Abstract
The conventional wisdom to tailor the properties of binary transition metal carbides by order-disorder phase transformation has been inapplicable for the machinable ternary carbides (MTCs) due to the absence of ordered phase in bulk sample. Here, the presence of an ordered phase with structural carbon vacancies in Nb4AlC3–x (x ≈ 0.3) ternary carbide is predicted by first-principles calculations and experimentally identified for the first time by transmission electron microscopy and micro-Raman spectroscopy. Consistent with the first-principles prediction, the ordered phase, o-Nb4AlC3, crystalizes in P63/mcm with a = 5.423 Å, c = 24.146 Å. Coexistence of ordered (o-Nb4AlC3) and disordered (Nb4AlC3–x) phase brings about abundant domains with irregular shape in the bulk sample. Both heating and electron irradiation can induce the transformation from o-Nb4AlC3 to Nb4AlC3–x. Our findings may offer substantial insights into the roles of carbon vacancies in the structure stability and order-disorder phase transformation in MTCs.
Similar content being viewed by others
Introduction
Binary transition metal carbides have an extraordinary ability to accommodate metalloid structural vacancies (different from the Frenkel, Schottky and anti-Schottky defects, structural vacancies change the stoichiometry of the crystal1) in either a disordered or ordered manner. Ordering of carbon vacancies in the binary carbides (NbC1–x, VC1–x, TaC1–x, etc.) could give rise to self-organized nanolamellar phases or domains, offering huge opportunities to modulate the microstructure-property space1,2,3,4,5.
Machinable ternary carbides (MTCs, having a general chemical formula MmAnCm–n, where M is an early transition metal element; A is an A group element; m and n are integers, m ≥ 2n)6,7,8,9,10 are the most investigated carbides in the last two decades. Crystallizing in the P63/mmc (for n = 1)6 or R3–m (for n = 2)10,11 space group, their crystal structures are closely related, which can be regarded as the periodically stacking of strongly bonded “MmCm–n” sheets and “A” atomic layers along [0001]. The building block of “MmCm–n” in the MTCs strongly resembles that in the binary carbides. The presence of structural carbon vacancies in the MTCs are widely postulated12 since monolithic MTCs can be synthesized only with certain degree of carbon deficiency13. The carbon vacancies have been believed to be disordered before the pioneering work by Etzkorn and coworkers14 on V4AlC3–x single crystal (with a dimension of 0.2 × 0.2 × 0.01 mm) grown by the auxiliary metal bath technique. They pointed out that the V4AlC3–x single crystal grown at 1500 °C holds 10% disordered carbon vacancies, while the carbon vacancies become ordered at 1300 °C, forming V12Al3C8. So far, the knowledge of the carbon vacancies in the interesting MTCs is quite limited12.
First-principles calculation is a powerful tool to investigate the point defects, crystal structure and properties of the MTCs7,15,16,17,18,19. However, it is frustrating for the phase stability of stoichiometric Nb4AlC3. Theoretically, Wang et al.20 argued that stoichiometric Nb4AlC3 is unstable and decomposes to Nb2AlC and NbC above 57 K. Experimentally, Hu et al.21 demonstrated that Nb4AlC3 has a good stability at 2000 K. Since Nb4AlC3 bears striking resemblance to V4AlC3, this puzzling and unsolved inconsistence necessitates the revisiting of the crystal structure of Nb4AlC3 with considerations of carbon vacancies.
Here, under the guidance of the first-principles prediction, an ordered phase bearing structural carbon vacancies in Nb4AlC3–x (x ≈ 0.3), o-Nb4AlC3 (Nb12Al3C8), is unambiguously identified in experiment, demonstrating the validity to investigate the carbon vacancies in the MTCs with the combination of first-principles calculations, electron diffractometry and Raman spectroscopy.
Results
Predication of ordered phase
In analogy with the carbon-vacancy ordered phase in V4AlC3–x (Ref. 14)ten hypothetical carbon-vacancy configurations (VCs) with a vacancy concentration of 1/9 were constructed based on a  supercell of Nb4AlC3 (Fig. 1a–j). There are two distinct types of Nb6C octahedrons in Nb4AlC3 (P63/mmc), involving the carbon atoms located at 4f sites with a Nb–C bond length of 2.21 Å (OCT-4f) and 2a sites with a Nb–C bond length of 2.28 Å (OCT-2a). VC1, VC8 and VC10 have two OCT-2a type carbon-vacant octahedrons in each unit cell. In contrast, VC3, VC4, VC5, VC6 and VC7 have two OCT-4f type carbon-vacant octahedrons. VC2 and VC9 own one OCT-2a and one OCT-4f type carbon-vacant octahedrons. The crystal structure information of the constructed VCs is provided in Supplementary Table 1. To evaluate the phase stability, the formation energy for VC (
supercell of Nb4AlC3 (Fig. 1a–j). There are two distinct types of Nb6C octahedrons in Nb4AlC3 (P63/mmc), involving the carbon atoms located at 4f sites with a Nb–C bond length of 2.21 Å (OCT-4f) and 2a sites with a Nb–C bond length of 2.28 Å (OCT-2a). VC1, VC8 and VC10 have two OCT-2a type carbon-vacant octahedrons in each unit cell. In contrast, VC3, VC4, VC5, VC6 and VC7 have two OCT-4f type carbon-vacant octahedrons. VC2 and VC9 own one OCT-2a and one OCT-4f type carbon-vacant octahedrons. The crystal structure information of the constructed VCs is provided in Supplementary Table 1. To evaluate the phase stability, the formation energy for VC ( ) is calculated by
) is calculated by  . EVC and
. EVC and  are total energies of the VC and Nb4AlC3 unit cell, respectively. The chemical potential of carbon, μC, is assumed to be that in graphite. Table 1 lists the calculated values. With lower total energies, VC8 and VC10 are the energetically most possible VCs. The
are total energies of the VC and Nb4AlC3 unit cell, respectively. The chemical potential of carbon, μC, is assumed to be that in graphite. Table 1 lists the calculated values. With lower total energies, VC8 and VC10 are the energetically most possible VCs. The  for VC8 and VC10 are negative (a brief discussion in the context of chemical potential is provided in Supplementary Note 1), indicating that stoichiometric Nb4AlC3 is metastable and prone to spontaneously forming ordered phases. With a more negative
for VC8 and VC10 are negative (a brief discussion in the context of chemical potential is provided in Supplementary Note 1), indicating that stoichiometric Nb4AlC3 is metastable and prone to spontaneously forming ordered phases. With a more negative  , VC8 isostructural with V12Al3C8 (Ref. 14) is slightly more favorable than VC10 from an energetic point of view. The mechanical stability of crystals requires the elastic constants cij to match the Born–Huang criterion. Specifically, the restrictions for hexagonal crystal system22,23 are:
, VC8 isostructural with V12Al3C8 (Ref. 14) is slightly more favorable than VC10 from an energetic point of view. The mechanical stability of crystals requires the elastic constants cij to match the Born–Huang criterion. Specifically, the restrictions for hexagonal crystal system22,23 are:  . According to the calculated elastic constants in Supplementary Table 2, VC8 and VC10 satisfy the mechanical stability criteria. In addition, the phonon dispersion curves in Supplementary Fig. 1 have no imaginary frequencies. VC8 and VC10 are therefore dynamically stable.
. According to the calculated elastic constants in Supplementary Table 2, VC8 and VC10 satisfy the mechanical stability criteria. In addition, the phonon dispersion curves in Supplementary Fig. 1 have no imaginary frequencies. VC8 and VC10 are therefore dynamically stable.
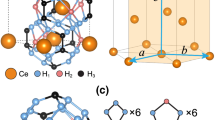
Illustration of constructed VCs.
The VCs were constructed by removing two carbon atoms in a  supercell of Nb4AlC3. (a) VC1, (b) VC2, (c) VC3, (d) VC4, (e) VC5, (f) VC6, (g) VC7, (h) VC8, (i) VC9, (j) VC10. As a reference, the projection of Nb4AlC3 supercell along [112– 0] is provided. The blue, red and purple balls denote the Nb, Al and C atoms. The grey squares denote the carbon vacancies.
supercell of Nb4AlC3. (a) VC1, (b) VC2, (c) VC3, (d) VC4, (e) VC5, (f) VC6, (g) VC7, (h) VC8, (i) VC9, (j) VC10. As a reference, the projection of Nb4AlC3 supercell along [112– 0] is provided. The blue, red and purple balls denote the Nb, Al and C atoms. The grey squares denote the carbon vacancies.
Identification of ordered phase and determination of crystal structure
To test the theoretical prediction, the phase component of the as-prepared Nb4AlC3–x (x ≈ 0.3) was revisited. Like other MTCs, grains of Nb4AlC3–x are elongated with ca. 54 μm in length and 10 μm in width, as shown in Fig. 2a. Indexing the low-index zone axis electron diffraction patterns (EDPs) in Fig. 2b–d results in a new hexagonal structure with a = 5.5 Å, c = 25.2 Å, which is different from that of previously reported Nb4AlC3 (a = 3.1 Å, c = 24.1 Å)24. For convenience, the new phase is denoted as o-Nb4AlC3. Then, Fig. 2b–d correspond to the EDP of [0001], [12– 10] and [01– 10] of o-Nb4AlC3, respectively. The reflection conditions are l = 2n for (hh– 0l) and (000l). The appearance of (000l) with l = 2n + 1 in Fig. 2d is caused by double diffractions, which can be verified by its disappearance in the EDP collected along [hki0] (Fig. 2e). The convergent beam electron diffraction (CBED) patterns with different convergence angles along [0001] in Fig. 2f,g demonstrate that there is a six-fold rotation axis and two mirror planes along [0001]. In addition, the CBED pattern collected along [hki0] in Fig. 2h indicates a mirror plane vertical to [0001]. Therefore, the corresponding point group is 6/mmm. Considering the reflection conditions, the space group of o-Nb4AlC3 is determined to be P63/mcm. Noteworthily, the diffraction spots marked by black arrows in Fig. 2b–d can be indexed with Nb4AlC3–x as well. Figure 3a,b present transmission electron microscopy (TEM) dark field morphologies imaged with (101–0) and (303– 0) of o-Nb4AlC3, respectively. Since (303– 0) of o-Nb4AlC3 coincides with (112– 0) of Nb4AlC3–x (Fig. 2b,c), the dark domains with irregular shape in Fig. 3a are Nb4AlC3–x; while the regions with bright contrasts correspond to o-Nb4AlC3. The worm-like ribbons marked by arrows are antiphase boundaries in o-Nb4AlC3.
Grain morphology and EDPs.
(a) Scanning electron microscopy image of the as-prepared Nb4AlC3–x. Inset is an electron backscatter diffraction image. The grains are elongated. Selected area EDPs belonging to (b) [0001], (c) [12– 10], (d) [01– 10] and (e) [hki0] of o-Nb4AlC3 are indexed in red fonts. The diffraction spots marked by black arrows coincide with those of Nb4AlC3–x, as denoted with black indices. CBED patterns were collected along (f,g) [0001] and (h) [hki0]. (f) and (g) were recorded with different convergence angles. A (0000) CBED disk was montaged in (h). “m” and “c*” stand for mirror plane and [0001] direction in reciprocal space, respectively.
As determined by electron-probe X-ray microanalysis, the molar ratio of Nb:Al in o-Nb4AlC3 is 4:1.05 (see Supplementary Table 3). The energy dispersive X-ray spectroscopy (EDS) mapping of Al (Fig. 3c) and Nb (Fig. 3d) demonstrates that there is no compositional difference of Al and Nb between o-Nb4AlC3 and Nb4AlC3–x. In addition, carbon is 10% deficient in the starting materials to synthesize monolithic Nb4AlC324. Then, the chemical formula of o-Nb4AlC3 is Nb24Al6+δC18–n (δ = 0.3) since the unit cell of o-Nb4AlC3 is three times that of Nb4AlC3–x (Fig. 4a). As the lowest multiplicity for the Wyckoff sites of P63/mcm (Ref. 25) is 2, the formation of o-Nb4AlC3 cannot be caused by an excess of Al, otherwise the minimum Al/Nb is 8/24 with δ = 2. Considering the nominal composition of the starting materials (Nb:C = 12:8.1) and restrictions on the Wyckoff sites of P63/mcm (Ref. 25), o-Nb4AlC3 is Nb12Al3C8 with the carbon vacancies occupying the 2b Wyckoff site. Namely, o-Nb4AlC3 has the VC8 configuration. The experimental EDPs and simulated patterns with the VC8 configuration are in excellent consistence (see Supplementary Fig. 2a–f). The crystal structure information of o-Nb4AlC3 is listed in Table 2. o-Nb4AlC3 can be constructed readily by removing the carbon atoms at (0, 0, 0) and (0, 0, 1/2) of the  supercell (Fig. 4b). The orientation relationship is: [12– 10] o-Nb4AlC3 ‖ [11–00] Nb4AlC3–x, [01–10] o-Nb4AlC3 ‖ [12– 10] Nb4AlC3–x.
supercell (Fig. 4b). The orientation relationship is: [12– 10] o-Nb4AlC3 ‖ [11–00] Nb4AlC3–x, [01–10] o-Nb4AlC3 ‖ [12– 10] Nb4AlC3–x.
Crystal structure and Raman spectrum of o-Nb4AlC3.
(a) Illustration of the unit-cell projection of o-Nb4AlC3 (red) and Nb4AlC3–x (black) along [0001]. The EDPs in Fig. 2c,d demonstrate that the lengths of c axis of o-Nb4AlC3 and Nb4AlC3–x in the present experiment are identical. (b) Unit cell of o-Nb4AlC3 (left) and the projection of edge-sharing Nb6C octahedrons along [01– 10] (median) and [12– 10] (right). The octahedral interstitial sites at the origin and (0, 0, 1/2) are not occupied by carbon atoms. The blue, red and purple balls illustrate the Nb, Al and C atoms. (c) Polarized and unpolarized Raman spectrums collected with a 600 lines per mm diffraction grating. (d,e) Unpolarized Raman spectrums collected with a 1800 lines per mm diffraction grating in the wavenumber ranges boxed in (c).
Raman spectroscopic verification of o-Nb4AlC3
To further verify the crystal structure, micro-Raman spectroscopic investigations were conducted. The polarized and unpolarized Raman spectra are presented in Fig. 4c–e. The group theory predicts the following symmetries for zone-center (Γ point) optical phonons: Γoptical = 7A1g + 3A1u + 5A2g + 7A2u + 4B1g + 8B1u + 7B2g + 4B2u + 11E2u + 12E2g + 11E1u + 11E1g, where A1g, E1g and E2g are Raman active modes. The experimental and theoretical Raman shifts calculated by first-principles (Table 3) are well consistent. In addition, the peaks located at 158 (ω3), 169 (ω4), 220 (ω11), 259 (ω17), 287 (ω19), 616 (ω25) and 681 cm–1 (ω30) disappear in the polarized Raman spectrum, indicating a symmetry of A1g (Ref. 11), which is exactly the same with that predicted by the first-principles calculations. Therefore, the Raman spectroscopic investigation unambiguously validates the crystal structure of o-Nb4AlC3 with the predicted VC8 configuration.
Statistics on the TEM dark field morphologies imaged with superlattice diffraction spots indicate that o-Nb4AlC3 accounts for ca. 81 vol.% of the as-prepared sample. The X-ray diffraction (XRD) pattern in Fig. 5a is indexed with o-Nb4AlC3. The superlattice peaks, (h0h–l) with h = 3n ± 1, are unidentifiable in the XRD pattern due to their remarkably low intensities (see Supplementary Table 4).
XRD pattern, dilatation of the carbon-vacant octahedron and extra Raman peaks.
(a) XRD pattern of the as-prepared Nb4AlC3–x. All identifiable peaks belonging to o-Nb4AlC3 and Nb4AlC3–x coincide. For the sake of conciseness, they are indexed with the cell of o-Nb4AlC3. (b) Change of diagonal distances of Nb (blue balls in the inset) in the carbon-vacant octahedron using the values of Nb6C octahedrons as references. Red balls and stars denote the octahedrons with a carbon vacancy located at the 2a and 4f sites of Nb4AlC3–x (P63/mmc), respectively. (c) Raman spectrum collected with a 1800 lines per mm diffraction grating. Arrows denote the extra weak Raman peaks not belonging to o-Nb4AlC3 or Nb4AlC3–x. Those peaks are believed to be generated by the vibration of VC10.
Discussion
Formation of a carbon vacancy within the Nb6C octahedron breaks six Nb–C bonds and destabilizes the structure. Meanwhile, the redistribution of the electron charge within the vacancy neighbors through the dilatation of the carbon-vacant octahedron strengthens the remaining Nb–C bonds around the carbon vacancy and stabilizes the structure. The triumph of the stabilizing factor over the destabilizing one gives rise to carbon-vacancy ordered phases26,27. With weaker Nb–C bonds, forming a carbon vacancy in OCT-2a costs less energy than that in OCT-4f (Fig. 5b). Similar features have been confirmed in Ta4AlC3 and Ti4AlN3 (Ref. 16,17). Generally speaking, the more the diagonal distances of the Nb atoms in the carbon-vacant octahedron expand, the stronger the remaining Nb–C bonds around the carbon vacancy become and then the more stable the carbon-vacant structure is (Fig. 5b). Therefore, VC8 and VC10 with carbon vacancies only in OCT-2a and most expansions of the carbon-vacant octahedrons have lower total energies than the other VCs.
The revisiting of the phase component in Nb4AlC3–x confirms the presence of carbon-vacancy ordered phase predicted by our first-principles calculations: o-Nb4AlC3 has the VC8 configuration. The difference of  between VC10 and VC8 is only 0.07 eV and it is therefore not unreasonable to anticipate the existence of VC10. Virtually, there are several weak Raman peaks belonging to neither o-Nb4AlC3 (VC8) nor Nb4AlC3–x in the Raman spectrum collected with a 1800 lines per mm diffraction grafting (Fig. 5c). These extra peaks are most likely generated by VC10 (see Supplementary Table 5). Since no EDPs belonging to VC10 (see Supplementary Fig. 2g–i) were identified in the present study, VC10 is believed to exist not in a highly ordered manner. The crystal structure information of VC10 is provided in Supplementary Table 6.
between VC10 and VC8 is only 0.07 eV and it is therefore not unreasonable to anticipate the existence of VC10. Virtually, there are several weak Raman peaks belonging to neither o-Nb4AlC3 (VC8) nor Nb4AlC3–x in the Raman spectrum collected with a 1800 lines per mm diffraction grafting (Fig. 5c). These extra peaks are most likely generated by VC10 (see Supplementary Table 5). Since no EDPs belonging to VC10 (see Supplementary Fig. 2g–i) were identified in the present study, VC10 is believed to exist not in a highly ordered manner. The crystal structure information of VC10 is provided in Supplementary Table 6.
Carbon-vacancy ordered phase is stable at low temperature1. When temperature rises and the contribution of entropy to the Gibbs free energy is appreciable, carbon vacancies tend to be in short-range order or disordered. Therefore, o-Nb4AlC3 is a low-temperature phase; while Nb4AlC3–x (with certain amounts of disordered carbon vacancies) is the corresponding high-temperature phase. As indicated by the first-principles calculations (Table 1, Fig. 5b) and Rietveld refinements of X-ray (neutron) diffraction patterns of V4AlC3–x (Ti4AlN3–x)14,28, the disordered vacancies in Nb4AlC3–x are most likely located at the 2a site of the P63/mmc space group. The existence of carbon-vacancy disordered Nb4AlC3–x at room temperature is due to the fact that the cooling rate during the sample synthesis is not slow enough and brings about disordered domains (Figs 3a and 6a). When o-Nb4AlC3 is heated above a critical temperature, transformation to Nb4AlC3–x occurs with the nucleation and growth of new disordered nanodomains in the ordered phase (Fig. 6b). For the sample quasi-quenched from 1400 °C after keeping 30 min, the amount of disordered domains (with dark contrasts) increases from ca. 19 vol.% (Fig. 6a) to 36 vol.% (Fig. 6b). Dwelling for 10 s at 1500 °C, nearly all o-Nb4AlC3 transforms to Nb4AlC3–x, leaving some ordered nanodomains (with bright contrasts, Fig. 6c). Consequently, the EDP (Fig. 6d) exhibits the features of short-range ordering. Thereby, similar to the carbon-vacancy disordering in V4AlC3–x where the disordering occurs in the range from 1300 °C to 1500 °C14, that in Nb4AlC3–x starts around 1400 °C and completes at 1500 °C.
Domain morphologies and electron irradiation.
TEM dark field image recorded with (101– 0) of o-Nb4AlC3 in the sample (a) as-prepared, (b) quasi-quenched from 1400 °C after dwelling for 30 min and (c) quasi-quenched from 1500 °C after dwelling for 10 s. o-Nb4AlC3 and Nb4AlC3–x are in bright and dark contrasts, respectively. Inset in (b) is an enlarged morphology demonstrating the disordered nanodomains. (d) An EDP with the features of short-range ordering. The superlattice diffraction spots become weak streaks. (e) Dependence of IS2/IS1 on the irradiation time. IS1 and IS2 are the intensities of the diffraction spots marked in the inset. (f,g) EDPs recorded after irradiated 120 s. (f,g) belong to [0001] and [11– 00] of Nb4AlC3–x, respectively. The electron dose for irradiation is approximately 0.04 e Å–2 s–1.
Resembling the ordered phase in binary carbides29,30, transformation from o-Nb4AlC3 to Nb4AlC3–x can be induced by electron irradiation, as shown in Supplementary Movies 1,2. The intensity of the superlattice diffraction spots decreases dramatically as the irradiation proceeds (Fig. 6e). Irradiated for approximately 120 s, the superlattice diffraction spots disappear (Fig. 6f,g) with the transformation from o-Nb4AlC3 to Nb4AlC3–x. The extremely electron irradiation sensitive nature possibly hides o-Nb4AlC3 and domains from being discovered before.
In summary, under the guidance of first-principles calculations, a new carbon-vacancy ordered phase, o-Nb4AlC3 (Nb12Al3C8) has been discovered. It crystalizes in the space group of P63/mcm with a = 5.423 Å, c = 24.146 Å. Coexistence of ordered (o-Nb4AlC3) and disordered (Nb4AlC3–x) phase brings about domains with irregular shape. Both heating and electron irradiation can induce the transformation from o-Nb4AlC3 to Nb4AlC3–x. The excellent consistency between the first-principles prediction and experimental results demonstrated in this work may inspire the theoretical investigation on the vacancies in over 70 machinable ternary carbides/nitrides. The unveiled domain structure likely ignites investigation enthusiasm on the order-disorder phase transformation as well.
Methods
First-principles calculations with CASTEP module
Electronic exchange-correlation energy was treated under the generalized gradient approximation (GGA–PBE)31,32. Interaction of electrons with ion cores was represented by norm-conserving pseudopotential33. The plane-wave cut off energy and Brillouin zone sampling were fixed at 770 eV and 5 × 5 × 2 Monkhorst-Pack-point meshes34, respectively. The Broyden-Fletcher-Goldfarb-Shanno minimization method was used for geometry optimization35, where the tolerances were selected as the difference in total energy within 1 × 10−8 eV per atom, maximum ionic Hellmann-Feynman force within 0.001 eV Å−1, maximum ionic displacement within 5 × 10−4 Å and maximum stress within 0.02 GPa. The elastic constants were determined by the method reported by Milman et al.36. Vibrational frequencies were determined with the finite displacement method37.
Sample preparation
Bulk Nb4AlC3–x (x ≈ 0.3) sample was synthesized by the method reported in Ref. 21,38. Briefly, Nb (−300 mesh), Al (−300 mesh) and graphite (D90 = 6.5 μm) powders with a molar ratio of 4 : 1.2 : 2.7 were homogenized with agate balls and absolute alcohol in an agate jar for 12 h and then dried at 70 °C for 24 h. After that, the blended powders were cold compressed in a graphite mold. Finally, the green compact together with the mold were put into a hot pressing furnace and sintered at 1900 °C for 1 h under a uniaxial pressure of 30 MPa with flowing Ar as protective gas.
Composition and microstructure characterization
Composition of thirty points in different regions of the as-prepared sample was analyzed by an electron-probe microanalyser (Shimadzu EPMA-1610, Kyoto, Japan). The phase components were investigated by XRD in an X-ray diffractometer (Rigaku D/max-2400, Tokyo, Japan) with Cu Kα radiation. The microstructural characterizations were performed on a transmission electron microscope (FEI Tecnai G2 F20, Oregon, USA) working at 200 kV with an energy dispersive spectroscopy detector and a high-angle annular dark-field detector in the scanning transmission electron microscopy system. Selected area electron diffraction and CBED patterns were taken in Tecnai T12 (FEI Tecnai T12, Oregon, USA).
Micro-Raman spectroscopic characterization
Unpolarized and polarized Raman spectrums were collected at room temperature on a LabRAM HR800 (Horiba Jobin Yvon, France) equipped with an air-cooled CCD array detector in a backscattering geometry and with diffraction gratings of 600 and 1800 lines per mm. A He–Ne laser (632.82 nm) with an incident power of ca. 20 mW was used as excitation source. Theoretical Raman shifts were obtained by lattice dynamics calculation. According to Zhang et al.11, the peaks with A1g symmetry disappear when  (
( is the angle between the z axis of hexagonal crystal and that of the system coordinates). Therefore, the grains with
is the angle between the z axis of hexagonal crystal and that of the system coordinates). Therefore, the grains with  were chosen to collect the polarized and unpolarized Raman spectrums.
were chosen to collect the polarized and unpolarized Raman spectrums.
Quasi-quenching
Quasi-quenching was realized by a thermomechanical simulator, Gleeble 3800. The cooling rate above 800 °C is near 200 °C s–1, as shown in Supplementary Fig. 3.
Additional Information
How to cite this article: Zhang, H. et al. Discovery of carbon-vacancy ordering in Nb4AlC3-x under the guidance of first-principles calculations. Sci. Rep. 5, 14192; doi: 10.1038/srep14192 (2015).
References
Gusev, A. I. Disorder and Order in Strongly Nonstoichiometric Compounds: Transition Metal Carbides, Nitrides and Oxides (Springer, 2001).
Demyashev, G. M. Review: Transition metal-based nanolamellar phases. Prog. Mater. Sci. 55, 629–674 (2010).
Yu, X. X., Thompson, G. B. & Weinberger, C. R. Influence of carbon vacancy formation on the elastic constants and hardening mechanisms in transition metal carbides. J. Eur. Ceram. Soc. 35, 95–103 (2015).
Gusev, A. I. & Rempel, A. A. Superconductivity in disordered and ordered niobium carbide. Phys. Status Solidi B 151, 211–224 (1989).
Nguyen, J., Glandut, N., Jaoul, C. D. & Lefort, P. Electrochemical hydrogen insertion in substoichiometric titanium carbide TiC0.6: influence of carbon vacancy ordering. Langmuir 29, 12036–12042 (2013).
Barsoum, M. W. The MN+1AXN phases: A new class of solids; Thermodynamically stable nanolaminates. Prog. Solid State Chem. 28, 201–281 (2000).
Wang, J. Y. & Zhou, Y. C. Recent progress in theoretical prediction, preparation and characterization of layered ternary transition-metal carbides. Annu. Rev. Mater. Res. 39, 415–443 (2009).
Eklund, P., Beckers, M., Jansson, U., Högberg, H. & Hultman, L. The Mn + 1AXn phases: materials science and thin-film processing. Thin Solid Films 518, 1851–1878 (2010).
Sun, Z. M. Progress in research and development on MAX phases: a family of layered ternary compounds. Int. Mater. Rev. 56, 143–166 (2011).
Zhang, H. et al. Crystal structure determination of nanolaminated Ti5Al2C3 by combined techniques of XRPD, TEM and ab initio calculations. J. Adv. Ceram. 1, 268–273 (2012).
Zhang, H., Wang, X. H., Xiang, H. M., Li, Z. J. & Zhou, Y. C. Micro-Raman spectroscopic study of nanolaminated Ti5Al2C3 . Appl. Phys. Lett. 104, 131903 (2014).
Barsoum, M. W. MAX Phases: properties of machinable ternary carbides and nitrides, pp. 50–52 (Wiley, 2013).
Wang, X. H. & Zhou, Y. C. Solid-liquid reaction synthesis of layered machinable Ti3AlC2 ceramic. J. Mater. Chem. 12, 455–460 (2002).
Etzkorn, J., Ade, M. & Hillebrecht, H. V2AlC, V4AlC3–x (x ≈ 0.31) and V12Al3C8: synthesis, crystal growth, structure and superstructure. Inorg. Chem. 46, 7646–7653 (2007).
Eklund, P. et al. Discovery of the ternary nanolaminated compound Nb2GeC by a systematic theoretical-experimental approach. Phys. Rev. Lett. 109, 035502 (2012).
Du, Y. L., Sun, Z. M., Hashimoto, H. & Tian, W. B. First-principles study of carbon vacancy in Ta4AlC3 . Mater. Trans. 49, 1934–1936 (2008).
Music, D., Ahuja, R. & Schneider, J. M. Theoretical study of nitrogen vacancies in Ti4AlN3 . Appl. Phys. Lett. 86, 031911 (2005).
Dahlqvist, M., Alling, B. & Rosén, J. Stability trends of MAX phases from first principles. Phys. Rev. B 81, 220102 (2010).
Wang, J. M., Liu, B., Wang, J. Y. & Zhou, Y. C. Theoretical investigation of thermodynamic stability and mobility of the intrinsic point defects in Ti3AC2 (A = Si, Al). Phys. Chem. Chem. Phys. 17, 8927–8934 (2015).
Wang, J. M., Wang, J. Y., Zhou, Y. C. & Hu, C. F. Phase stability, electronic structure and mechanical properties of ternary-layered carbide Nb4AlC3: an ab initio study. Acta Mater. 56, 1511–1518 (2008).
Hu, C. F. et al. In situ reaction synthesis, electrical and thermal and mechanical properties of Nb4AlC3 . J. Am. Ceram. Soc. 91, 2258–2263 (2008).
Born, M. & Huang, K. Dynamical theory of crystal lattices (Oxford University Press, 1954).
Nye, F. Physical properties of crystals (Oxford University Press, 1964).
Hu, C. F. et al. Nb4AlC3: a new compound belonging to the MAX phases. Scr. Mater. 57, 893–896 (2007).
Hahn, T. International tables for crystallography, space-group symmetry, pp. 601 (Wiley, 2005).
Korzhavyi, P. A., Pourovskii, L. V., Hugosson, H. W., Ruban, A. V. & Johansson, B. Ab initio study of phase equilibria in TiCx . Phys. Rev. Lett. 88, 015505 (2001).
Tan, K. E. et al. Carbon vacancies in titanium carbide. Model. Simul. Mater. Sci. Eng. 5, 187 (1997).
Rawn, C. J. et al. Structure of Ti4AlN3—a layered Mn+1AXn nitride. Mater. Res. Bull. 35, 1785–1796 (2000).
Murata, Y., Yukawa, N., Mori, H. & Fujita, H. Electron irradiation induced disordering of short-range ordered (Ti, Mo)Cx carbide. J. Less-Common Met. 141, 309–319 (1988).
Won, J., Valdez, J. A., Naito, M., Ishimaru, M. & Sickafus, K. E. Transmission electron microscopy study of an electron-beam-induced phase transformation of niobium nitride. Scr. Mater. 60, 799–802 (2009).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Perdew, J. P. et al. Atoms, molecules, solids and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 46, 6671 (1992).
Rappe, A. M., Rabe, K. M., Kaxiras, E. & Joannopoulos, J. D. Optimized pseudopotentials. Phys. Rev. B 41, 1227 (1990).
Pack, J. D. & Monkhorst, H. J. “Special points for Brillouin-zone integrations” –a reply. Phys. Rev. B 16, 1748 (1977).
Pfrommer, B. G., Côté, M., Louie, S. G. & Cohen, M. L. Relaxation of crystals with the quasi-newton method. J. Comput. Phys. 131, 233–240 (1997).
Milman, V. & Warren, M. C. Elasticity of hexagonal BeO. J. Phys.: Condens. Matter 13, 241 (2001).
Refson, K., Tulip, P. R. & Clark, S. J. Variational density-functional perturbation theory for dielectrics and lattice dynamics. Phys. Rev. B 73, 155114 (2006).
Zheng, L. Y. et al. Preparation, microstructure and mechanical properties of Nb4AlC3–Nb5(Si, Al)3 composites. J. Am. Ceram. Soc. 96, 365–368 (2013).
Acknowledgements
The authors would like to thank Xu Wang for the help in the electron backscatter diffraction experiment. This work was supported by the Chinese Academy of Sciences (CAS) and Shenyang National Laboratory for Materials Science, Institute of Metal Research, CAS.
Author information
Authors and Affiliations
Contributions
H.Z. carried out the sample synthesis, microstructure characterization and structure determination. T.H. conducted the first-principles calculations. Z.J.L. and M.M.H. collected the Raman spectrums. X.H.W. conceived and designed the project. H.Z., X.H.W., E.D.W. and Y.C.Z. wrote the paper. All authors contributed to data analysis and scientific discussion.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhang, H., Hu, T., Wang, X. et al. Discovery of carbon-vacancy ordering in Nb4AlC3–x under the guidance of first-principles calculations. Sci Rep 5, 14192 (2015). https://doi.org/10.1038/srep14192
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep14192
This article is cited by
-
Sintering of MAX-phase materials by spark plasma and other methods
Journal of Materials Science (2021)
-
From structural ceramics to 2D materials with multi-applications: A review on the development from MAX phases to MXenes
Journal of Advanced Ceramics (2021)
-
Synthesize of V4AlC3 Based MAX Phase Composites by Reactive Spark Plasma Sintering of V2O5:Al:C
Metals and Materials International (2021)
-
First-principles study on the mechanical properties of M2CT2 (M = Ti, Zr, Hf; T = O, F, OH) MXenes
Nuclear Science and Techniques (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.