (NGS) of the coding exons and intron-exon boundaries of SHOX (MiSeq, Illumina, San Diego, CA)23. Primers and PCR conditions used for the amplification of the coding regions of SHOX are available upon request.
Upstream qPCR-based copy number profiling
CNV analysis was performed as previously described with minor modifications (Supplementary Data S6)18. Three amplicons, one for each upstream CNE (CNE-5, CNE-3 and CNE-2), were designed and subjected to stringent in silico and in vitro validations to guarantee their specificity and efficiency39. Next, qPCR reactions were carried out on the LightCycler 480 Instrument II (Roche Applied Science, Penzberg, Germany) and data-analysis was subsequently performed with the commercially available qBasePlus software (Biogazelle NV, Zwijnaarde, Belgium)40. Two reference genes, ZNF80 and GPR15, were used for normalization of the relative quantities and three positives controls with known copy number were used as a reference to calculate the copy numbers.
Array-based comparative genomic hybridization (arrayCGH)
Genome-wide copy number profiling was performed on 180 K oligonucleotide arrays (Agilent Technologies, Santa Clara, CA). In addition, a custom high-resolution 8 × 60 K Agilent microarray was designed using the online design tool eArray, targeting a region of 693 kb around SHOX (chrX:247,599–940,876; UCSC, Human Genome Browser, hg19). Hybridizations were performed according to manufacturer’s instructions with minor modifications. The results were subsequently visualized in arrayCGHbase41.
Chromosome conformation capture assays (4C-seq)
The experimental chicken procedures have been performed following the protocols approved by the Ethical Committee for Animal Research from Consejo Superior de Investigaciones (CSIC) according to the European Union regulations. The 4C-seq assay was performed as previously reported33,42,43,44. HH28 chicken embryos were dissected and limbs were processed to obtain approximately 10 million isolated cells. Human U2OS cells were processed to obtain 4.5 million cells. Human and chicken samples were equally processed. Cells were lysed (lysis buffer: 10 mM Tris-HCl pH 8, 10 mM NaCl, 0.3% IGEPAL CA-630 (Sigma-Aldrich, St. Louis, MO), 1X protease inhibitor cocktail (Roche Applied Science), the DNA digested with DpnII endonuclease (New England Biolabs, Ipswich, MA) and ligated with T4 DNA ligase (Promega). Subsequently, Csp6I endonuclease (Thermo Fisher Scientific, Waltham, MA) was used in a second round of digestion and the DNA was ligated again. Specific primers were designed at the Shox promoter with Primer3 v. 0.4.045 (Supplementary Data S7). Illumina adaptors were included in the primers sequence and 16 PCRs were performed with Expand Long Template PCR System (Roche Applied Science) and pooled together. This library was purified with a High Pure PCR Product Purification Kit (Roche Applied Science); its concentration measured using the Quanti-iTTM PicoGreen dsDNA Assay Kit (Life technologies) and sent for deep sequencing. The 4C-seq data were analysed as previously described43. Briefly, chicken and human raw sequencing data were de-multiplexed and aligned using the Chicken May 2006 assembly (galGal3) and Human February 2009 (hg19) respectively, as the reference genome. Reads located in fragments flanked by two restriction sites of the same enzyme, or in fragments smaller than 40 bp were filtered out. Mapped reads were then converted to reads-per-first-enzyme-fragment-end units and smoothed using a mean running window algorithm. Data was submitted to GEO under accession GSE65959.
Additional Information
How to cite this article: Verdin, H. et al. Profiling of conserved non-coding elements upstream of SHOX and functional characterisation of the SHOX cis-regulatory landscape. Sci. Rep. 5, 17667; doi: 10.1038/srep17667 (2015).
References
Rao, E. et al. Pseudoautosomal deletions encompassing a novel homeobox gene cause growth failure in idiopathic short stature and Turner syndrome. Nat Genet 16, 54–63 (1997).
Rao, E. et al. The Leri-Weill and Turner syndrome homeobox gene SHOX encodes a cell-type specific transcriptional activator. Hum Mol Genet 10, 3083–3091 (2001).
Clement-Jones, M. et al. The short stature homeobox gene SHOX is involved in skeletal abnormalities in Turner syndrome. Hum Mol Genet 9, 695–702 (2000).
Kleinjan, D. A. & Van Heyningen, V. Long-range control of gene expression: emerging mechanisms and disruption in disease. Am J Hum Genet 76, 8–32 (2005).
Fukami, M., Kato, F., Tajima, T., Yokoya, S. & Ogata, T. Transactivation function of an approximately 800-bp evolutionarily conserved sequence at the SHOX 3′ region: implication for the downstream enhancer. Am J Hum Genet 78, 167–170 (2006).
Sabherwal, N. et al. Long-range conserved non-coding SHOX sequences regulate expression in developing chicken limb and are associated with short stature phenotypes in human patients. Hum Mol Genet 16, 210–222 (2007).
Durand, C. et al. Enhancer elements upstream of the SHOX gene are active in the developing limb. Eur J Hum Genet 18, 527–532 (2010).
Benito-Sanz, S. et al. Identification of the first recurrent PAR1 deletion in Leri-Weill dyschondrosteosis and idiopathic short stature reveals the presence of a novel SHOX enhancer. J Med Genet 49, 442–450 (2012).
Kenyon, E. J., McEwen, G. K., Callaway, H. & Elgar, G. Functional Analysis of Conserved Non-Coding Regions Around the Short Stature hox Gene (shox) in Whole Zebrafish Embryos. PLoS ONE 6, e21498 (2011).
Cotney, J. et al. The evolution of lineage-specific regulatory activities in the human embryonic limb. Cell 154, 185–196 (2013).
Albuisson, J. et al. Clinical utility gene card for: Leri-Weill dyschondrosteosis (LWD) and Langer mesomelic dysplasia (LMD). 20, 1–4 (2012).
Shears, D. J. et al. Mutation and deletion of the pseudoautosomal gene SHOX cause Leri-Weill dyschondrosteosis. Nat Genet 19, 70–73 (1998).
Belin, V. et al. SHOX mutations in dyschondrosteosis (Leri-Weill syndrome). Nat Genet 19, 67–69 (1998).
Flanagan, S. F. et al. Prevalence of mutations in the short stature homeobox containing gene (SHOX) in Madelung deformity of childhood. J Med Genet 39, 758–763 (2002).
Huber, C., Rosilio, M., Munnich, A. & Cormier-Daire, V . French SHOX GeNeSIS Module. High incidence of SHOX anomalies in individuals with short stature. J Med Genet 43, 735–739 (2006).
Hirschfeldova, K. et al. SHOX gene defects and selected dysmorphic signs in patients of idiopathic short stature and Léri–Weill dyschondrosteosis. Gene 491, 123–127 (2012).
Rappold, G. A. et al. Deletions of the homeobox gene SHOX (short stature homeobox) are an important cause of growth failure in children with short stature. J Clin Endocrinol Metab 87, 1402–1406 (2002).
D’haene, B. et al. Improved Molecular Diagnostics of Idiopathic Short Stature and Allied Disorders: Quantitative Polymerase Chain Reaction-Based Copy Number Profiling of SHOX and Pseudoautosomal Region 1. J Clin Endocrinol Metab 95, 3010–3018 (2010).
Fukami, M., Okuyama, T., Yamamori, S., Nishimura, G. & Ogata, T. Microdeletion in the SHOX 3′ region associated with skeletal phenotypes of Langer mesomelic dysplasia in a 45,X/46,X,r(X) infant and Leri-Weill dyschondrosteosis in her 46,XX mother: Implication for the SHOX enhancer. Am J Med Genet A 137A, 72–76 (2005).
Benito-Sanz, S. et al. A novel class of pseudoautosomal region 1 deletions downstream of SHOX is associated with Leri-Weill dyschondrosteosis. Am J Hum Genet 77, 533–544 (2005).
Benito-Sanz, S. et al. Clinical and Molecular Evaluation of SHOX/PAR1 Duplications in Leri-Weill Dyschondrosteosis (LWD) and Idiopathic Short Stature (ISS). J Clin Endocrinol Metab 96, E404–E412 (2011).
Benito-Sanz, S. et al. Identification of the first PAR1 deletion encompassing upstream SHOX enhancers in a family with idiopathic short stature. Eur J Hum Genet 20, 125–127 (2012).
De Leeneer, K. et al. Flexible, Scalable and Efficient Targeted Resequencing on a Benchtop Sequencer for Variant Detection in Clinical Practice. Hum Mutat 36, 379–387 (2015).
MacDonald, J. R., Ziman, R., Yuen, R. K. C., Feuk, L. & Scherer, S. W. The database of genomic variants: a curated collection of structural variation in the human genome. Nucleic Acids Res 42, D986–992 (2014).
Firth, H. V. et al. DECIPHER: Database of Chromosomal Imbalance and Phenotype in Humans Using Ensembl Resources. Am J Hum Genet 84, 524–533 (2009).
Pang, A. W. C., MacDonald, J. R., Yuen, R. K. C., Hayes, V. M. & Scherer, S. W. Performance of high-throughput sequencing for the discovery of genetic variation across the complete size spectrum. G3 4, 63–65 (2014).
Teague, B. et al. High-resolution human genome structure by single-molecule analysis. Proc Natl Acad Sci USA 107, 10848–10853 (2010).
Redon, R. et al. Global variation in copy number in the human genome. Nature 444, 444–454 (2006).
Delpretti, S. et al. Multiple enhancers regulate Hoxd genes and the Hotdog LncRNA during cecum budding. Cell Rep 5, 137–150 (2013).
Andrey, G. et al. A switch between topological domains underlies HoxD genes collinearity in mouse limbs. Science 340, 1234167 (2013).
Montavon, T. et al. A regulatory archipelago controls Hox genes transcription in digits. Cell 147, 1132–1145 (2011).
Bulger, M. & Groudine, M. Looping versus linking: toward a model for long-distance gene activation. Genes Dev 13, 2465–2477 (1999).
Dekker, J., Rippe, K., Dekker, M. & Kleckner, N. Capturing chromosome conformation. Science 295, 1306–1311 (2002).
Spielmann, M. & Klopocki, E. CNVs of noncoding cis-regulatory elements in human disease. Curr Opin Genet Dev 23, 249–256 (2013).
Rao, S. S. P. et al. A 3D Map of the Human Genome at Kilobase Resolution Reveals Principles of Chromatin Looping. Cell 159, 1665–1680 (2014).
Dixon, J. R. et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 485, 376–380 (2012).
Tsuchiya, T. et al. Compound heterozygous deletions in pseudoautosomal region 1 in an infant with mild manifestations of langer mesomelic dysplasia. Am J Med Genet A 164, 505–510 (2014).
Bunyan, D. J., Taylor, E.-J., Maloney, V. K. & Blyth, M. Homozygosity for a novel deletion downstream of the SHOX gene provides evidence for an additional long range regulatory region with a mild phenotypic effect. Am J Med Genet A 164, 2764–2768 (2014).
D’haene, B., Vandesompele, J. & Hellemans, J. Accurate and objective copy number profiling using real-time quantitative PCR. Methods 50, 262–270 (2010).
Hellemans, J., Mortier, G., De Paepe, A., Speleman, F. & Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 8, R19 (2007).
Menten, B. et al. arrayCGHbase: an analysis platform for comparative genomic hybridization microarrays. BMC Bioinformatics 6, 124–124 (2005).
Hagège, H. et al. Quantitative analysis of chromosome conformation capture assays (3C-qPCR). Nat Protoc 2, 1722–1733 (2007).
Noordermeer, D. et al. The dynamic architecture of Hox gene clusters. Science 334, 222–225 (2011).
Splinter, E., De Wit, E., Van de Werken, H. J. G., Klous, P. & De Laat, W. Determining long-range chromatin interactions for selected genomic sites using 4C-seq technology: from fixation to computation. Methods 58, 221–230 (2012).
Rozen, S. & Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132, 365–386 (2000).
Acknowledgements
We wish to thank Eveline Debals, Dimitri Broucke, Peter Degrave, Machteld Baetens and Brecht Crombez for their expert technical assistance. We are most grateful to the families who participated in this study. This study makes use of data generated by the DECIPHER Consortium. A full list of centres who contributed to the generation of the data is available from http://decipher.sanger.ac.uk and via email from decipher@sanger.ac.uk. H.V. is a postdoctoral fellow of the FWO. E.D.B. and B.C. are Senior Clinical Investigators of the FWO. This study is supported by FWO grant G079711N (CIS-CODE). Spanish and Andalusian government grants BFU2010-14839, BFU2013-41322-P and BIO-396 to J.L.G.S. and SAF2012-30871 to K.E.H. and S.B.S. Andalusian government supports A.F.M. as the scientific manager of the CABD´s Aquatic Vertebrates Platform. CIBERER supports S.B.S. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
H.V. and E.D.B. designed the study; S.J., B.C., K.D.W., J.D.S. and I.F. collected the patient samples and clinical data; H.V., A.F.M. and S.B.S. conducted the experiments; H.V., A.F.M., S.B.S., B.M., K.E.H., J.L.G.S. and E.D.B. analysed and interpreted the results; H.V., K.E.H., J.L.G.S. and E.D.B. drafted the manuscript. All authors reviewed and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Verdin, H., Fernández-Miñán, A., Benito-Sanz, S. et al. Profiling of conserved non-coding elements upstream of SHOX and functional characterisation of the SHOX cis-regulatory landscape. Sci Rep 5, 17667 (2016). https://doi.org/10.1038/srep17667
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep17667
This article is cited by
-
Rare dosage abnormalities flanking the SHOX gene
Egyptian Journal of Medical Human Genetics (2021)
-
Identification of a limb enhancer that is removed by pathogenic deletions downstream of the SHOX gene
Scientific Reports (2018)
-
Identification of 15 novel partial SHOX deletions and 13 partial duplications, and a review of the literature reveals intron 3 to be a hotspot region
Journal of Human Genetics (2017)
-
Copy Number Variation Disorders
Current Genetic Medicine Reports (2017)
-
Systematic molecular analyses of SHOX in Japanese patients with idiopathic short stature and Leri–Weill dyschondrosteosis
Journal of Human Genetics (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.