Abstract
The transition energies and formation energies of N, C, F, Cl and S as substitutional dopants in Ag3PO4 are studied using first-principles calculations based on the hybrid Hartree-Fock density functional, which correctly reproduces the band gap and thus provides the accurate defect states. Our results show that NO and CO act as deep acceptors, FO, ClO and SP act as shallow donors. NO and CO have high formation energies under O-poor condition therefore they are not suitable for p-type doping Ag3PO4. Though FO, ClO and SP have shallow transition energies, they have high formation energies, thus FO, ClO and SP may be compensated by the intrinsic defects (such as Ag vacancy) and they are not possible lead to n-type conductivity in Ag3PO4.
Similar content being viewed by others
Introduction
Since Honda and Fujishima first discovered the photocatalytic water splitting into H2 and O21, the photocatalysis of water splitting has become an active research field and a potential way to solve the severe environmental crisis and energy shortage issues2,3. TiO2 is the earliest photocatalyst used in water splitting, the intrinsic wide band gap of pure TiO2 (~3.2 eV for anatase and ~3.0 eV for rutile) confines its photon absorption to the ultraviolet (UV) region, severely limiting solar energy utilization to ~5%4. Metal oxides are considered as potential candidates for photoelectrochemical (PEC) water splitting because of their resistance to oxidization and possible stability in aqueous solutions5.
Recently, Ye et al. reported that cubic structure semiconductor Ag3PO4, which exhibits strong oxidation power leading to O2 production from water and its quantum yield achieve up to nearly 90% under visible light6. This is intriguing because most photocatalysts give much poorer quantum yields of ~20%7. Theoretical studies have also been performed to understand their origin7,8,9,10,11. Reunchan and Umezawa suggest that native point defects are unlikely to be responsible for an intrinsic conductivity of Ag3PO4, which an n-type character was observed in the previous report, but Ag3PO4 could feasibly be doped in n-type fashion7.
First-principles density functional theory (DFT) calculations have been commonly used to study the electronic properties of point defects insulators and semiconductors. The local density approximation (LDA)12 or the generalized gradient approximation (GGA)13 functional are typically employed to describe the exchange-correlation energy within DFT. A major shortcoming of LDA and GGA calculations is the large uncertainty in the position of defect levels (and hence also formation energies) due to the severe underestimation of the semiconductor band gap. Heyd et al. recently proposed hybrid Hartree-Fock (HF) density functional14, the hybrid functional has been used to accurately reproduce the band gap of insulators and semiconductors, therefore, the use of the hybrid functional is rationalized for the description of defect physics15,16,17,18.
In this paper, we perform first-principles calculations based on the hybrid HF density functional to investigate the influence of N, C, F, Cl and S impurities on the electronic properties of Ag3PO4. Because interstitials of these impurities usually have large formation energies, we will only consider substitutional defects. The paper is organized as follow: Details of the calculations are provided in Sec. II. The electronic properties of each impurity are described in Sec. III. Finally, Sec. IV summarizes the results.
Methodology
The density functional calculations were performed in the Vienna ab initio simulation package (VASP)19,20. Interaction between the valence and core electrons was described using the projector augmented wave (PAW) approach21. A plane-wave basis set was used to expand the wave functions up to a kinetic energy cutoff value of 300 eV.
We used the Heyd-Scuseria-Ernzerhof hybrid functional (HSE06)14,22, which adopts a screened Coulomb potential. Hence, greatly improving the description of structural properties and band structures, including band gaps. Both of these aspects are particularly important for defects. The HSE exchange is derived from the PBE0 (Perdew-Burke-Ernzerhof (PBE) functional containing 25% exact exchange)23 exchange by range separation and then by elimination of counteracting long-range contributions as4.

Where a is the mixing coefficient and ω is the range-separation parameter. A consistent screening parameter of ω = 0.2 Å−1 is used for the semilocal PBE exchange as well as for the screened nonlocal exchange as suggested for the HSE06 functional24. We find that a proportion of 33% HF exchange with 67% PBE exchange produces accurate values for lattice constants and the band gap in Ag3PO4.
We used a 128-atom supercell constructed by 2 × 2 × 2 replication of the cubic Ag3PO4 unit cell (space group  ), which ensures sufficient spatial separation between the periodic images of the impurities. Various dopings of Ag3PO4 have been modeled by substitution of S at P or Y (Y = N, C, F, Cl) at O sites. For geometry optimizations and electronic structure calculations, the Brillouin zone was sampled with a 2 × 2 × 2 mesh of Monkhorst-Pack special k-points25. Both the atomic positions and cell parameters were optimized until residual forces were below 0.01 eV/Å.
), which ensures sufficient spatial separation between the periodic images of the impurities. Various dopings of Ag3PO4 have been modeled by substitution of S at P or Y (Y = N, C, F, Cl) at O sites. For geometry optimizations and electronic structure calculations, the Brillouin zone was sampled with a 2 × 2 × 2 mesh of Monkhorst-Pack special k-points25. Both the atomic positions and cell parameters were optimized until residual forces were below 0.01 eV/Å.
Formation energies and transition levels
To determine the defect formation energies and defect transition energy levels, we follow the procedure in Ref. 26. The defect formation energy ∆Hf(α, q) as a function of the electron Fermi energy27 EF as well as the atomic chemical potentials28,29 μi is as follows:

Where

E(α, q) is the total energy for the studied supercell containing defect α in charge state q and E (host) is the total energy of the same supercell without the defect. ni indicates the number of atoms of type i (host atoms or impurity atoms) that have been added to (ni < 0) or removed from (ni > 0) the supercell and q is the number of electrons transferred from the supercell to the reservoirs in forming the defect cell. EF is the electron Fermi level referenced to the valence-band maximum (VBM) of host, εVBM(host) and varies from the valence-band maximum to the conduction-band minimum (CBM). μi is the chemical potential of constituent i referenced to its elemental solid or gas with energy E(i).
The defect transition energy level εα(q/q′) is the EF in Eq. (1), at which the formation energy ∆Hf(α, q) of defect α in charge state q is equal to that of another charge q′ of the same defect, i.e.,

In this paper, we used a hybrid scheme to combine the advantages of both special k-points and Γ-point-only approaches12. In this scheme, for acceptor level (q < 0), the transition energy level with respect to VBM is given by:

For donor level (q >0), the ionization energy referenced to the CBM is given by:

Where εDk(0) and εDΓ(0) are the defect levels at the special k-points (averaged) and at the Γ-point, respectively; εVBMΓ(host) and εCBMΓ(host) are the VBM and CBM energies, respectively, of the host at the Γ-point; and εgΓ(host) is the calculated bandgap at the Γ-point.The formation energy of a charged defect is then given by

Where ∆Hf(α, 0) is the formation energy of the charge-neutral defect. More details of calculation methods for formation energies and transition energies of defects are described elsewhere30.
Chemical potentials
Under thermal equilibrium growth conditions, the steady production of host material, Ag3PO4, should satisfy the following equation:

where μAg, μP and μO are the chemical potentials of Ag, P and O source, respectively and ∆Hf is the formation energy for Ag3PO4 per formula. In order to avoid the precipitation of the host elements, the chemical potential μi must be bound by

To avoid the formation of secondary phases (such as Ag2O and P2O5), μAg, μP and μO must satisfy further constrains:


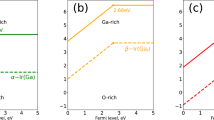
Considering Eqs. (6)–(9), the accessible ranges for μAg, μP and μO are limited and are presented as the shaded area in Fig. 1. As shown in Eqs. (5), the calculated formation energies of charged defects depend sensitively on the selected values for μAg, μP and μO and the Fermi-level positions. Here, the calculated values at two representative chemical potential points are labeled as O-rich and O-poor in Fig. 1. The exact value of chemical potentials at points O-rich condition and O-poor condition are (−0.84, −7.93, 0) and (0, −3.71, −1.69) for μAg, μP and μO, respectively.
For impurity doping, the chemical potentials of impurities also need to satisfy other constraints to avoid the formation of impurity-related phases, for example




The formation enthalpies of the AgCl, Ag2SO4, AgF and CO2 compounds obtained using the present HSE06 functional are listed in Table 1. The formation enthalpies obtained with the present HSE06 functional calculations are agreement with the experimental values.
Results and Discussion
Bulk properties
We first present the results for the structural and electronic properties of defect-free bulk Ag3PO4. The crystal structure of Ag3PO4 has a cubic structure with space group  , its basic structural unit is constructed by PO4 tetrahedron and AgO4 tetrahedron. The interaction between phosphorus and oxygen is mainly by covalent bond, while the interaction between silver and oxygen is formed mainly by ionic bond8. The optimized cell parameters are a = b = c = 6.02 Å and excellent agreement with the experimental values of a = b = c = 6.00 Å8. The P-O, Ag-O and Ag-Ag bond lengths are calculated to be 1.56 Å (experimental value11: 1.56 Å), 2.37 Å (experimental value11: 2.36 Å), 3.01 Å (experimental value11: 3.00 Å), respectively. The calculated indirect band gap (M−Γ) is 2.33 eV and the direct gap at Γ is 2.45 eV, in excellent agreement with the experimental value of 2.36 eV and 2.43 eV6, respectively.
, its basic structural unit is constructed by PO4 tetrahedron and AgO4 tetrahedron. The interaction between phosphorus and oxygen is mainly by covalent bond, while the interaction between silver and oxygen is formed mainly by ionic bond8. The optimized cell parameters are a = b = c = 6.02 Å and excellent agreement with the experimental values of a = b = c = 6.00 Å8. The P-O, Ag-O and Ag-Ag bond lengths are calculated to be 1.56 Å (experimental value11: 1.56 Å), 2.37 Å (experimental value11: 2.36 Å), 3.01 Å (experimental value11: 3.00 Å), respectively. The calculated indirect band gap (M−Γ) is 2.33 eV and the direct gap at Γ is 2.45 eV, in excellent agreement with the experimental value of 2.36 eV and 2.43 eV6, respectively.
N, C, F, Cl and S impurities in Ag3PO4
In this section, we discuss the different impurities in Ag3PO4 individually. The calculated thermodynamic transition energy levels for all impurities are listed in Fig. 2.
Nitrogen
The electronic properties of substitutional N at O sites (NO) has been confirmed by several reports4,5,33. The conductivity of a semiconductor depends not only on the thermodynamic transition levels of donor and acceptors, but also on their formation energies. The thermodynamic transition level corresponds to the intersection of the formation energies for the different charge states. Formation energy as a function of Fermi level for NO in the O-rich and O-poor limit are shown in Fig. 3. When the Fermi level is low, the neutral charge state (NO0) is energetically preferable, as the Fermi level rises, above the Fermi level of 0.46 eV, the negative charge state (NO−1) becomes more favorable. The thermodynamic transition level of ε(−/0) is located at 0.46 eV above VBM. To obtain further details, the squared wave function  of the neutral defect state at Γ point is visualized in Fig. 4(a). The neutral defect state is highly confined around the N atom, which suggests that NO0 induces a localized defect state.
of the neutral defect state at Γ point is visualized in Fig. 4(a). The neutral defect state is highly confined around the N atom, which suggests that NO0 induces a localized defect state.
Spatial distribution of the squared wave functions  of the neutral defect states created by (a) NO, (b) CO, (c) FO, (d) ClO, (e) SP, where the isosurface values34 are 0.05, 0.05, 0.005, 0.005, 0.005 e/Å3, respectively. The silver, pink, red balls represent Ag, P, O atoms, respectively.
of the neutral defect states created by (a) NO, (b) CO, (c) FO, (d) ClO, (e) SP, where the isosurface values34 are 0.05, 0.05, 0.005, 0.005, 0.005 e/Å3, respectively. The silver, pink, red balls represent Ag, P, O atoms, respectively.
The N 2p orbital energy is 1.9 eV higher than the O 2p orbital energy, this imply that upon N substitution on O, the N 2p will create a partially filled impurity state at the Fermi level35. Thus, we examined the total density of states and projected density of states of NO0 (not shown). The neutral defect state has the main contribution from s orbitals and d orbitals of Ag, p orbitals of N and O, minor contribution from p orbitals of P. We also analyzed the local lattice relaxations around NO0 and NO−1. In the neutral state, the neighboring Ag atoms relax inward, resulting in a N-Ag bond length (2.11 Å) that is 11% shorter than the equilibrium O-Ag bond length, while the N-P bond length (1.65 Å) become 6% longer than the equilibrium O-P bond length. In the negative state, the N-Ag bond length is 2.11 Å, while the N-P bond length 1.64 Å.
Figure 3 shows that the formation energy of NO0 is more stable than NO−1 under O-poor condition but still relatively high even the Fermi level is near the conduction band. The high formation energy of the NO indicates that the N-doped Ag3PO4 system using an N2 source may not readily to produce p-type conductivity, which is consistent with the N-doped ZnO system36.
Carbon
As carbon atom has four valence electrons which is two electrons less than oxygen atom, the substitution of C on O site (CO) will act as a double acceptor. The 2p orbital energy of carbon is 3.8 eV higher than the O 2p orbital35. Consequently, CO has two distinct transition levels in the band gap: a ε(−/0) transition at 1.54 eV above the VBM and a ε(2−/0) transition at 1.39 eV above the VBM as shown in Fig. 2. This implies that CO in Ag3PO4 is a negative-U system. A defect often has a negative U if the atomic position of the defect depends sensitively on its charge state37, U refers to the additional energy upon charging of the defect with an additional electron38. In the neutral charge state (CO0), the three Ag nearest neighbors relax inward by 13% (of the equilibrium O-Ag bond length) while one nearest P atom slightly relaxes outward by about 13% (of the equilibrium O-P bond length).
Figure 4(b) plots the squared wave function of the neutral CO defect level. One can see that the squared wave function is localized around C atom, consistent with the deep level feature. The neutral defect state has p orbitals of C and O, s orbitals and d orbitals of Ag contribute primarily, p orbitals of P contribute in a small part. The formation energies of CO in the neutral, −1 and −2 charge states as a function of the Fermi level are shown in Fig. 5. Even the formation energies of CO under O-poor condition is significantly lower than under O-rich condition but still relatively high, therefore C is not suitable for p-type doping Ag3PO4.
Fluorine
Shifting the Fermi level towards the conduction band, or in other words n-type conditions, likely enhance the photocatalytic activities in Ag3PO47. In contrast to the NO and CO, the substitutional F at O site (FO) exhibits distinct characteristics. Unlike the NO and CO, where the defect states are highly localized at impurities, the defect state induced by FO is spatially distributed away from F as the delocalized state [see Fig. 4(c)]. The defect state has main contribution from s and d orbitals of Ag followed by s orbitals of F, O and P. For FO0 the three nearest neighbor Ag atoms relax outward by 17%, while for FO+1 the relaxation are outward by 18% of the equilibrium Ag-O bond length.
The formation energy of FO in different charge states as a function of the Fermi level is shown in Fig. 6. When the Fermi level is low, the positive charge state (FO+1) is energetically preferable, as the Fermi level rises, the formation energy approaches that of the neutral charge state (FO0), above the Fermi level of 2.41 eV, the neutral charge state becomes more favorable. The thermodynamic transition level of ε(0/+) is located 2.41 eV above the VBM (0.04 eV below the CBM). Though the Fermi level drops to the VBM the formation energy of FO becomes very small for the favorable growth condition (O-poor condition), but when the Fermi level is near the CBM, the formation energy of FO is still relatively high, thus, FO may be compensated by Ag vacancy and it is not possible lead to n-type conductivity in Ag3PO4.
Chlorine
Chlorine atom is a famous n-type dopant37,39. The formation energy of substitutional Cl (ClO) against the Fermi level is shown in Fig. 7 for the two extreme cases. For ClO, no transition level is find in the gap (a ε(0/+) transition at 0.14 eV above the CBM) and the +1 charge state is energetically favorable for the whole range of the Fermi level. The extra electron from ClO0 occupies a conduction-band-like state, i.e., an extended state that is only slightly perturbed by the presence of the impurity33. Therefore, ClO is a shallow donor.
We have plotted the squared wave function of the neutral defect state at the Γ point in Fig. 4(d). It is seen that the squared wave function associated with the donor level distributed not only around Cl atom, but also around O atoms and Ag atoms away from the Cl atom, indicating a delocalized feature, which is consistent with the result that ClO is a shallow donor. The defect state has main contribution from s and d orbitals of Ag followed by s and p orbitals of Cl, O and P. The formation energy of ClO is relatively high even under O-poor condition, therefore chlorine is not suitable for n-type doping Ag3PO4.
Sulfur
Sulfur is a possible candidate for n-type doping when substituted for P7. In the case of the S substituting on the P site, the transition level ε(0/+) is located at 2.37 eV (0.08 eV below the CBM). The defect state is spatially away from SP0 [Fig. 4(e)], which is consistent with the result that SP is a shallow donor. The defect state has main contribution from s and d orbitals of Ag followed by s orbitals of S, O and P. It is also clearly from the shape of wave function that s and d orbitals are the main contributor to the defect state.
Sulfur is surrounded by four O atoms, for SP0 these four nearest neighbor O atoms relax inward by 4% of the equilibrium P-O bond length. Formation energies of SP in its various charge state are shown in Fig. 8. We note, SP has lower formation energy than FO and ClO for both O-rich and O-poor conditions. When the Fermi level near the VBM, SP is stable in the +1 charge state. In n-type Ag3PO4, where the Fermi level is near the CBM, SP is stable in the neutral charge state, but the formation energies in the n-type regime are high under O-poor condition for this sulfur is not suitable candidate for n-type doping Ag3PO4.
Conclusion
Using hybrid density functional calculations we have investigated the electrical properties of N, C, F, Cl and S impurities in Ag3PO4. We found that NO and CO act as deep acceptors, FO, ClO and SP act as shallow donors. NO and CO have high formation energies even under most equilibrium condition (O-poor condition) therefore they are not suitable for p-type doping Ag3PO4. Though FO, ClO and SP have shallow transition energies, they have high formation energies, thus FO, ClO and SP may be compensated by Ag vacancy and they are not possible lead to n-type conductivity in Ag3PO4.
Additional Information
How to cite this article: Huang, Y. et al. The electronic properties of impurities (N, C, F, Cl and S) in Ag3PO4: A hybrid functional method study. Sci. Rep. 5, 12750; doi: 10.1038/srep12750 (2015).
References
Fujishima, A. & Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature (London) 238, 37 (1972).
Lai, K., Zhu, Y., Dai, Y. & Huang, B. Intrinsic defect in BiVO4: A density functional theory study. J. Appl. Phys. 112, 043706 (2012).
Wang, D. F., Zou, Z. G. & Ye, J. H. Photocatalytic Water Splitting with the Cr-Doped Ba2In2O5/In2O3 Composite Oxide Semiconductors. Chem. Mater. 17, 3255 (2005).
Çelik, V. & Mete, E. Range-separated hybrid exchange-correlation functional analyses of anatase TiO2 doped with W, N, S, W/N, or W/S. Phys. Rev. B 86, 205112 (2012).
Yin, W. J. et al. Doping properties of monoclinic BiVO4 studied by first-principles density-functional theory. Phys. Rev. B 83, 155102 (2011).
Yi, Z. et al. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation. Nat. Mater. 9, 559 (2010).
Reunchan, P. & Umezawa, N. Native defects and hydrogen impurities in Ag3PO4 . Phys. Rev. B 87, 245205 (2013).
Liu, J. J. et al. Electronic structure and optical properties of Ag3PO4 photocatalyst calculated by hybrid density functional method. Appl. Phys. Lett. 99, 191903 (2011).
Ma, X. et al. Origin of photocatalytic activation of silver orthophosphate from first-principles. J. Phys. Chem. C 115, 4680 (2011).
Ma, Z., Yi, Z., Sun, J. & Wu, K. Electronic and Photocatalytic Properties of Ag3PC4VI (C = O, S, Se): A Systemic Hybrid DFT Study. J. Phys. Chem. C 116, 25074 (2012).
Umezawa, N., Shuxin, O. & Ye, J. Theoretical study of high photocatalytic performance of Ag3PO4 . Phys. Rev. B 83, 035202 (2011).
Ceperley, D. M. & Alder, B. J. Ground State of the Electron Gas by a Stochastic Method. Phys. Rev. Lett. 45, 566 (1980).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 77, 3865 (1996).
Heyd, J., Scuseria, G. E. & Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 124, 219906 (2006).
Shimada, T., Ueda, T., Wang, J. & Kitamura, T. Hybrid Hartree-Fock density functional study of charged point defects in ferroelectric PbTiO3 . Phys. Rev. B 87, 174111 (2013).
Lyons, J. L., Janotti, A. & Van de Walle, C. G. Effects of carbon on the electrical and optical properties of InN, GaN and AIN. Phys. Rev. B 89, 035204 (2014).
Chen, S., Narang, P., Atwater, H. A. & Wang, L.-W. Phase Stability and Defect Physics of a Ternary ZnSnN2 Semiconductor: First Principles Insights. Adv. Mater. 26, 311 (2014).
Choi, M., Janotti, A. & Van de Walle, C. G. Native point defects in LaAlO3: A hybrid functional study. Phys. Rev. B 88, 214117 (2013).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758 (1999).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953 (1994).
Kohn, W. & Sham, L. J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 140, A1133 (1965).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 77, 3865 (1996); 78, 1396 (1997).
Krukau, A. V., Vydrov, O. A., Izmaylov, A. F. & Scuseria, G. E. Influence of the exchange screening parameter on the performance of screened hybrid functionals. J. Chem. Phys. 125, 224106 (2006)
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188 (1976).
Wei, S.-H. Comput. Overcoming the doping bottleneck in semiconductors. Comput. Mater. Sci. 30, 337 (2004).
Baraff, G. A. & Schluter, M. Electronic Structure, Total Energies and Abundances of the Elementary Point Defects in GaAs. Phys. Rev. Lett. 55, 1327 (1985).
Zhang, S. B. & Northrup, J. E. Chemical potential dependence of defect formation energies in GaAs: Application to Ga self-diffusion. Phys. Rev. Lett. 67, 2339 (1991).
Laks, D. B. et al. Native defects and self-compensation in ZnSe. Phys. Rev. B 45, 10965 (1992).
Yan, Y. & Wei, S.-H. Phys. Doping asymmetry in wide-bandgap semiconductors: Origins and solutions. Phys. Stat. Sol. (b) 245, 641 (2008).
David R. Lide, ed., CRC Handbook of Chemistry and Physics, 90th Edition (Internet Version 2010), CRC Press/Taylor and Francis, Boca Raton, FL. (2010)
Jung, In-Ho & Hudon, P. Thermodynamic Assessment of P2O5 . J. Am. Ceram. Soc., 95, 3665 (2012)
Varley, J. B., Janotti, A. & Van de Walle, C. G. Group-V impurities in SnO2 from first-principles calculations. Phys. Rev. B 81, 245216 (2010).
Momma, K. & Izumi, F. VESTA: a three-dimensional visualization system for electronic and structural analysis. J. Appl. Crystallogr., 41, 653 (2008).
Wang, B. C. et al. Band gap engineering in BiNbO4 for visible-light photocatalysis. Appl. Phys. Lett. 100, 182102 (2012).
Duan, X. M., Stampfl, C., Bilek, M. M. M. & Mackenzie, D. R. Codoping of aluminum and gallium with nitrogen in ZnO: A comparative first-principles investigation. Phys. Rev. B 79, 235208 (2009).
Wei, Su-Huai & Zhang, S. B. Chemical trends of defect formation and doping limit in II-VI semiconductors: The case of CdTe. Phys. Rev. B 66, 155211 (2002).
Grundmann, M. The Physics of Semiconductors, 2nd ed. (Springer, Berlin, 2010).
Varley, J. B., Weber, J. R., Janotti, A. & Van de Walle, C. G. Oxygen vacancies and donor impurities in β-Ga2O3 . Appl. Phys. Lett. 97, 142106 (2010).
Acknowledgements
We sincerely thank Dr. Jyh-Pin Chou for his valuable discussions. This work was supported by National Natural Science Foundation of China (Grant No. 61366007 and No. 11164032 and No. 61066005), Program for New Century Excellent Talents in University (Grant No. NCET-12-1080), Applied Basic Research Foundation of Yunnan Province (Grant No. 2011CI003 and No. 2013FB007), Program for Excellent Young Talents in Yunnan University. Computational resources were provided by the High Performance Computing Center of Yunnan University.
Author information
Authors and Affiliations
Contributions
Yang Huang carried out the DFT calculations and prepared the manuscript. T.M., Q.C., C.C. and Y.H. Contributed the discussion and suggestions. All authors read the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Huang, Y., Ma, T., Chen, Qy. et al. The electronic properties of impurities (N, C, F, Cl and S) in Ag3PO4: A hybrid functional method study. Sci Rep 5, 12750 (2015). https://doi.org/10.1038/srep12750
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep12750
This article is cited by
-
Cadmium and lithium doping in silver orthophosphate: An ab initio study
Scientific Reports (2016)
-
Comparative Study of the Magnetic Structure of BaFe2As2 Doped with Co or Ni
Journal of Superconductivity and Novel Magnetism (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.