Abstract
Recent innovations in ambient ionization technology for the direct analysis of various samples in their native environment facilitate the development and applications of mass spectrometry in natural science. Presented here is a novel, convenient and flame-based ambient ionization method for mass spectrometric analysis of organic compounds, termed as the ambient flame ionization (AFI) ion source. The key features of AFI ion source were no requirement of (high) voltages, laser beams and spray gases, but just using small size of n-butane flame (height approximately 1 cm, about 500 oC) to accomplish the rapid desorption and ionization for direct analysis of gaseous-, liquid- and solid-phase organic compounds, as well as real-world samples. This method has high sensitivity with a limit of detection of 1 picogram for propyphenazone, which allows consuming trace amount of samples. Compared to previous ionization methods, this ion source device is extremely simple, maintain-free, low-cost, user–friendly so that even an ordinary lighter (with n-butane as fuel) can achieve efficient ionization. A new orientation to mass spectrometry ion source exploitation might emerge from such a convenient, easy and inexpensive AFI ion source.
Similar content being viewed by others
Introduction
The features of high specificity, high speed and high sensitivity make mass spectrometry a powerful and versatile tool in the field of the analytical science. To meet the ever-increasing demand of nature science, it is urgent to develop new and effective ionization technologies of mass spectrometry to produce target ions. Traditional soft ionization methods, e.g., electrospray ionization (ESI)1,2,3, matrix-assisted laser desorption ionization (MALDI)4,5,6, have largely expanded the scopes of MS ionization, capable to handle various molecules with wide range of polarities and molecular weights, for example proteins, bacteria and metabolites. However, they require intricate sample pretreatments and complex devices, which render disturbance of analyte environment, requirement of multi-parameter adjustment and specialized training. The inventions of desorption electrospray ionization (DESI)7,8,9 and direct analysis in real time (DART)10,11 have triggered overwhelming development breakthrough of mass spectrometry. Namely, a new family of ionization methods occurs, known as ambient mass spectrometry without tedious sample preparation and operation in sample natural environment12,13,14,15,16,17,18,19,20. The past ten years witnessed the rapid development of ambient ionization methods which are becoming increasingly prosperous. Although ambient ionization technologies have simplified analytical procedure and improved ionization capabilities, some are still inconvenient given that the auxiliary gases, solvents, laser beams, electrical power consumption, etc. are required for operation. Several of these ambient ionization methods include matrix-assisted laser desorption electrospray ionization (MALDESI)21, laser ablation metastable-induced chemical ionization(LAMICI)22, desorption electrospray/metastable-induced ionization(DEMI)23, plasma assisted multiwavelength laser desorption ionization(PAMLDI)24 and ambient pressure thermal desorption ionization (APTDI)25,26, etc27,28,29. For further enlarging the application scopes of ambient ionization technologies, there is still a great necessity to engage in developing a more convenient and simpler ionization method to make whenever and wherever in-situ MS analysis feasible.
As we all know, the use of fire has paramount significance in the human civilization history and the applications of flame in the field of analytical science also have abundant history. The widely used flame-based analytical technologies need high temperature (usually up to thousands of degrees) to provide enough energy to atomize the analytes for further spectroscopy analyses, such as flame atomic absorption spectroscopy (up to 2000 oC)30, flame atomic emission spectroscopy31, flame atomic fluorescence spectroscopy32 and inductively coupled plasma mass spectrometry (up to 4000 oC)33. These technologies have been widely applied to constant and trace element analysis in the fields of metallurgy, geology, mining, medicine, sanitation and food. Flame-based chromatographic detectors (e.g. flame ionization detector34, nitrogen phosphorus detection35, flame photometric detector36) used in gas chromatography (GC), high performance liquid performance (HPLC) and thin layer chromatography (TLC) have been extensively reported. These detectors take advantage of flame to combust organic compounds separated from GC, HPLC, TLC and detect electrical signal stemming from the transformation of ion currents. Some papers reported flame-based ionization sources, for example air-acetylene flame37 and air-hydrogen flame38, which also reached to thousands of temperature and were used for the determination of isotope ratio of element.
Inspired by the idea of the ion generation processes from the flame, herein we describe the development and application of a highly convenient ambient ionization technology, ambient flame ionization (AFI) ion source. The flame temperature of AFI-MS is comparatively lower at about 500 oC (when n-butane was used as fuel and with low flow rate about 15 ml/min) and the experimental results showed that AFI-MS was an ideal soft ionization technology for analysis of organic compounds (usually generating quasi-molecular ion) by fast touching the samples with outer flame without obvious signals caused by degradation, oxidation and pyrolysis. AFI only requires ambient flame, that is, operation in a laser-, high voltage-, spray gases -free condition. The capability of AFI was verified by analyzing organic compounds and various real-world samples without intricate pretreatment prior to analysis. The applicability of AFI in analytical science was demonstrated by the direct and convenient analysis of real-world samples with high sensitivity, for example, pharmaceutical tablets, xenobiotics in fruit peels and vegetable surfaces and even pork fat.
Results
AFI instrumentation
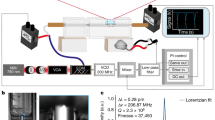
Figure 1 shows the photograph of AFI-MS device. In this device, the size of flame is controlled by pressure controlling valve and exactly adjusted by the flow micro-adjusting valve. An array of sample rods are prone to performing sequence sample analysis. In the AFI, the process of fuel combustion (here is n-butane) produces abundant energies which are released and translated to kinetic energy. The high-speed collisions of fast-moving reactive species in the n-butane flame go through various chemical ionization reactions. The reactive species in the flame of alkane were reported, such as: CHO+ and H3O+39,40,41,42,43. The ion-molecule reactions of these reactive species in the n-butane flame with analytes were important for the ionization of AFI-MS. The experimental results showed that the major ionization mode of AFI-MS is protonation of various organic compounds. The ions in the gas phase formed in the outer flame region could be easily transferred into MS entrance caused by the vacuum of mass spectrometer (Supplementary Fig. S1). Therefore, the distance of the flame to the MS entrance is important for the sensitivity of the AFI-MS method. The result demonstrated that the optimal distance between the center of flame and the inlet to mass spectrometer was 1 cm (Supplementary Fig. S2a).
The photograph of ambient flame ionization mass spectrometry (AFI-MS) apparatus for direct analysis of the sample.
1. sample rods, 2. butane transferring pipe, 3. flow micro-adjust valve, 4. pressure controlling valve. The analytes could be desorbed and ionized rapidly in the outer flame region and the ions generated by AFI could be transferred and detected by the mass spectrometry. The optimal distance between the centre of flame and the inlet to mass spectrometer is 10 mm. The ideal size of butane flame (height about 1 cm) is controlled by pressure controlling valve and exactly adjusted by flow micro-adjusting valve.
Flames of different fuels were compared, such as alkane, alcohol, ester and ketone (Supplementary Fig. S2b). The results demonstrated that the optimal fuel was n-butane. Compared with n-butane, they give much poor performance in the same experiment protocol. What is more, the gas nature of n-butane is easy to control by changing flow rate; however, the generation of flame about liquid fuels, such as alcohol, ethyl acetate and acetone have to use cotton thread and the flame size is not easy to control. Considering the most important ionization is protonation in AFI-MS analysis, it was proposed that the much lower proton affinity of n-butane (the data of proton affinity of n-butane is not available, data of iso-butane is referred)44 than the analytes might be a critical reason for the higher ionization performance of n-butane. In the process of analysis, different introduction points of flame were compared (supplementary Table S1 and Supplementary Fig. S1). The result showed that samples directly subjected to the outer flame were optimal in view of relative standard deviation and signal/noise. It is possible that the combustion of outer flame generates high temperature and more active species, which was beneficial for desorption and ionization of samples (Supplementary Fig. S2). The nature of flame can be controlled by changing the parameters of combustion, such as fuel flow rate and flame size (supplementary Table S2). In the AFI-MS analysis, the flow of butane is low (usually 15 mL/min) and no assisting air or oxygen flow is applied. Thus the flame temperature in the AFI-MS experiments usually remains at about 500 oC and is ideal for fast analysis of organic compounds, which is able to accomplish the rapid desorption and ionization of analyte and usually does not cause the obvious thermal degradation and oxidation of the analytes.
What is more, the device of AFI ion source is extremely simple and portable which only calls for ordinary flame to perform ionization without assistances of (high) voltages, laser beams and spray gases. Rather amazingly, the lighter (with n-butane as fuel) was also able to accomplish ionization (Supplementary Fig. S3). As an ionization source, the lighter is easy to carry and operate, lightweight, universally available and convenient, especially combining with portable mass spectrometry45,46,47 for in-situ and in-field analysis.
Analysis of trace vapor molecules
Initial experiments were carried out to show the convenience of AFI-MS by direct analysis of trace vapor molecules. The vial containing liquid analyte with low vapor pressure was positioned approximately 1 m away from the AFI and opened for a few seconds to expose trace above vapors to ambient air, while the flame was lit and mass spectrometer kept operating. After several seconds, resulting mass spectra were recorded with high sensitivity. Methyl salicylate, a chemical warfare agent simulant, was detected in its protonated form [M+H]+ of m/z 153 (Fig. 2a). The signal observed for dimethyl sulfoxide was the protonated dimer (m/z 157) as shown in Fig. 2b, whereas for 2-methoxyethyl ether the protonated monomer (m/z 135) was mainly observed as shown in Fig. 2c. To demonstrate the stability of AFI-MS in volatile sample analysis, the relative standard deviation (%RSD) and signal/noise (S/N) of dimethyl sulfoxide, 2-methoxyethyl ether and methyl salicylate were provided (see supplementary Table S3). On the other hand, the trace vapor drift introduction mode was compared to the in-flame mode, that is, the sample rod dipped with approximately 0.5 uL dimethyl sulfoxide was subjected directly to flame. Both modes are able to obtain clear mass spectra and ideal signal/noise ratio (supplementary Table S4). These results demonstrate that AFI is able to directly detect trace chemical vapors. Based on this advantage, the AFI has potential to be used for direct and in-situ analysis of dangerous chemicals such as chemical warfare agents and toxic industrial chemicals, which pose serious risks to public security and human health. The analytical process of chemical vapors is extremely easy, that is, only a small pot of n-butane gas or just an ordinary lighter is required to accomplish ionization without any specialized training. What is more, no signal disturbance and environment pollution occur in the analysis process in terms of solvent-free analysis.
Liquid sample analysis
Another paramount application of AFI-MS is direct analysis of diverse solution of organic compounds containing polar, nonpolar and organometallic compounds. In the analysis of polar and high polar compounds, [M+H]+ is normally the primary spectra characteristic, such as 6-chloroguanine, sudan1 and H-ALA-GLY-OH (Supplementary Fig. S4 and Supplementary Fig. S5). Figure 3a showed the signal at m/z 203, identified as protoned phenyl sulfoxide with a high-quality mass spectrum. Figure 3b shows the peak of high polar H-PHE-PHE-OH at m/z 313, which was detected as a protonated molecule. The AFI can also be able to test organometallic compounds such as ferrocene and nonpolar compounds like anthracene which of the major species shown in the spectra were the molecule ions respectively at m/z 186 (Fig. 3c) and m/z178 (Supplementary Fig. S6). These findings suggest that AFI is a soft ionization method that M+· and [M+H]+ are normally the principal spectra characteristic with little or no fragmentation ions. AFI-MS is also capable of direct analysis of solid of organic compounds on the top of sample rod (Supplementary Fig. S7). Because helium and nitrogen might bring the powder samples into mass spectrometer, the direct analysis of solid compounds in powder state using DART is liable to cause contaminations and memory effects. While the analytical process of AFI-MS without assisting gas does not have such problem. What is more, the AFI is simpler and more convenient to perform the in-situ and “green” of solvent-free analysis.
Direct analysis of real-world samples
The capability of AFI was demonstrated by convenient analysis of real-world samples without prior treatment via fast touching the samples in the outer flame. The movie presents a live demonstration of AFI-MS where various real-world samples such as fruit, vegetable, meat, beverage, etc. were rapidly and directly detected while the synchronous computer screen captures the signal responses of mass spectrometry in real time (see Supplementary Movie S1). The food safety issue is crucial for public health and has been focused on momentous attention taking into account ever-increasing food scandals. A vital application of the AFI-MS is direct and rapid analysis of xenobiotics (e.g., illegal additives and pesticide residues) in fruit peels and vegetable surfaces without any treatment and chemical contamination. The susceptible fruit and vegetable are generally treated with fungicides to protect them from rotting for long-time storage48,49. Figure 4a shows the characteristic isotopic cluster peaks at m/z 297 and m/z 299, corresponding to protonated imazalil50, a kind of widely used fungicide. The confirmation of imazalil was supported by signal accurate mass measurement, the isotopic pattern of two chlorides and accurate mass element composition (Supplementary Table S7). Animal fat sample was also analyzed directly by AFI. Major constituents of fat are triacylglycerols (TAGs) and their determinations are important due to their vital impact to health51,52,53. Although many technologies are available for determination of TAGs, some of these methods need long and tedious sample pretreatment process54,55,56. Figure 4b showed, as an instance, direct analysis of pork fat using AFI-MS without any pretreatment except cutting fat to pieces. The pivotal finding was that triglycerides were detected (depicted in Supplementary Table S5). Aside from TAGs, some diglycerides (DAGs) were also detected (Supplementary Table S6).
AFI-MS rapid analysis of real-world samples by fast touching samples to the outer flame.
(a) The peel of apple without any pretreatment. (b) Small pieces of pork fat with length 5 mm and width 5mm. (c) Drug tablet containing 4-acetamidophenol (325 mg), (1S,2S)-(+)pseudoephedrine hydrochloride (30 mg), N-(2-Diphenylmethoxyethyl)-N,N-dimethylamine hydrochloride (25 mg), dextromethorphan hydrobromide monohydrate (15 mg). Drug tablets were scraped off a thin layer of tablet and expose the subsurface active materials. Four scans were combined to produce the mass spectrum in AFI-MS analysis of this drug tablet.
The advantageous applications of AFI-MS in the analysis of real-world samples have been further proved by direct and rapid analysis of active ingredients of pharmaceuticals in the form of tablet, for example, azithromycin dispersible tablets, metronidazole tablets and compound paracetamol tablets (II) (Supplementary Fig. S8 and Supplementary Fig. S9). In the analysis of tablets containing paracetamol, pseudoephedrine hydrochloride, diphenhydramine hydrochloride and dextromethorphan hydrobromide, all of four active ingredients were simultaneously detected, as shown in Fig. 4c. The peaks at m/z 152, 166, 256 and 272 were respectively corresponded to protonated active ingredients of 4-acetamidophenol, pseudoephedrine, diphenhydramine and dextromethorphan, respectively. The detection of active ingredients in tablets was further confirmed by tandem mass spectrometry (Supplementary Fig. S10), consistent with authentic compounds. In order to explore the analysis sensitivity of AFI-MS, the limit of detection of AFI-MS in detection of propyphenazone was obtained at 1 picogram level (see Supplementary Fig. S11). AFI can be also used to direct detect allicin, a primary thiosulfate in the garlic. The peaks at m/z 163 and m/z 180, respectively corresponded to [M+H]+ and [M+NH4]+ (see Supplementary Fig. S13). These results shown here demonstrate that analytical procedures of real-world samples are solvent-free, that is, without any chemical contamination, which presents a “green” analytical process.
Analysis of the sample in the negative ion mode
After testing the application of AFI-MS in positive ion mode, the negative ion mode was also performed. The performance of AFI-MS in negative ion mode is not good as the positive ion mode. For instance, only weak signal of [M-H]– at m/z 139 (Supplementary Fig. S14) was obtained from AFI-MS analysis of 3-fluorobenzoic acid. It is possible that the negative ions with lone pair electrons are not stable in oxidation environment of AFI-MS. We are still working to search a better condition for AFI-MS analysis of organic compound in negative ion mode.
Discussion
A novel ambient ionization technique, ambient flame ionization source, has been developed for only requirement of ambient flame without other assistances. The ionization process in the AFI is gentle, generating charged intact molecular species with rare or no fragmentation ions. In addition, AFI is compatible with other atmospheric pressure ionization mass spectrometer, such as triple-quadrupole mass spectrometer (Supplementary Fig. S15). The unique characters of AFI-MS relied on the following critical points: the small flame by the combustion of n-butane in open air condition provides a focused heating zone (about 500 oC) for fast desorption of organic compounds and meanwhile the gaseous reactive species from the flame rapidly promoted the spontaneous ionization of the organic compound. Of course there are some potential competing processes might occur at same time, such as thermal degradation, oxidation, pyrolysis of analytes. Therefore, in order to get the better results and controlling side effects of competing oxidation and degradation, many parameters of AFI-MS could be optimized, such as: the choice of fuel, fuel flow, size of flame, sample introduction mode, sample introduction point in the flame and the distance between the flame and the inlet of MS.
AFI-MS technology have some similarity and differences with some previously reported thermal desorption/ionization techniques such as, ambient pressure thermal desorption ionization (APTDI)25 and pyrolysis mass spectrometry (PyMS)57. The APTDI is a popular ambient ionization method by rapid heating of the sample with the help of heated nitrogen gas flow (100 ~ 450 oC) for transferring the generated ions to the mass spectrometer25,26. The pyrolysis mass spectrometry (PyMS) mainly studies the decomposition products of macromolecular samples in high temperature pyrolysis (even to 600 oC) condition by suitable mass spectrometry methods. The similar point is that high temperature of outer flame (about 500 oC when n-butane was applied as fuel) in AFI-MS was also important factor to achieve rapid desorption of the organic compounds, but the different points are that the reactive species in the n-butane flame might be critical for the ionization process of AFI-MS. In the process of AFI-MS analysis, the melting and boiling phenomena were not obvious, but it is believed that they are important factors for sample desorption step. The reasons might be that: (1) the high sensitivity of AFI-MS required only small or trace amount of samples, (2) the focused heating zone of outer flame (about 500 oC) could provide enough energy in very short time to initiate the fast desorption and ionization of analytes. Most organic compounds responded fast in AFI-MS analysis and we found that normally the volatile organic compounds responded slightly faster than the solid samples with higher melt and boil point. In the AFI-MS analysis, the fast touching of samples with outer flame zone usually was applied in very short time usually at about 1–3 seconds.
The experimental results showed that the AFI-MS was an ideal method for mass spectrometric analyses of organic compounds and the applicability of AFI-MS technique has close relationship with the physicochemical properties of the analytes. Because the fast sample desorption process in the outer flame has close relationship to the melting and boiling point and the vapour pressure in ambient condition of the analyte. The ionization process of the gaseous analyte mostly depended on the ionization energy and proton affinity. The capabilities of AFI are demonstrated through rapid, direct, in-situ mass spectrometric analysis of sample in various states, such as volatile organic compounds (e.g. phenyl sulfoxide, 6-chloroguanine), medicine molecules in tables (e.g. azithromycin dispersible tablets, metronidazole tablets), amino acids, lipid molecules (e.g. pork fat). Because most of these organic compounds could be desorbed and ionized with the help of flame, most of these organic compounds were protonated in AFI-MS; however, the compound with low ionization energy, such as ferrocene (6.82 eV)58, anthracene (7.439 eV)59 give radical cations. All data demonstrated that AFI-MS is a promising approach in the related research area since it is fast, simple and also carries great potential for portability. As such, AFI-MS will be of interest to the analytical community at large, such as public security, environmental protection, therapeutic drug monitoring and food quality monitoring. Researches on the complicated combustion process in AFI-MS are still going on to harness its advantages for optimizing AFI-MS conditions and to reduce the competing side-processes, such as: degradation, oxidation, pyrolysis and combustion of analytes. In view of convenience and versatility, the AFI is potentially an attractive candidate to couple portable or miniature mass spectrometry to perform in-field analysis of target samples in their undisturbed environment and native states. Such simple and high-efficiency AFI method might have a bright future in direct, high-throughput and on-site analysis.
Methods
Sample collection
Chemical reagents were directly used without any further purification. Food was purchased from local stores without any further treatment. All of food was directly exposed to the flame without any treatment. Drug tablets were bought from local pharmacy. Coated tablets needed to scrape off a thin layer of the tablet and expose the subsurface active materials, whereas uncoated tablets were directly detected without any treatment. (See the Supplementary Information)
AFI-MS
Experiments were performed on a liner ion trap Fourier transform-ion cyclotron resonance ULTRA XL mass spectrometer (Thermo Fisher Scientific) and Finnigan TSQ Quantum Access™ triple-quadrupole mass spectrometer (Thermo Fisher Scientific, Waltham, MA) installed with a homemade AFI ion source. The basic operation conditions of a liner ion trap fourier transform-ion cyclotron resonance ULTRA XL mass spectrometer were set as follow: capillary voltage: 9 V; the capillary temperature, 250 oC; tube lens voltage: 100 V. The ion optics conditions were set as follow: multipole 00 offset voltage, −4 V; multipole 0 offset voltage, −4.5 V; multipole 1 offset voltage, −15.5 V; lens 0 voltage, −4.5 V; lens 1 voltage, −40.0 V; gate lens voltage, −48.0 V; front lens voltage, −5.5 V. Peak integration and data acquisition were performed through the instrument embedded Xcalibur® software. The optimal distance between the center of butane flame and the inlet to mass spectrometer is 1 cm (Supplementary Fig. 2a). The AFI-MS experiments could also performed in Thermo TSQ Quantum AccessTM triple-quadrupole mass spectrometer (Thermo-Fisher Scientific, Waltham, USA) (Supplementary Figure S15).
Additional Information
How to cite this article: Liu, X.-P. et al. Direct and Convenient Mass Spectrometry Sampling with Ambient Flame Ionization. Sci. Rep. 5, 16893; doi: 10.1038/srep16893 (2015).
References
Fenn, J. B. Electrospray wings for molecular elephants (nobel lecture). Angew. Chem. Int. Ed. 42, 3871–3894 (2003).
Whitehouse, C. M., Dreyer, R. N., Yamashita, M. & Fenn, J. B. Electrospray interface for liquid chromatographs and mass spectrometers. Anal. Chem. 57, 675–679 (1985).
Fenn, J. B., Mann, M., Meng, C. K., Wong, S. F. & Whitehouse, C. M. Electrospray ionization for mass spectrometry of large biomolecules. Science 246, 64–71 (1989).
Karas, M., Bachmann, D. & Hillenkamp, F. Influence of the wavelength in high-irradiance ultraviolet laser desorption mass spectrometry of organic molecules. Anal. Chem. 57, 2935–2939 (1985).
Karas, M., Bachmann, D., Bahr, U. & Hillenkamp, F. Matrix-assisted ultraviolet-laser desorption of nonvolatile compounds. Int. J. Mass Spectrom. Ion Processes 78, 53–68 (1987).
Harvey, D. J. Matrix-assisted laser desorption/ionization mass spectrometry of carbohydrates. Mass Spectrom. Rev. 18, 349–450 (1999).
Takáts, Z., Wiseman, J. M., Gologan, B. & Cooks, R. G. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science 306, 471–473 (2004).
Cooks, R. G., Ouyang, Z., Takáts, Z. & Wiseman, J. M. Ambient mass spectrometry. Science 311, 1566–1570 (2006).
Chen, H., Cotte-Rodrı́guez, I. & Cooks, R. G. Cis-diol functional group recognition by reactive desorption electrospray ionization (desi). Chem. Commun., 597– 599 (2006).
Cody, R. B., Laramée, J. A. & Durst, H. D. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal. Chem. 77, 2297–2302 (2005).
Gross, J. H. Direct analysis in real time-a critical review on dart-ms. Anal. and Bioanal. Chem . 406, 63–80 (2014).
Gómez-Rı́os, G. A. & Pawliszyn, J. Development of coated blade spray ionization mass spectrometry for the quantitation of target analytes present in complex matrices. Angew. Chem. Int. Ed. 53, 14503– 14507 (2014).
Santos, V. G. et al. Venturi easy ambient sonic-spray ionization. Anal. Chem. 83, 1375–1380 (2011).
Chen, H. W., Wortmann, A., Zhang, W. H. & Zenobi, R. Rapid in vivo fingerprinting of nonvolatile compounds in breath by extractive electrospray ionization quadrupole time-of-flight mass spectrometry. Angew. Chem. Int. Ed. 46, 580–583 (2007).
Harris, G. A., Galhena, A. S. & Fernandez, F. M. Ambient sampling/ionization mass spectrometry: Applications and current trends. Anal. Chem. 83, 4508–4538 (2011).
Nudnova, M. M., Sigg, J., Wallimann, P. & Zenobi, R. Plasma ionization source for atmospheric pressure mass spectrometry imaging using near-field optical laser ablation. Anal. Chem. 87, 1323–1329 (2015).
Zhang, J. T., Wang, H. Y., Zhu, W., Cai, T. T. & Guo, Y. L. Solvent-assisted electrospray ionization for direct analysis of various compounds (complex) from low/nonpolar solvents and eluents. Anal. Chem. 86, 8937–8942 (2014).
Haddad, R., Sparrapan, R. & Eberlin, M. N. Desorption sonic spray ionization for (high) voltage-free ambient mass spectrometry. Rapid Commun. Mass Spectrom. 20, 2901–2905 (2006).
Huang, M. Z., Cheng, S. C., Cho, Y. T. & Shiea, J. Ambient ionization mass spectrometry: A tutorial. Anal. Chim. Acta 702, 1–15 (2011).
Liu, P. Y., Forni, A. & Chen, H. Development of solvent-free ambient mass spectrometry for green chemistry applications. Anal. Chem. 86, 4024–4032 (2014).
Sampson, J. S., Hawkridge, A. M. & Muddiman, D. C. Generation and detection of multiply-charged peptides and proteins by matrix-assisted laser desorption electrospray ionization (maldesi) fourier transform ion cyclotron resonance mass spectrometry. J. Am. Soc. Mass Spectrom . 17, 1712–1716 (2006).
Galhena, A. S., Harris, G. A., Nyadong, L., Murray, K. K. & Fernández, F. M. Small molecule ambient mass spectrometry imaging by infrared laser ablation metastable-induced chemical ionization. Anal. Chem. 82, 2178–2181 (2010).
Nyadong, L., Galhena, A. S. & Fernández, F. M. Desorption electrospray/metastable-induced ionization: A flexible multimode ambient ion generation technique. Anal. Chem. 81, 7788–7794 (2009).
Zhang, J. L. et al. Thin layer chromatography/plasma assisted multiwavelength laser desorption ionization mass spectrometry for facile separation and selective identification of low molecular weight compounds. Anal. Chem. 84, 1496–1503 (2012).
Chen, H., Ouyang, Z. & Cooks, R. G. Thermal production and reactions of organic ions at atmospheric pressure. Angew. Chem. Int. Ed. 45, 3656–3660 (2006).
Demoranville, L. T. & Brewer, T. M. Ambient pressure thermal desorption ionization mass spectrometry for the analysis of substances of forensic interest. Analyst 138, 5332–5337 (2013).
Shiea, J. et al. Electrospray-assisted laser desorption/ionization mass spectrometry for direct ambient analysis of solids. Rapid Commun. Mass Spectrom. 19, 3701–3704 (2005).
Sampson, J. S. & Muddiman, D. C. Atmospheric pressure infrared (10.6 μm) laser desorption electrospray ionization (ir-ldesi) coupled to a ltq fourier transform ion cyclotron resonance mass spectrometer. Rapid Commun. Mass Spectrom. 23, 1989–1992 (2009).
Nemes, P. & Vertes, A. Laser ablation electrospray ionization for atmospheric pressure, in vivo and imaging mass spectrometry. Anal. Chem. 79, 8098–8106 (2007).
Willis, J. B. The development of the nitrous oxide acetylene flame for atomic absorption spectroscopy - a personal account. Spectrochim. Acta B . 52, 667–674 (1997).
Wacker, W. E. C., Fuwa, K. & Iida, C. Accuracy of determinations of serum magnesium by flame emission atomic absorption spectrometry. Nature 202, 659-& (1964).
Kirkbrig, G. Application of non-flame atom cells in atomic-absorption and atomic-fluorescence spectroscopy - review. Analyst 96, 609-& (1971).
Houk, R. S. Mass spectrometry of inductively coupled plasmas. Anal. Chem . 58, 97A–105A (1986).
McWilliam, I. G. & Dewar, R. A. Flame ionization detector for gas chromatography. Nature 181, 760–760 (1958).
Shin, H. S., Kim, S., Myung, S. W. & Park, J. S. Iodoacetonitrile and bromoacetonitrile as alkylating reagents for the nitrogen phosphorus detector. Anal. Chem. 67, 1853–1859 (1995).
Pandey, S. K. & Kim, K.-H. The fundamental properties of the direct injection method in the analysis of gaseous reduced sulfur by gas chromatography with a pulsed flame photometric detector. Anal. Chim. Acta 615, 165–173 (2008).
Taylor, H. E., Garbarino, J. R. & Koirtyohann, S. R. Flame ionization mass-spectrometry - isotope ratio determinations for potassium. Appl. Spectrosc. 45, 886–889 (1991).
Yu, L. L., Turk, G. C. & Koirtyohann, S. R. Characterization of a hydrogen flame as an ion source form mass spectrometry. J. Anal. At. Spectrom. 14, 669–674 (1999).
Calcote, H. F. Ion and electron profiles in flames. Symposium (International) on Combustion 9, 622–637 (1963).
Shcherbakov, N. D., Kabichev, G. I. & Serov, V. V. Mechanism of primary reactions of chemical ionization in hydrocarbon flames. Combust. Explos. Shock Waves 25, 429–432 (1989).
Porter, R. P., Clark, A. H., Kaskan, W. E. & Browne, W. E. A study of hydrocarbon flames. Symposium (International) on Combustion 11, 907–917 (1967).
Blades, A. T. Ion formation in hydrocarbon flames. Can. J. Chem. 54, 2919–2924 (1976).
Holm, T. Aspects of the mechanism of the flame ionization detector. J. Chromatogr. A 842, 221–227 (1999).
Hunter, E. P. L. & Lias, S. G. Evaluated gas phase basicities and proton affinities of molecules: An update. J. Phys. Chem. Ref. Data 27, 413–656 (1998).
Gao, L., Sugiarto, A., Harper, J. D., Cooks, R. G. & Ouyang, Z. Design and characterization of a multisource hand-held tandem mass spectrometer. Anal. Chem. 80, 7198–7205 (2008).
Ouyang, Z. et al. Rectilinear ion trap: Concepts, calculations and analytical performance of a new mass analyzer. Anal. Chem. 76, 4595–4605 (2004).
Gao, L., Song, Q., Patterson, G. E., Cooks, R. G. & Ouyang, Z. Handheld rectilinear ion trap mass spectrometer. Anal. Chem. 78, 5994–6002 (2006).
Thurman, E. M. et al. Discovering metabolites of post-harvest fungicides in citrus with liquid chromatography/time-of-flight mass spectrometry and ion trap tandem mass spectrometry. J. Chromatogr. A 1082, 71–80 (2005).
Navarro, M., Picó, Y., Marı́n, R. & Mañes, J. Application of matrix solid-phase dispersion to the determination of a new generation of fungicides in fruits and vegetables. J. Chromatogr. A 968, 201– 209 (2002).
Picó, Y., Farré, M. l., Soler, C. & Barceló, D. Identification of unknown pesticides in fruits using ultra-performance liquid chromatography–quadrupole time-of-flight mass spectrometry: Imazalil as a case study of quantification. J. Chromatogr. A 1176, 123–134 (2007).
Abbas, O., Pierna, J. A. F., Codony, R., Holst, C. V. & Baeten, V. Assessment of the discrimination of animal fat by ft-raman spectroscopy. J. Mol. Struct. 924-26, 294–300 (2009).
Syahariza, Z. A., Man, Y. B. C., Selamat, J. & Bakar, J. Detection of lard adulteration in cake formulation by fourier transform infrared (ftir) spectroscopy. Food Chem. 92, 365–371 (2005).
Dugo, P., Kumm, T., Fazio, A., Dugo, G. & Mondello, L. Determination of beef tallow in lard through a multidimensional off-line non-aqueous reversed phase-argentation lc method coupled to mass spectrometry. J. Sep. Sci. 29, 567–575 (2006).
Marcos Lorenzo, I., Pérez Pavón, J. L., Fernández Laespada, M. E., Garcı́a Pinto, C. & Moreno Cordero, B. Detection of adulterants in olive oil by headspace–mass spectrometry. J. Chromatogr. A 945, 221-230 (2002).
Parcerisa, J., Casals, I., Boatella, J., Codony, R. & Rafecas, M. Analysis of olive and hazelnut oil mixtures by high-performance liquid chromatography–atmospheric pressure chemical ionisation mass spectrometry of triacylglycerols and gas–liquid chromatography of non-saponifiable compounds (tocopherols and sterols). J. Chromatogr. A 881, 149–158 (2000).
Řezanka, T. & Řezanková, H. Characterization of fatty acids and triacylglycerols in vegetable oils by gas chromatography and statistical analysis. Anal. Chim. Acta 398, 253–261 (1999).
Otto, S. et al. Application of pyrolysis–mass spectrometry and pyrolysis–gas chromatography–mass spectrometry with electron-ionization or resonance-enhanced-multi-photon ionization for characterization of crude oils. Anal. Chim. Acta 855, 60–69 (2015).
Ryan, M. F., Eyler, J. R. & Richardson, D. E. Adiabatic ionization energies, bond disruption enthalpies and solvation free energies for gas-phase metallocenes and metallocenium ions. J. Am. Chem. Soc. 114, 8611–8619 (1992).
Hager, J. W. & Wallace, S. C. Two-laser photoionization supersonic jet mass spectrometry of aromatic molecules. Anal. Chem. 60, 5–10 (1988).
Acknowledgements
Authors are grateful for financial supports from the National Natural Science Foundation of China (21172250, 21475145 and 21472228) and Youth Innovation Promotion Association CAS (2013171).
Author information
Authors and Affiliations
Contributions
Y.L.G. and H.Y.W. conceived and designed the research; Y.L.G. contributed importantly to the discussion of results and manuscript refinement. X.P.L. performed the experiments with some assistance from H.Y.W. and H.Z. The manuscript was written by X.P.L. with contributions from H.Y.W., J.T.Z., M.X.W. and Q.W.S. All authors discussed and commented on the manuscript and supplementary information.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Liu, XP., Wang, HY., Zhang, JT. et al. Direct and Convenient Mass Spectrometry Sampling with Ambient Flame Ionization. Sci Rep 5, 16893 (2015). https://doi.org/10.1038/srep16893
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep16893
This article is cited by
-
Fast Eruption Desorption Ionization for Mass Spectrometric Analysis
Journal of the American Society for Mass Spectrometry (2018)
-
Sampling and analyte enrichment strategies for ambient mass spectrometry
Analytical and Bioanalytical Chemistry (2018)
-
Use of Interrupted Helium Flow in the Analysis of Vapor Samples with Flowing Atmospheric-Pressure Afterglow-Mass Spectrometry
Journal of the American Society for Mass Spectrometry (2017)
-
Direct Analysis and Quantification of Metaldehyde in Water using Reactive Paper Spray Mass Spectrometry
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.