Abstract
A near-infrared sensor for cyanide ion (CN−) was developed via internal charge transfer (ICT). This sensor can selectively detect CN− either through dual-ratiometric fluorescence (logarithm of I414/I564 and I803/I564) or under various absorption (356 and 440 nm) and emission (414, 564 and 803 nm) channels. Especially, the proposed method can be employed to measure β-glucosidase by detecting CN− traces in commercial amygdalin samples.
Similar content being viewed by others
Introduction
Cyanide is an important raw material of pharmaceuticals, insecticides and fertilisers, as well as an indispensable reagent in metallurgy and gilded industry1,2. However, CN− can bind into mitochondrial cytochrome oxidase and inhibit critical enzymatic cascades in organisms, thus, CN− is extremely detrimental. Consequent intracellular hypoxia often leads to central nervous system disorders, cardiovascular impairment or even death by suffocation within seconds to minutes3. It should be noted that CN− can be derived from a variety of sources such as those from industrial accidents, pharmaceuticals, fire smoke and even some common foods4. For example, ingested amygdalin, which is one of the most typical cyanogenic glycosides found in kernels such as peach, apricot, cherry and almond, would be hydrolysed by the widely distributed natural β-glucosidase and generated hydrogen cyanide (HCN) in living organisms, leading to disruption of normal physiological activities and may result in death caused by rapid suffocation5. Therefore, CN− monitoring has attracted much attention and many techniques such as voltammetry, titrimetry, electrochemical and chromatography have been developed6,7,8,9,10. Unfortunately, most of these methods require expensive equipment and are complicated for real-time analyses.
Recently, fluorometric detection has been increasingly used because of its low cost and rapid operation. In 2005, Geddes et al. reported a set of sensors for CN− detection based on complexation between boronic acid and CN−11. In 2008, Qin et al. described a reversible probe for CN− based on the conversion of zinc ions in the presence of Cu2+ and CN−12. On the basis of nucleophilic attack of the hydrogen-bonded carbonyl groups, Lee et al. developed a new method to detect CN− in 200913. In 2011, Lee et al. selectively detected CN− based on the addition reaction between indole moiety and CN−14. With a similar mechanism, Shiraishi et al. recently synthesised a new fluorescent probe to selectively and sensitively detect CN−15. Most of these probes are designed on ON-OFF and OFF-ON mechanisms16,17,18,19,20,21, wherein detection depends only on the changes in single emission and might be affected by external factors including excitation power, detector sensitivity and environment22.
According to literature14,15,23, CN− can conjugate with fluorescent dyes to modulate the ICT state and also elicit selective fluorescence changes. Thus, a novel fluorometric sensor SY (Fig. 1) was designed. SY displays an instantaneous (<5 s) fluorescence signal change in the presence of CN− (0–10 μM) in acetonitrile and a unique dual-ratiometric fluorescence (I414/I564 and I803/I564) can be achieved for more accurate detection, especially that CN− can be measured through multi-channels (under two absorption wavelengths of 356, 440 nm and three emission wavelengths of 414, 564, 803 nm), offering versatility during measurement. The applicability of SY was verified by its successful application in measuring β-glucosidase based on the above-mentioned typical hydrolysing ability towards amygdalin, which is useful in monitoring β-glucosidase level in living organisms because abnormal changes in β-glucosidase are linked to Gaucher’s disease, Parkinson disease, pulmonary hypertension and even central nervous system disorders24,25.
Results
UV-vis absorption titration spectra of SY upon addition of CN−
Firstly, the reaction time between SY and CN− was tested. As shown in Fig. S1 (ESI†), SY can complete the reaction with CN− within 5 s, indicating a rapid response of SY to CN−, which is beneficial in real-time detection. The UV-vis absorption titration spectra of SY (5 μM) were measured in acetonitrile at room temperature. As shown in Fig. 2a, the absorbance at 440 nm gradually decreased with the addition of CN− and a new peak at 356 nm appeared, indicating that a chemical reaction occurred between CN− and the indole group of SY. A colour change from yellow to colourless was dependent on CN− concentration (Fig. S6) and 10 μM CN− led to saturation. The logarithm of the absorbance ratio (A356/A440) produced a linear relationship (R2 = 0.996) with CN− concentration ranging from 0 μM to 7.5 μM (Fig. 2b) and the detection limit for CN− based on 3σ/slope was 9.1 × 10−8 M26. In addition, the absorption values at each single peak also showed excellent linear relationship with R2 = 0.998 (356 nm, 0–10 μM CN−) and R2 = 0.996 (440 nm, 0–10 μM CN−) respectively (Fig. S2). The detection limit was 5.8 × 10−7 M at 356 nm and 3.9 × 10−7 M at 440 nm under similar condition.
Fluorescence titration studies of SY upon addition of CN−
Secondly, fluorescence titration studies on SY (5 μM) towards CN− (0–10 μM) were also performed and a trend similar to that in UV-vis absorption titration was observed. With the addition of CN−, the peak at 564 nm gradually decreased and a new emission peak at 414 nm was recorded. It’s worth noting that a concentration-dependent emission at 803 nm gradually and evidently increased owing to the nucleophilic attack of CN−, which destroyed the conjugation of SY and blocked the ICT (Fig. 3a, Fig. S9–14)27. Accordingly, an excellent linear relationship (R2 = 0.996) was fitted between the logarithm of the fluorescence ratio (I803/I564) and CN− concentration from 0 μM to 9 μM (Fig. 3b). The detection limit (3σ/slope) for CN− was 7.3 × 10−8 M28. A second linear relationship (R2 = 0.998) was also obtained between the logarithm of the fluorescence ratio (I414/I564) and CN− concentration ranging from 0 μM to 10 μM at a detection limit of 5.4 × 10−8 M (Fig. 3c). As shown in Fig. S3 and S4, SY exhibited a good corresponding linear relationship with CN− under each of the following emission channels: R2 = 0.998 (414 nm, 0–10 μM CN−), 0.998 (564 nm, 0–10 μM CN−) and 0.995 (803 nm, 0–10 μM CN−). The SY performance was subsequently studied in a mixing solvent of acetonitrile and increasing amount of water (0–20%, V/V, Fig. S5). Overall, the results verified the stability of SY.
The fluorescence titration studies of SY towards CN−.
(a) Fluorescence spectra of SY (5 μM) upon addition of CN− (0–10 μM) at room temperature. Inset: enlarged fluorescence spectra from 750 nm to 900 nm, λex = 360 nm. (b) The corresponding linear relationship between the logarithm of the fluorescence ratio (I803/I564) in the presence of CN− (0–9 μM). (c) The corresponding linear relationship between the logarithm of the fluorescence ratio (I414/I564) in the presence of CN− (0–10 μM).
Competitive experiments for CN− selectivity
Furthermore, S2−, CO32−, ACO−, S2O32−, SO42−, I−, Br−, Cl−, H2PO4−, F−, SCN−, NO2−, glutathione (GSH), homocysteine (Hcy) and cysteine (Cys) were selected as interference ions to evaluate the selectivity of SY15,22. As shown in Fig. S7, with the addition of these various analytes (25 μM for each), SY did not show any significant fluorescence changes at 414 and 803 nm. By contrast, the addition of only 5 μM CN− enhenced fluorescence at 414 and 803 nm, indicating that SY can selectively detect CN−.
Discussion
1H NMR and MS were used to verify the mechanism of SY in CN− detection. As shown in Fig. S15a, the protons of the benzene ring in SY exhibited an upfield shift upon addition of CN−. Similar results were observed on the protons of methyl and methylene groups (Fig. S15b), indicating the function of CN−. The mass spectra (Fig. S24) further proved the SY-CN formation, m/z 462.36 (calc. = 462.20) corresponding to [SY + CN + H]+.
Application for measuring enzyme activity in vitro
To confirm the applicability of the proposed method, SY was employed to study the activity of β-glucosidase by using cyanogenic glycoside amygdalin as indicator and SY as signal receptor29. Moreover, β-Glucosidase catalysed the hydrolysis of amygdalin to free CN−, initiating a signal change in the fluorescence spectrometer resulting from the reaction between SY and CN− (Fig. 4). As shown in Table 1, the amount of CN− detected by SY (the logarithm of the fluorescence ratio I414/I564, I803/I564) matched the concentrations of β-glucosidase. The relative standard deviations (RSDs) of I414/I564 and I803/I564 were lower than 4.9% and indicative of fine accuracy, further demonstrating the potential application of this method in CN− determination. An alternative method (I414, I564 and I803, Table S1) was also provided to quantify CN−. However, the RSDs of I414, I564 and I803 were larger than those of I414/I564 and I803/I564, indicating that enhanced precision can be achieved by ratiometric method.
In conclusion, a novel near-infrared probe SY for CN− was successfully developed. SY exhibits a unique colorimetric in UV-vis and dual-ratiometric in fluorescence towards CN−, resulting in more accurate detection. Moreover, its multi-channel detection offers versatility during measurement. The minimum detection limit of SY was 5.4 × 10−8 M. Furthermore, SY was successfully used to measure β-glucosidase. Overall, the results indicate that SY is a selective sensor for CN− and a potential indicator of β-glucosidase.
Methods
Synthesis material and instruments
All solvents and chemical reagents were analytical grade and purchased from commercial suppliers. 1H NMR and 13C NMR spectra were recorded on a Bruker 500 AVANCE III spectrometer with chemical shifts reported in ppm at room temperature. Mass spectra were obtained with Thermo Fisher LCQ Fleet mass spectrometer (USA) and a LC/Q-Tof MS spectrometry (USA). Absorption spectra were collected by using a Shimadzu 1750 UV-visible spectrometer (Japan). Fluorescence spectra were measured with a Shimadzu RF-5301 fluorescence spectrometer (Japan) with both excitation and emission slits set at 10 nm if without further demonstration.
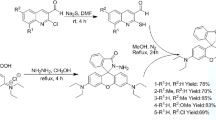
Compound (A) and (B) is prepared according to the reported literature30,31. Synthesis of (A) A mixture of 2-methylbenzimidazole (1.33 g, 5 mmol), p-phthaldialdehyde (1.34 g, 5 mmol) were added into 1.5 mL acetic anhydride and 1 mL acetic acid, stirring at 120°C for 6 h. Cooled to room temperature, 5 mL concentrated hydrochloric acid was added. Then 30% aqueous sodium hydroxide solution to make pH = 7, filtered and washed with water. The crude product was purified by column chromatography on silica gel using DCM to obtain a yellowish green crystal, 65% yield. 1H NMR (500 MHz, DMSO-d6) δ 10.02 (s, 1 H), 8.11 (d, J = 6.6 Hz, 1 H), 8.00 (dd, J = 6.6, 2.3 Hz, 3 H), 7.94 (d, J = 6.6 Hz, 2 H), 7.78 (q, J = 13.2 Hz, 2 H), 7.58–7.49 (m, 1 H), 7.48–7.40 (m, 1 H).
Synthesis of (B) 2,3,3-trimethylindolenine (1 mL, 5.3 mmol) and iodoethane (0.9 mL, 10.6 mmol) were mixed in CH3CN (15 mL), refluxing for 24 h. Cooled to room temperature, filtered, washed with ethyl acetate (3 × 10 mL) to obtain a pink crystal, 85% yield.
Synthesis of SY A mixture of (A) (0.5 mmol), (B) (0.5 mmol) and anhydrous sodium acetate (1 mmol) were added into 3 mL acetic anhydride, stiring at room temperature for 10 h. And then filtered, washed with water. The crude product was purified by column chromatography on silica gel using CH2Cl2 and ethyl acetate (1:1, V/V) to obtain a red solid, 60% yield. 1H NMR (500 MHz, DMSO-d6) δ 8.47 (d, J = 13.0 Hz, 1 H), 8.32 (d, J = 6.5 Hz, 2 H), 8.13 (d, J = 6.5 Hz, 1 H), 8.01 (t, J = 6.5 Hz, 3 H), 7.96 (dd, J = 4.9, 2.3 Hz, 1 H), 7.93–7.86 (m, 2 H), 7.77 (dd, J = 13.0, 2.0 Hz, 2 H), 7.64 (dd, J = 4.9, 2.3 Hz, 2 H), 7.57–7.50 (m, 1 H), 7.49–7.43 (m, 1 H), 4.76 (q, J = 5.7 Hz, 2 H), 1.84 (s, 6 H), 1.50 (t, J = 5.7 Hz, 3 H). 13C NMR (125 MHz, DMSO-d6) δ 181.82, 166.49, 153.99, 153.30, 144.61, 140.91, 140.45, 136.55, 135.66, 134.83, 131.71, 130.11, 129.69, 128.86, 127.22, 126.29, 125.11, 123.66, 123.27, 122.83, 115.78, 113.37, 52.88, 42.89, 26.03, 14.38. HRMS: [M-I]+, calculated for [C29H27N2S], 435.1889, found: 435.1887.
Additional Information
How to cite this article: Xing, P. et al. Ratiometric and colorimetric near-infrared sensors for multichannel detection of cyanide ion and their application to measure β-glucosidase. Sci. Rep. 5, 16528; doi: 10.1038/srep16528 (2015).
References
Nguyen, H. H., Tran, T. & Wong, P. L. M. A kinetic study of the cementation of gold from cyanide solutions onto copper. Hydrometallurgy 46, 55–69 (1997).
Chaaban, M. A. Hazardous waste source reduction in materials and processing technologies. J. Mater. Process. Technol. 119, 336–343 (2001).
Hamel, J. A Review of Acute Cyanide Poisoning With a Treatment Update. Crit. Care Nurse 31, 72–82 (2011).
Baud, F. J. Cyanide: critical issues in diagnosis and treatment. Hum. Exp. Toxicol. 26, 191–201 (2007).
Rauws, A. G., Olling, M. & Timmerman, A. The pharmacokinetics of amygdalin. Arch. Toxicol. 49, 311–319 (1982).
Ding, G. et al. Electrofluorochromic Detection of Cyanide Anions Using a Nanoporous Polymer Electrode and the Detection Mechanism. Chem. Eur. J. 20, 13226–13233 (2014).
Xu, Z., Chen, X., Kim, H. N. & Yoon, J. Sensors for the optical detection of cyanide ion. Chem. Soc. Rev. 39, 127–137 (2010).
Breuer, P. L., Sutcliffe, C. A. & Meakin, R. L. Cyanide measurement by silver nitrate titration: Comparison of rhodanine and potentiometric end-points. Hydrometallurgy 106, 135–140 (2011).
Lou, B., Chen, Z. Q., Bian, Z. Q. & Huang, C. H. Multisignaling detection of cyanide anions based on an iridium(III) complex: remarkable enhancement of sensitivity by coordination effect. New J. Chem. 34, 132–136 (2010).
Mak, K. K. W., Yanase, H. & Renneberg, R. Cyanide fishing and cyanide detection in coral reef fish using chemical tests and biosensors. Biosens. Bioelectron. 20, 2581–2593 (2005).
Badugu, R., Lakowicz, J. R. & Geddes, C. D. Enhanced fluorescence cyanide detection at physiologically lethal levels: reduced ICT-based signal transduction. J. Am. Chem. Soc. 127, 3635–3641 (2005).
Lou, X., Zhang, L., Qin, J. & Li, Z. An alternative approach to develop a highly sensitive and selective chemosensor for the colorimetric sensing of cyanide in water. Chem. Commun. 44, 5848–5850 (2008).
Jo, J. & Lee, D. Turn-on fluorescence detection of cyanide in water: activation of latent fluorophores through remote hydrogen bonds that mimic peptide beta-turn motif. J. Am. Chem. Soc. 131, 16283–16291 (2009).
Kim, H. J., Ko, K. C., Lee, J. H., Lee, J. Y. & Kim, J. S. KCN sensor: unique chromogenic and ‘turn-on’ fluorescent chemodosimeter: rapid response and high selectivity. Chem. Commun. 47, 2886–2888 (2011).
Shiraishi, Y., Nakamura, M., Yamamoto, K. & Hirai, T. Rapid, selective and sensitive fluorometric detection of cyanide anions in aqueous media by cyanine dyes with indolium-coumarin linkages. Chem. Commun. 50, 11583–11586 (2014).
Robbins, T. F., Qian, H., Su, X., Hughes, R. P. & Aprahamian, I. Cyanide Detection Using a Triazolopyridinium Salt. Org. Lett. 15, 2386–2389 (2013).
Chung, S. Y., Nam, S. W., Lim, J., Park, S. & Yoon, J. A highly selective cyanide sensing in water via fluorescence change and its application to in vivo imaging. Chem. Commun. 20, 2866–2868 (2009).
Jung, H. S., Han, J. H., Kim, Z. H., Kang, C. & Kim, J. S. Coumarin-Cu(II) Ensemble-Based Cyanide Sensing Chemodosimeter. Org. Lett. 13, 5056–5059 (2011).
Dong, M., Peng, Y., Dong, Y.-M., Tang, N. & Wang, Y.-W. A Selective, Colorimetric and Fluorescent Chemodosimeter for Relay Recognition of Fluoride and Cyanide Anions Based on 1,1′-Binaphthyl Scaffold. Org. Lett. 14, 130–133 (2011).
Wang, P., Yao, Y. & Xue, M. A novel fluorescent probe for detecting paraquat and cyanide in water based on pillar[5]arene/10-methylacridinium iodide molecular recognition. Chem. Commun. 50, 5064–5067 (2014).
Chen, X. et al. A near-infrared fluorescent sensor for detection of cyanide in aqueous solution and its application for bioimaging. Chem. Commun. 46, 8953–8955 (2010).
Li, H., Wen, Z., Jin, L., Kan, Y. & Yin, B. A coumarin-Meldrum’s acid conjugate based chemodosimetric probe for cyanide. Chem. Commun. 48, 11659–11661 (2012).
Lv, X. et al. Ratiometric fluorescence detection of cyanide based on a hybrid coumarin-hemicyanine dye: the large emission shift and the high selectivity. Chem. Commun. 47, 12843–12845 (2011).
Cullen, V. et al. Acid β-glucosidase mutants linked to gaucher disease, parkinson disease and lewy body dementia alter α-synuclein processing. Ann. Neurol. 69, 940–953 (2011).
Lieberman, R. L. et al. Structure of acid β-glucosidase with pharmacological chaperone provides insight into Gaucher disease. Nat. Chem. Biol. 3, 101–107 (2007).
Sun, H. et al. A colorimetric lead (II) ions sensor based on selective recognition of G-quadruplexes by a clip-like cyanine dye. Talanta 136, 210–214 (2015).
Goswami, S., Paul, S. & Manna, A. Carbazole based hemicyanine dye for both “naked eye” and ‘NIR’ fluorescence detection of CN− in aqueous solution: from molecules to low cost devices (TLC plate sticks). Dalton Trans. 42, 10682–10686 (2013).
Xu, Y. et al. “ICT-not-quenching” near infrared ratiometric fluorescent detection of picric acid in aqueous media. Chem. Commun. 49, 4764–4766 (2013).
Jose, D. A., Elstner, M. & Schiller, A. Allosteric Indicator Displacement Enzyme Assay for a Cyanogenic Glycoside. Chem. Eur. J. 19, 14451–14457 (2013).
Kong, F. et al. A highly sensitive near-infrared fluorescent probe for cysteine and homocysteine in living cells. Chem. Commun. 49, 9176–9178 (2013).
Huang, Z. L. et al. Novel heterocycle-based organic molecules with two-photon induced blue fluorescent emission. J. Mater. Chem. 13, 708–711 (2003).
Acknowledgements
This work was financially supported by the Scientific Research Foundation of Northwest A&F University (Z111021103 and Z111021107), the National Natural Science Foundation of China (No. 21206137, 21272030, 201205095 and 21476185), Shaanxi Province Science and Technology (No. 2013K12-03-23), State Key Laboratory of Chemo/Biosensing and Chemometrics, Hunan University (No. 2013005).
Author information
Authors and Affiliations
Contributions
S.G.S. supervised and interpreted the research. P.F.X. performed the measurements and wrote the manuscript. Y.Q.X., H.J.L., S.H.L. and A.P.L. helped with interpreted data and wrote the manuscript. All authors discussed the results and commented on the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Xing, P., Xu, Y., Li, H. et al. Ratiometric and colorimetric near-infrared sensors for multi-channel detection of cyanide ion and their application to measure β-glucosidase. Sci Rep 5, 16528 (2015). https://doi.org/10.1038/srep16528
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep16528
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.