Abstract
Carbon dots, generally small carbon nanoparticles with various forms of surface passivation, have achieved the performance level of semiconductor quantum dots in the green spectral region, but their absorption and fluorescence in red/near-IR are relatively weaker. Conceptually similar to endofullerenes, host-guest carbon dots were designed and prepared with red/near-IR dyes encapsulated as guest in the carbon nanoparticle core. Beyond the desired enhancement in optical properties, the host-guest configuration may significantly broaden the field of carbon dots.
Similar content being viewed by others
Introduction
Carbon dots (also called carbon quantum dots in some literature reports despite the absence of classically defined quantum confinement) have emerged as a new class of photoactive nanomaterials1,2,3,4, with their fluorescence properties resembling those typically found in conventional semiconductor nanocrystals or quantum dots (QDs)5. The structure of a carbon dot is relatively simple, generally a small carbon nanoparticle with various forms of surface passivation, among which the more effective has been the chemical functionalization with organic or polymeric species (Fig. 1) for carbon dots of bright fluorescence emissions4. In the green over the spectral region covered by green fluorescent protein (GFP), for example, the performance of existing carbon dots in terms of fluorescence quantum yields in solution or the image brightness at the individual dot level on a substrate has been found to be competitive to that of the presently dominating CdSe/ZnS QDs6. According to available experimental results, carbon dots are nontoxic2,3,4,7, certainly without the toxicity concerns associated with the heavy metal-containing semiconductor QDs. Therefore, there has been a growing interest in potential applications of carbon dots for fluorescence bioimaging in vitro and in vivo2,3,4,7,8,9,10,11,12. However, despite the extensive effort in the relevant research community, the development of carbon dots of high fluorescence quantum yields in the biologically more significant near-IR spectral region has found only limited success. This, combined with the generally lower absorptivity of carbon nanoparticles in the red/near-IR, suggests that new strategies are necessary in order to use the carbon dots platform for fluorescence probes of the desired red/near-IR performance.
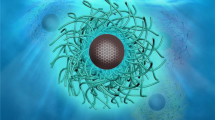
In the work reported here we “borrowed” the concept from the field of endofullerenes13 by considering the core carbon nanoparticle in a carbon dot as a “solid-state pool” (versus the cavity in a fullerene) to trap or encapsulate chromophoric species of strong red/near-IR absorption and emissions. This host-guest configuration takes advantage of the small carbon nanoparticle as host being optically largely transparent in the corresponding spectral regions, which has actually been identified above as a shortcoming of currently available carbon dots in their serving as red/near-IR probes. The resulting host-guest carbon dots, denoted as G@CDots (Fig. 1), exhibited the desired absorption and fluorescence properties, as designed and expected.
Results and Discussion
The thermal carbonization of organic precursors has been a popular approach for the synthesis of carbon dots2,3,4, in which a portion of the precursor organic species is converted into carbon nanoparticles and the remaining serves the function of surface passivation agents. Among various thermal processing options is the use of microwave irradiation14,15,16,17,18,19,20,21, which in principle creates carbonized seeds for their preferential absorption of the subsequent microwave energy towards the formation of the targeted carbon dot structure of a carbon nanoparticle with organic species on the surface for passivation. The microwave processing was adopted in this work for the “one-pot” synthesis of the G@CDots, with G denoting the selected fluorescent dyes of cresyl violet (CV), nile blue (NB) and zinc phthalocyanine (ZnPc).
Experimentally for the synthesis of CV@CDots, CV (20 mg) in an ethanol solution was mixed well with oligomeric polyethylene glycol of molecular weight ~900 (PEG900, 2 g), followed by the removal of ethanol via purging with nitrogen gas. The resulting mixture was placed in a commercial microwave oven and irradiated at 300 W for 20 min. Then, water was added to the reaction mixture with sonication to obtain a dark colored aqueous solution. The solution was centrifuged at 20,000 g, from which only a negligible amount of precipitate was observed and discarded. The supernatant was dialyzed in a membrane tubing (cutoff molecular weight ~1,000) against fresh water to remove unreacted starting materials and other small molecular species, yielding CV@CDots in an aqueous solution. The same processing protocol was applied to the preparation of NB@CDots and ZnPc@CDots, except that for the latter a 1:1 mixture of PEG900 and oligomeric polypropionylethyleneimine instead of neat PEG900 was in the mixture with ZnPc for microwave irradiation. The sample solutions were used for optical spectroscopy measurements and microscopy characterization.
For all three host-guest carbon dots, the absorption spectra in aqueous solutions exhibited contributions from carbon nanoparticles (more significantly in the blue/green spectral region, comparable with the absorption of carbon dots from the carbonization of PEG900 without the dye encapsulation, Fig. 2) and the guest dye molecules (Figs 2 and 3). However, the absorption bands of the encapsulated dyes are somewhat different from those of their corresponding free molecules, likely reflecting effects of the different environment in the hosting carbon nanoparticles. For example, the absorption of the CV in CV@CDots is much broader in comparison with that of the free dye molecules, both in aqueous solutions (Fig. 2). Similar encapsulation effects were observed in fluorescence spectra of the host-guest carbon dots. The spectra were found to be excitation wavelength dependent, as shown in Fig. 2 for example, which might be as expected considering the solid-like environment around the guest dye molecules in the hosting carbon nanoparticles (namely the molecules are each in a slightly different surrounding in a “solid-state solution”, a classical case for excitation wavelength dependent fluorescence emissions). Similarly for ZnPc@CDots in aqueous solution excited at its absorption peak, the fluorescence band is broader and red-shifted from that of the free ZnPc molecules (Fig. 3).
The absorption (ABS) spectrum of CV@CDots (—) and corresponding fluorescence (FLSC) spectra (excitation at 570 nm: —, 600 nm: -.- and 620 nm: -..-) in aqueous solution.
The spectra of free CV (- - -) and carbon dots from the carbonization of PEG900 without any encapsulation (…) in aqueous solutions are also shown for comparison. Inset: Photographs of an aqueous solution of the sample under UV light in the dark (left) and under natural day light (right).
Absorption (ABS) and fluorescence (FLSC) spectra of NB@CDots (top, —) and ZnPc@CDots (bottom, —) and the corresponding free dyes (- - -) in aqueous solutions (except for free ZnPc in DMSO).
Insets: Photographs of aqueous solutions of the corresponding samples under UV in the dark (left) and under natural day light (right).
The aqueous solutions of the host-guest carbon dots were diluted for the preparation of specimens on mica substrate for atomic force microscopy (AFM) characterization. Shown in Fig. 4 are the results for CV@CDots, NB@CDots and ZnPc@CDots. According to image height analyses, these host-guest carbon dots synthesized from thermal carbonization reactions are not as uniform in size as those from the surface chemical functionalization of pre-processed carbon nanoparticles reported previously6,22, though still relatively narrowly distributed. Most of these host-guest carbon dots are small, with their overall size profiles on the order of 10 nm or less (Fig. 4).
The carbon nanoparticle cores in the host-guest carbon dots are likely somewhat smaller than the overall dot profiles estimated from the height analysis of AFM images, as the latter may also include contributions of the organic species on carbon particle surface that survived the thermal carbonization processing. The expected significant contrast between the carbon core and surface organic species was exploited in the probing of the carbon nanoparticles by using transmission electron microscopy (TEM). For NB@CDots as an example, the TEM specimen was prepared such that a few drops of a dilute sample solution were deposited onto a silicon oxide-coated copper grid, followed by careful evaporation of the solvent. The imaging experiments were performed on a high-resolution TEM instrument (Hitachi H-9500). The results shown in Fig. 5 suggest that the NB-encapsulated carbon dots with residual PEG molecules as surface passivation moieties (confirmed by the significant PEG carbon peaks in 13C NMR analyses) are well-dispersed and that the carbon nanoparticle cores are size-wise small and relatively narrowly distributed.
For the host-guest carbon dots in aqueous solutions, the fluorescence quantum yields of the encapsulated dyes were evaluated against those of their free counterparts. Mechanistically, the observed fluorescence emissions from the guest dyes were due to their intrinsic electronic transition properties, not induced by the host carbon dots. However, the carbon pool environment in the hosting carbon dots could have meaningful effects on the fluorescence properties of the guest dyes. Among the three selected dyes, CV is soluble in water23, NB less so and only weakly fluorescent in an aqueous environment24 and ZnPc soluble in organic solvents23. Generally the results suggested that the fluorescence quantum yields of CV and NB as guests in the host-guest carbon dots were similar to those of free CV and NB molecules, respectively, all in aqueous solutions. More specifically for CV, it is known in the literature that its fluorescence quantum yields in aqueous solutions are somewhat concentration dependent, higher in a more dilute solution, yet overall about 40% lower than the yields in methanol25. The observed similar fluorescence quantum yields between the encapsulated and free CV molecules might be due to the opposing effects of a relatively higher CV concentration and more non-aqueous environment in CV@CDots, which decreases and enhances the quantum yields, respectively. However, for NB@CDots in an aqueous solution, the estimated fluorescence quantum yields of the guest NB were higher than that of free NB molecules in water (on the order of 0.01)24 but still significantly lower than that in ethanol (around 0.27)23, probably suggesting that the environment for the encapsulated NB is not entirely free from water. Similarly, the fluorescence quantum yields of ZnPc as guest in the host-guest carbon dots in an aqueous solution were also significantly lower than those of free ZnPc molecules in organic solvents, likely also due to the exposure of the encapsulated ZnPc to water (because the ZnPc fluorescence in a polar organic solvent is apparently quenched efficiently by the addition of water). Therefore, in further investigations the fluorescence properties of these water-sensitive dyes may be used to study the local environment in the core carbon nanoparticles in the host-guest carbon dots. Experimentally, more effort is needed to correct the light scattering effect in aqueous solutions of the host-guest carbon dots for a more accurate determination of the fluorescence quantum yields of the encapsulated dyes.
Conceptually similar to endofullerenes that have expanded the horizons of the fullerene field13, the host-guest carbon dots represent a new QD-like nanoarchitecture for materials properties and functions beyond those achieved with the original carbon dots. The extension of absorption and fluorescence coverage of carbon dots into the red/near-IR spectral regions with the relevant dyes as guest in this work serves as a representative example for the potential and versatile nature of the host-guest carbon dots platform. Such a new platform is expected to significantly broaden the reach of the already rapidly advancing carbon dots research and development.
Additional Information
How to cite this article: Sun, Y.-P. et al. Host-Guest Carbon Dots for Enhanced Optical Properties and Beyond. Sci. Rep. 5, 12354; doi: 10.1038/srep12354 (2015).
References
Sun, Y.-P. et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 128, 7756–7757 (2006).
Luo, P. G. et al. Carbon “quantum” dots for optical bioimaging. J. Mater. Chem. B 1, 2116–2127 (2013).
Wang, Y. & Hu, A. Carbon quantum dots: synthesis, properties and applications. J. Mater. Chem. C 2, 6921–6939 (2014).
Luo, P. G. et al. Carbon-based quantum dots for fluorescence imaging of cells and tissues. RSC Adv . 4, 10731–10807 (2014).
Kairdolf, B. A. et al. Semiconductor Quantum Dots for Bioimaging and Biodiagnostic Applications. Annu. Rev. Anal. Chem. 6, 143–162 (2013).
Wang, X. et al. Bandgap-Like Strong Fluorescence in Functionalized Carbon Nanoparticles. Angew. Chem. Int. Ed. 49, 5310–5314 (2010).
Yang, S.-T. et al. Carbon Dots as Nontoxic and High-Performance Fluorescence Imaging Agents. J. Phys. Chem. C 113, 18110–18114 (2009).
Yang, S.-T. et al. Carbon Dots for Optical Imaging in vivo. J. Am. Chem. Soc. 131, 11308–11309 (2009).
Ding, C., Zhu, A. & Tian, Y. Functional surface engineering of C-dots for fluorescent Biosensing and in vivo bioimaging. Acc. Chem. Res. 47, 20–30 (2014).
Cao, L. et al. Competitive Performance of Carbon “Quantum” Dots in Optical Bioimaging. Theranostics 2, 295–301 (2012).
Tao, H. et al. In Vivo NIR Fluorescence Imaging, Biodistribution and Toxicology of Photoluminescent Carbon Dots Produced from Carbon Nanotubes and Graphite. Small 8, 281–290 (2012).
Huang, X. et al. Effect of Injection Routes on the Biodistribution, Clearance and Tumor Uptake of Carbon Dots. ACS Nano 7, 5684–5693 (2013).
Popov, A. A., Yang, S. & Dunsch. L. Endohedral fullerenes. Chem. Rev. 113, 5989–6113 (2013).
Zhu, H. et al. Microwave synthesis of fluorescent carbon nanoparticles with electrochemiluminescence properties. Chem. Commun. 45, 5118–5120 (2009).
Liu, C. et al. One-step synthesis of surface passivated carbon nanodots by microwave assisted pyrolysis for enhanced multicolor photoluminescence and bioimaging. J. Mater. Chem. 21, 13163–13167 (2011).
Jaisawl, A., Ghosh, S. S. & Chattopadhyay, A. One step synthesis of C-dots by microwave mediated caramelization of poly (ethylene glycol). Chem. Commun. 48, 7955–7957 (2012).
Zhai, X. et al. Highly luminescent carbon nanodots by microwave-assisted pyrolysis. Chem. Commun. 48, 7955–7957 (2012).
Chandra, S., Das, P., Bag, S., Laha, D. & Pramanik. P. Synthesis, functionalization and bioimaging applications of highly fluorescent carbon nanoparticles. Nanoscale 3, 1533–1540 (2012).
Chandra, S. et al. Tuning of photoluminescence on different surface functionalized carbon quantum dots. RSC Adv . 2, 3602–3606 (2012).
Sachdev, A. et al. A novel one-step synthesis of PEG passivated multicolour fluorescent carbon dots for potential biolabeling application. RSC Adv . 3, 16958–16961 (2013).
Liu, C. et al. Nano-carrier for gene delivery and bioimaging based on carbon dots with PEI-passivation enhanced fluorescence. Biomaterials 33, 3604–3613 (2012).
LeCroy, G. E. et al. Toward Structurally Defined Carbon Dots as Ultracompact Fluorescent Probes. ACS Nano 8, 4522–4529 (2014).
Brouwer, A. M. Standards for photoluminescence quantum yield measurements in solution. Pure Appl. Chem. 83, 2213–2228 (2011).
Jose, J., Ueno, Y. & Burgess, K. Water-Soluble Nile Blue Derivatives: Syntheses and Photophysical Properties. Chem. Euro. J . 15, 418–425 (2009).
Isak, S. & Eyring, E. M. Fluorescence quantum yield of cresyl violet in methanol and water as a function of concentration. J. Phys. Chem. 96, 1738–1742 (1992).
Acknowledgements
The support from Air Force Office of Scientific Research (AFOSR, through the program of Dr. Charles Lee) is gratefully acknowledged. Z.L. was a visiting student from Prof. Fushen Lu’s group at Shantou University in China, M.J.M. a visiting professor from Northwest Missouri State University supported by the South Carolina Space Grant Consortium and Y.L. on leave from Technical Institute of Physics and Chemistry in Beijing, China with a visiting scholarship provided by Chinese Academy of Sciences.
Author information
Authors and Affiliations
Contributions
Y.-P.S. came up with the idea and supervised the performance of the work; P.W., Z.L. and F.Y. performed various tasks of the work; M.J.M. and H.Q. contributed to the sample analyses; and G.E.L. and Y.L. assisted the performance of the work.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Sun, YP., Wang, P., Lu, Z. et al. Host-Guest Carbon Dots for Enhanced Optical Properties and Beyond. Sci Rep 5, 12354 (2015). https://doi.org/10.1038/srep12354
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep12354
This article is cited by
-
Turning date palm fronds into biocompatible mesoporous fluorescent carbon dots
Scientific Reports (2018)
-
Cottontail rabbits shed clade 2.3.4.4 H5 highly pathogenic avian influenza A viruses
Archives of Virology (2018)
-
Engineering carbon quantum dots for photomediated theranostics
Nano Research (2018)
-
Nitrogen-doped Carbon Dots Mediated Fluorescent on-off Assay for Rapid and Highly Sensitive Pyrophosphate and Alkaline Phosphatase Detection
Scientific Reports (2017)
-
A facile ultrasonic-assisted fabrication of nitrogen-doped carbon dots/BiOBr up-conversion nanocomposites for visible light photocatalytic enhancements
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.