Abstract
We explore the electrical rectification of large amplitude fluctuating signals by an asymmetric nanostructure operating in aqueous solution. We show experimentally and theoretically that a load capacitor can be charged to voltages close to 1 V within a few minutes by converting zero time-average potentials of amplitudes in the range 0.5–3 V into average net currents using a single conical nanopore. This process suggests that significant energy conversion and storage from an electrically fluctuating environment is feasible with a nanoscale pore immersed in a liquid electrolyte solution, a system characteristic of bioelectronics interfaces, electrochemical cells and nanoporous membranes.
Similar content being viewed by others
Introduction
In biological processes, nanoscale energy and information flows occur under significant environmental fluctuations. Enzymes operate in rapidly changing environments1,2 and the cell membrane is equipped with protein aqueous pores (ion channels3) which allow noisy electrical and biochemical signals to be transduced into net directional fluxes4,5,6. These facts suggest that artificial nanostructures could also perform useful information and energy transduction processes in highly fluctuating environments. The above question is of significance because external fluctuations are usually considered a nuisance, not an opportunity, in applications of nanoscale devices. In particular, although the correlation between the state of a system and its environmental fluctuations precludes the systematic harvesting of energy from equilibrium random fluctuations, this is not the case of non-equilibrium external fluctuations uncorrelated to the system state.
We study here the electrical rectification of large amplitude fluctuating potentials using a single nanopore immersed in an ionic aqueous solution. To provide an appropriate context, we summarize first other relevant studies. On the basis of previous work by Siwy et al.7,8, we have demonstrated the transduction of periodic electrical signals with zero time-averages into net average currents in the cases of a biomimetic nanopore functionalized with amino acid groups9, a cylindrical pore that can be blocked by a nanoparticle10 and the (wide pore) bacterial porin channel of Escherichia coli reconstituted on a planar lipid bilayer5. However, the scientific and technically relevant question of energy conversion and storage has not been addressed in the above cases. We experimentally and theoretically demonstrate here a simple procedure allowing the conversion of external random signals (electric potentials) into directional average fluxes (net currents) using a single conical nanopore. The net current obtained is used to charge an external load capacitor, demonstrating significant energy conversion and storage from a highly fluctuating external environment. Analogously to the case of the cell membrane ion channels, the nanopore immersed in a liquid electrolyte solution shows ionic selectivity and electrical rectification characteristics5. The experimental data can be described theoretically by simulating the capacitor charging and discharging processes.
Because we use a single biomimetic pore operating in an aqueous salt solution which is electrically coupled to a commercial capacitor, the reported experiments can be of relevance to bioelectronics, information processing and energy conversion processes using electrochemical nanodevices11,12,13,14,15,16.
Results and Discussion
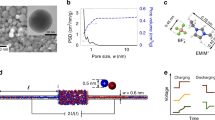
Figure 1 schematically shows the rectification characteristics obtained using a single conical pore with a nanoscale tip radius which separates two identical 0.1 M KCl aqueous solutions. The pore is obtained by using asymmetric track-etching methods17,18 and its surface is functionalized with carboxylate residues that are ionized at pH = 7. The nanostructure has an asymmetric, negative fixed charge distribution along the axis which gives the electrical rectification observed in the steady-state current (I) – potential (VN) curve of the pore (Fig. 1a)18. A time (t)-dependent, randomly fluctuating electric potential VR(t) of amplitude V0 (white noise) is externally applied by means of a couple of Ag|AgCl electrodes immersed in the bathing solution (Fig. 1b). The output electric current I(t) shows a non-zero time average value <I> (Fig. 1c) as a result of the pore electrical rectification shown in Fig. 1a. The electric current I(t) through the pore shows no time delay with respect to VR(t) if the potential period is much longer than the relaxation time of the system. Because of the small pore volume involved, the above condition is approximately valid in nanopores9 and ion channels5 for the case of low frequency signals.
Scheme of the energy conversion and storage process using a single conical pore.
(a) Steady-state current (I) - potential (VN) curves showing electrical rectification (pH = 7) and ohmic response (pH = 3, control experiment). (b) A randomly fluctuating electric potential VR(t) is externally applied using a voltage source. (c) The output electric current I(t) for pH = 7 gives a non-zero time average current <I>. (d) This net current <I> allows the charging of an external load capacitor connected in series to the nanopore. As the capacitor is charged, a potential difference (voltage) VC(t) which drives a reverse current opposing the charging process is set up.
We use now the net current <I> to charge a commercial capacitor connected in series to the single nanopore (Fig. 1d), showing that steady-state capacitor voltages of the order of 0.2 V can be obtained for amplitudes V0 of the order of 1 V within a few minutes. Note finally that a control experiment for the same pore sample at the (low) pH = 3.0 shows an ohmic behavior (Fig. 1a) because the conical pore is in neutral form at this pH value. The absence of current rectification gives a zero net current in this case.
Figure 2 shows typical charge (a) and discharge (b) curves measured with an external load capacitor of capacitance C = 0.1 μF and an input fluctuating potential of amplitude V0 = 3 V. The net current <I> as a function of V0 (c) and the capacitor charge curves at different values of V0 (d) are also shown. Fig. 2a shows that for the charged pore (pH = 7) at sufficiently long times t→∞, a maximum steady-state voltage VC(∞) is reached when the reverse current opposing the charging process is equal to the net charging current. The conical nanopore behaves then as a voltage-controlled current source: the maximum current corresponds to the initially uncharged capacitor, VC(t = 0) = 0, decreasing to zero when the capacitor reaches the voltage VC(∞). In the case of the neutral pore (pH = 3, control experiment in Fig. 2a), the capacitor voltage cannot reach significant average values because of the absence of current rectification (see Fig. 1a). Also, independent tests with other periodically fluctuating potentials (triangular and square zero-average potentials) were carried out, giving net currents and then significant capacitor charging.
Experiments.
(a) The capacitor potential vs. time charging for pH = 7 (top) and pH = 3 (bottom, control experiment) curves and (b) the discharging curve for pH = 7. In both cases the potential amplitude V0 = 3 V. (c) The average (net) current vs. the external potential amplitude. (d) The capacitor charging curves at different amplitudes of the potential. In all experiments the external load capacitance was C = 0.1 μF. The curves in Fig. 2d show identical time patterns because the input potential pulses are generated using the same scheme, scaling the single point values to obtain the desired amplitude. This procedure allows discarding the presence of internal noise sources due to the wiring, capacitor and voltage source that might influence the phenomena studied.
When the external potential is disconnected, the capacitor begins to discharge following a characteristic exponential decay (Fig. 2b). As expected, increasing the amplitude V0 of the input signals leads to higher net currents <I>, as shown in Fig. 2c. The value of <I> does not depend on the random pulse lifetime because there is no time delay between the instantaneous current and the external potential (see Figs. 1a–c and Ref. 9 for details). The average currents <I> of Fig. 2c can be obtained directly from the experimental instantaneous currents I(t). Because the values of the input pulses are equally probable, these average currents should be approximately equal to those obtained by dividing the area limited by the I(VN) curve of Fig. 1a and the potential axis between −V0 and +V0 by the peak to peak value of this potential. We have checked that this is indeed the case, which gives further support to the validity of our assumptions. Note also that all curves in Fig. 2d show identical time patterns because the fluctuating signals are generated by the voltage source of Fig. 1d according to the same scheme. This procedure clearly suggests that the charging process arises only from the rectification of the input signal by the single nanostructure and is not significantly influenced by internal noise sources due to the electrical equipment.
Because of the increase of <I> with V0, the charging curves of Fig. 2d give steady capacitor voltages VC(∞) which increase with the input signal amplitude. Remarkably, VC(∞) is of the order of 1 V within a few minutes using a commercial capacitor. A measure of the charging process efficiency is given by the energy ratio  . From the experimental results of Fig. 2d, we can assume an effective total time T = 3τch for the capacitor voltage saturation, where τch is the characteristic charging time. The above energy ratio can then be obtained from the recorded experimental data and takes values between e = 0.016 (V0 = 0.5 V) and e = 0.071 (V0 = 3 V), which are small but not negligible. In this latter case, approximately 7% of the input energy from the external fluctuating signal can be recovered (i. e., 93% of the input energy is dissipated).
. From the experimental results of Fig. 2d, we can assume an effective total time T = 3τch for the capacitor voltage saturation, where τch is the characteristic charging time. The above energy ratio can then be obtained from the recorded experimental data and takes values between e = 0.016 (V0 = 0.5 V) and e = 0.071 (V0 = 3 V), which are small but not negligible. In this latter case, approximately 7% of the input energy from the external fluctuating signal can be recovered (i. e., 93% of the input energy is dissipated).
The single pore resistance in Fig. 1a is of the order of 1 V/1 nA = 1 GΩ and the capacitance of the load capacitor in Fig. 1d is 0.1 μF, giving characteristic times of the order of 109 Ω × 10−7 F = 100 s, in agreement with the experimental curves of Fig. 2. In particular, the different characteristic times observed in the charging and discharging curves can be compared with the equivalent circuit times τch = RchC (charging) and τdi = RdiC (discharging), where Rch and Rdi are the effective single pore resistances for the charging and discharging processes. These times are of the order of 50 s and 200 s in Figs. 2a and 2b, respectively, in agreement with the different resistances of Fig. 1a obtained for forward (VN > 0) and reverse (VN < 0) pore polarization.
The results of Fig. 1a and 2a clearly show that it is the current rectification that gives the capacitor charging. As a consequence, this charging effect should be enhanced by increasing the pore rectification, which in turn depends on the pore geometry, the asymmetrical charge distribution and the solution characteristics9,15,18. In particular, the absolute value of the positive and negative currents should decrease with the pore length and increase with the pore diameter. However, the net current that results from the difference between these absolute currents depends crucially on the pore rectification, which requires long pores and narrow tip diameters15.
The charging process can be simulated assuming that the nanopore is a potential dependent resistance connected in series to the capacitor. The whole circuit is fed with a (white noise) potential signal of amplitude V0. The equation describing the charging is dVC(t)/dt = I(t)/C, where VC(t) and I(t) are the instantaneous capacitor potential and the current through the circuit, respectively. We introduce the initial condition VC(t = 0) = 0, establish a constant time step Δt for the potential VR(t) to take random values between −V0 and +V0 and update the capacitor voltage as VC→VC + I(VN)Δt/C, where I(VN) is the measured current (Fig. 1a) at the potential VN = VR – VC. This procedure leads to the final maximum voltage VC(∞). Then, we introduce VR(t) = 0 in the algorithm in order to simulate the discharging process. Figure 3 shows that the theoretical charging (Fig. 3a) and discharging (Fig. 3b) curves reproduce the observed behavior, which gives further support to the experimental data reported.
Conclusion
A single asymmetric pore acting as a soft matter nanoscale version of the macroscopic solid-state diode allows charging a commercial capacitor to significant output potentials within a few minutes by rectifying an external, randomly fluctuating signal. The experimental data are correctly described by the theoretical simulations. The input signal amplitudes needed are dictated by the minimum voltage required for the nanostructure rectification to be significant and could then be decreased to 0.1 V in the case of (wide pore) biological ion channels (e.g., the OmpF porin of Escherichia coli bacteria5) at the price of a decreased physico-chemical robustness. Also, the use of these channels could be useful to quantify the long-term accumulative effects of large amplitude and low frequency external signals.
The nanopore shows rectifying properties similar to those of wide pore biological ion channels3,5 and is immersed in the electrolyte solutions characteristic of nanopore-based sensors19,20 and bioelectronics interfaces11. The results suggest that physical mechanisms such as rectification can be exploited in the design of energy conversion and storage schemes based on soft matter nanostructures electrically coupled to conventional electronic elements.
Methods
Nanopore characteristics
Polyethylene terephthalate (PET) polymer foils (Hostaphan RN 12, Hoechst) of thickness 12 μm were irradiated with single swift heavy ions (Pb, U and Au) having an energy of 11.4 MeV per nucleon at the linear accelerator UNILAC (GSI, Darmstadt) (PET). The single conical pore is obtained by using asymmetric track-etching methods described with detail elsewhere17,18. These methods give typical radii in the range 10–20 nm (cone tip) and 100–200 nm (cone basis). In our pore, the approximate radii were 11 nm and 155 nm, respectively, obtained from the steady-state current (I) – potential (VN) curve, where VN = VR – VC18. The etching process gives carboxylate residues on the pore wall surface which are ionized at pH = 7 in a 0.1 M KCl aqueous solution. Because of the approximately conical geometry, the nanostructure has an asymmetric (negative) fixed charge distribution18 along the axis which gives the electrical rectification observed in the I – VN curve of the pore (Fig. 1a). Also, additional control measurements were carried out at pH values low enough (pH = 3) for the pore to be in neutral form. The pH was controlled by a Crison GLP22 pH-meter before and after each measurement.
Electrical measurements
The fluctuating electric potential is externally applied by means of a couple of Ag|AgCl electrodes immersed in the bathing solutions (Fig. 1d). Random pulse lifetimes in the range of 0.5 s were applied. The electrical rectification is due to the fact that the current entering the pore through the (narrow) tip opening experiences an electrical resistance lower than that entering the pore through the (wide) basis opening. Pore electrical measurements were recorded with one picoammeter/voltage source (Keithley 6487/E). The voltage across the commercial capacitor of capacitance C = 0.1 μF was measured with a multimeter (Keithley 2000/E). The average currents <I> of Fig. 2c were obtained directly from the recorded instantaneous currents I(t).
Theoretical simulations
The charging process was simulated taking the nanopore as a potential dependent resistance connected in series to the capacitor. The whole circuit was fed with a (white noise) potential signal of amplitude V0. The equation describing the charging was dVC(t)/dt = I(t)/C, where VC(t) and I(t) were the instantaneous capacitor potential and the current through the circuit, respectively. We introduced the initial condition VC(t = 0) = 0 and established a (constant) time step Δt = 0.1 s because this value is of the same order of magnitude as the experimental step used in the measurements. At each step Δt, VR(t) took random values between −V0 and +V0 and the capacitor voltage was updated as VC→VC + I(VN)Δt/C where I(VN) is the experimental current of Fig. 1a. The simulations were carried out with different values of the time step Δt. After completion of the charging process, a maximum voltage VC(∞) was reached. At this moment, we set VR(t) = 0 in the numerical algorithm to simulate the discharging process. All simulations were performed using a Python numerical code.
References
Astumian, R. D. Stochastic conformational pumping: A mechanism for free-energy transduction by molecules. Annu. Rev. Biophys. 40, 289–313 (2011).
Qian, H. Cooperativity in cellular biochemical processes: Noise-enhanced sensitivity, fluctuating enzyme, bistability with nonlinear feedback and other mechanisms for sigmoidal responses. Annu. Rev. Biophys. 41, 179–204 (2012).
Hille, B. Ionic Channels of Excitable Membranes (Sinauer Associates Inc., Sunderland, MA, 1992).
Levin, M. Molecular bioelectricity in developmental biology: new tools and recent discoveries: control of cell behavior and pattern formation by transmembrane potential gradients. Bioessays 34, 205–217 (2012).
Queralt-Martín, M. et al. Electrical pumping of potassium ions against an external concentration gradient in a biological ion channel. Appl. Phys. Lett. 103, 043707 (2013).
Hudspeth, A. J., Choe, Y., Mehta, A. D. & Martin, P. Putting ion channels to work: Mechanoelectrical transduction, adaptation and amplification by hair cells. Proc. Nat. Acad. Sci. U.S.A. 97, 11765–11772 (2000).
Siwy, Z. & Fuliński, A. Fabrication of a Synthetic Nanopore Ion Pump. Phys. Rev. Lett. 89, 198103 (2002).
Siwy, Z. & Fuliński, A. A nanodevice for rectification and pumping ions. Am. J. Phys. 72, 567–574 (2004).
Ramirez, P., Gomez, V., Ali, M., Ensinger, W. & Mafe, S. Net currents obtained from zero-average potentials in single amphoteric nanopores. Electrochem. Commun. 31, 137–140 (2013).
Ali, M. et al. Current rectification by nanoparticle blocking in single cylindrical nanopores. Appl. Phys. Lett. 104, 043703 (2014).
Misra, N. et al. Bioelectronic silicon nanowire devices using functional membrane proteins. Proc. Natl. Acad. Sci. U.S.A. 106, 13780–13784 (2009).
Ramirez, P., Ali, M., Ensinger, W. & Mafe, S. Information processing with a single multifunctional nanofluidic diode. Appl. Phys. Lett. 101, 133108 (2012).
Hou, Y., Vidu, R. & Stroeve, P. Solar energy storage methods. Ind. Eng. Chem. Res. 50, 8954–8964 (2011).
Guo, W. et al. Energy harvesting with single-ion-selective nanopores: A concentration-gradient-driven nanofluidic power source. Adv. Funct. Mater. 20, 1339–1344 (2010).
Cervera, J., Ramirez, P., Mafe, S. & Stroeve, P. Asymmetric nanopore rectification for ion pumping, electrical power generation and information processing applications. Electrochim. Acta, 56, 4504–4511 (2011).
Tybrandt, K., Forchheimer, R. & Berggren, M. Logic gates based on ion transistors. Nat. Commun., 3, 871 (2012)
Apel, P. Track etching technique in membrane technology. Radiat. Meas. 34, 559–566 (2001).
Ali, M., Ramirez, P., Mafe, S., Neumann, R. & Ensinger, W. A pH-tunable nanofluidic diode with a broad range of rectifying properties. ACS Nano 3, 603–608 (2009).
Albrecht, T. How to Understand and Interpret Current Flow in Nanopore/Electrode Devices. ACS Nano 5, 6714–6725 (2011).
Ali, M. et al. Carbohydrate-Mediated Biomolecular Recognition and Gating of Synthetic Ion Channels. J. Phys. Chem. C 117, 18234–18242 (2013).
Acknowledgements
We acknowledge the support from the Ministry of Economic Affairs and Competitiveness and FEDER (project MAT2012-32084) and the Generalitat Valenciana (project Prometeo/GV/0069).
Author information
Authors and Affiliations
Contributions
S.M. and P.R. designed the experiments, M.A., S.N. and W.E. fabricated and functionalized the nanopores, P.R. and V.G. performed the experiments, J.C. performed the simulations, P.R. and S. M. wrote the paper. All authors contributed to the concept of the paper and the discussion of the results.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Gomez, V., Ramirez, P., Cervera, J. et al. Charging a Capacitor from an External Fluctuating Potential using a Single Conical Nanopore. Sci Rep 5, 9501 (2015). https://doi.org/10.1038/srep09501
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep09501
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.