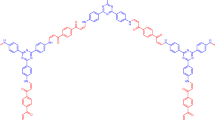
Abstract
Ion-controlled switchable progress is very important in many biological behaviors. Here, we reported K+-controlled switch, this switch system exhibited excellent carbaryl (G) binding/release by fluorescent (FL), ultraviolet-visible (UV) spectrums and 1H NMR spectroscopy. More importantly, the K+-controlled G binding/release switch based on C4C5 not only in the solution, but also on the surface, promising for the application for the pesticide controlled release.
Similar content being viewed by others
Introduction
Ion-controlled switchable process is very important in many biological behaviors, for example, metal ion-directed protein folding and self-assembly, Ca2+ induced contraction or relaxation of the human heart and the Na+ stimulated nerve impulses1,2,3,4,5,6. Recently, those in which the binding substrate could be “on” or “off” by ion, have attracted great interest in host-guest complex7,8,9, due to their extensive potential application not only in the construction of artificial molecular machines but also in the development of sensing and controllable drug-delivery system10,11,12,13,14,15,16,17,18,19,20,21,22,23. For example, riptycene-derived containing two dibenzo-[18]-crown-6 interacted with paraquat derivatives which can be combined and released by Li+24. To our knowledge, most biological ion-controlled switches realize on the membrane interface, but the ion-controlled switch on the surface has been scarcely reported.
Controlling surface wettability has attracted interest in recent years25,26,27,28, as it is important for range of biological and chemical application29,30, thus, chemical, thermal and pH-sensitive wettability changed have been reported. These changes own to their inherent physicochemical properties under environmental conditions. These means can provide a general method to realize ion-controlled switch between hydrophobicity and hydrophilicity. For example, wettability provides a method for calix[4]azacrown modified Si-surface response to [C4min]Cl31. Among of these, the design and synthesis of response molecules play an important role in constructing wettable functional surface. As we all known, calixarene, because of its adjustable cavity and modifying substituents of the upper and lower rims, have been a good choice of functional response molecules. Calixcrown make a family of calixarenes that exhibit a superior recognition of organic molecules and metal ions via the cooperation of both the calixarene and crown moieties32,33,34,35,36,37,38,39. Carbaryl (G) is a pesticide which have naphthalene group can interact with calixarene through π-π stacking. Crown ether was employed to selectively bind K+ in aqueous solution40. It was known that 18C6 is a very strong sequestering agent for potassium ion41,42, which has strong binding affinity toward K+. Therefore, we designed a wettable responsive switch based on K+-controlled calixcrown binding/release G. All were shown in the figure 1b. As a consequence of its outstanding properties, the K+-controlled switch has formed attractive application in many fields, especially on a silicon surface.
Results
Synthesize of C4C5
Dialkyne 143,44,45 (0.5 g, 9.8 mmol) and NaH (0.067 g, 40 mmol) and OTS glycol (0.35 g, 10 mmol) were stirred in the anhydrous THF (70 mL), see in the figure 1a. The mixture reaction was stirred at 50°C for 15 h. When the flask was cooled to room temperature, methanol was added to remove the no reacting NaH. The residue solvent was evaporated by suction filtration to give white powder C4C5 (0.45 g, 73%). The structure and conformation of C4C5 were confirmed by 1H NMR, 13C NMR studies (Supplementary Fig. S1, Fig. S2), elemental analyses and EI(+)MS spectra (Supplementary Fig. S3). All of these indicated the cone conformation of C4C546,47,48,49. Base on the interaction between the cavity of the C4C5 and naphthalene group, we obtained [C4C5+G] complex with a conjugation ratio of 1:1, by UV, 1H NMR spectroscopy and density functional calculation studies. Further, in the situ [C4C5+G] complex was added K+. From fluorescence and 1H NMR spectroscopy we found the C4C5 binding/release G was controlled by K+. Even further, the K+-controlled G binding/release switch had been successfully applied on the functional micro-silicon surface by the simple click reaction. Moreover, the K+-controlled G binding/release could act as a convenient and effective wettability switch by contact angle (CA) measurement on the silicon surface.
The K+-controlled G binding/release in the solution
To provide support for the K+-controlled binding/release G, some experiments were done in the solution phase. First of all, a fluorescence experiment of the C4C5 and G, K+ were carried out using an excitation wavelength of 280 nm. The fluorescence of G (1.0 × 10−5 M, 2.0 mL) enhanced with the addition of the C4C5 (1.0 × 10−3 M, 20 μL) in CH3CN because of their interaction. This indicated the [C4C5+G] complex was successfully obtained. To further study of the C4C5 with G carried out by K+, the same equiv. of K+ was added for the interaction with [C4C5+G] which showed fluorescence recovered (Fig. 2). Then, ultraviolet-visible (UV) spectrums of C4C5, [C4C5+G], [C4C5+K+] were also done in CH3CN solution. From the spectrum of UV (Supplementary Fig. S4), the absorption of C4C5 was increased when G added and recovered when K+ added. The fluorescence spectrum and UV spectrum all demonstrated K+- controlled binding/release G can come true in the solution.
(a) Fluorescence intensity changes of G, binding G and release G in CH3CN upon irradiation with 334 nm light (λex = 280 nm, black line represents the fluorescence of G; red line represents binding G; green line represents release G). (b) FL variation [ΔI = (I-I0)] (I0 is the FL intensity of G) histogram for the [C4C5+G] complex and [C4C5+G] with K+ at 334 nm.
In order to illustrate the details of formation of [C4C5+G] in the solution, the binding stoichiometry of the complex formed between C4C5 and G was 1:1 from Job's plot by ultraviolet-visible (UV) spectrum, which had a peak at 277.9 nm with a molar fraction of 0.5 (Supplementary Fig. S5). The binding stoichiometry of the complex formed between C4C5 and K+ was also 1:1 from the Job's plot. The liner relationship between intensity of UV at 277.9 nm and different concentration of G was carried (Supplementary Fig. S6). We found the [C4C5+K+] binding stoichiometry was also 1:1 (Supplementary Fig. S6). The host C4C5 was 1 × 10−4 M, 2.0 mL CH3CN solution and then the different amount of G (1 × 10−3 M, CH3CN solution) was gradually adding into the host solution. At the same time, the liner relationship between intensity of UV 277.9 nm and different concentration of K+ was also carried using the same method (Supplementary Fig. S7). The association constant of C4C5 and G is 6.22 × 103 M−1, however, C4C5 and K+ is 1.04 × 105 M−1, these results clearly suggested the complex of [C4C5+K+] was much stable than the complex of [C4C5+G].
The K+-controlled G binding/release characterized by NMR
To obtain insights into the mechanism, NMR experiments were carried out, 6.0 mM C4C5 and 1.0 equiv. of G and K+ in CD3CN, as showed in Fig. 3. The protons of the napthalene in the G underwent upfield shift of 0.05 ppm in the presence of C4C5. The result also indicated that the [C4C5+G] complex was successfully formed. When K+ added, the protons of naphthalene underwent an upfield shift of 0.05 ppm and recovered to original chemical shift, moreover, the protons of crown have underwent the protons of crown ether unit of C4C5 Ha, Hb, Hc and Hd underwent downfield shift of 0.04 ppm, 0.18 ppm, 0.14 ppm and 0.17 ppm respectively. According to the NMR, we deduced that before K+ was added, the crown ring of C4C5 was stretched, when K+ added, K+ entered into crown space, which made the lower space of crown become smaller and this reaction has made the space of the upper of the C4C5 become bigger and led to G released.
We also found the 18C6 was added (Supplementary Fig. S8e), the chemical shifts of Ha, Hb, Hc, Hd recovered to original values, moreover there appeared a new in peak in the spectrum, we supposed the new peak is the complex of 18C6 and K+. Based on above experiments, we have obtained the K+-controlled G binding/release switch.
The K+-controlled G binding/release characterized by ESI-MS
To further verify the binding between C4C5 and G, the ESI-MS analysis was carried out. The peak observed at m/z = 1084.72 in the ESI mass spectrum (Fig. 4a) can be attributed to [C4C5+G] complex. Furthermore, the peak observed at m/z = 921.61 in the ESI mass spectrum (Fig. 4b) can be attributed to [C4C5+K+] complex, we also found m/z = 883.58 in the ESI mass spectrum (Fig. S9) can be attributed to K+ released from C4C5. The whole ESI-MS study fully explained the binding/release process and also confirmed the 1:1 complex formation C4C5 and G, C4C5 and K+.
The Gaussian calculation of C4C5, [C4C5+G] and [C4C5 and K+]
Further, the binding of C4C5 and G, K+ were also examined by Gaussian03. The results were shown in Fig. 5. The host C4C5 was yellow, the guest carbaryl was green, oxygen atoms were red, nitrogen atoms were blue, hydrogen atoms were white, purple atom was potassium. The structure of C4C5 and the complex of [C4C5+G], [C4C5+K+] have been optimized by going through a cascade process starting from HF/3-21G → HF/6-31G → B3LYP/3-21G → B3LYP/6-31G. Meanwhile, the frequency analysis calculations were performed and the absence of imaginary frequencies indicated the low energy minimum of the structures obtained. All the energy were obtained by the equation of ΔE (binding energy) = E (host − guest) − [(E (host) + E (guest)], [C4C5+G] binding energy is −0.0014 a.u./mol, [C4C5+G+K+] binding energy is 0.05789 a.u./mol, while [C4C5+K+] is −0.9438 a.u./mol, comparing to these energy, which indicated that [C4C5+K+] complex is much stable than [C4C5+G] complex. The space of [C4C5+G] is 6.1Å, 7.1Å, while [C4C5+K+] is 6.4Å, 7.2Å, which indicated that K+ interacted with C4C5 made the space of become bigger and G can released from the space. These results of molecular mechanics calculation were generally consistent with the UV, 1H NMR experimental results. All the calculation details were shown in Supplementary Fig. S10, Fig. S11, Fig. S12.
(a) The side view on the optimized structure of [C4C5+G] complex, the binding energy was −0.0014 a.u./mol (in the C4C5 the yellow atoms represent carbon, red atoms represent oxygen, blue atoms represent nitrogen, white atom represents hydrogen; in the G, the cyan atoms represent carbon, the blue atoms represent nitrogen, red atoms represent oxygen). (b) The top view on the optimized structure of [C4C5+K+], the binding energy was −0.9438 a.u./mol (the yellow atoms represent carbon, red atoms represent oxygen, purple atom represents potassium). Which demonstrated the complex of [C4C5+K+] was much stable than [C4C5+G]. The space of [C4C5+K+] is 6.4Å, 7.2Å, which is much bigger than [C4C5+G] and make G release from the space.
K+- controlled G binding/release on the surface
More importantly, the K+-controlled binding/release carbaryl switch had an important and potential application by measurement of the contact angle (CA) on a functional micro- silicon surface. Because micro-structured and functional silicon surface can amplify the signal output with the respect of wettability50, it could reversibly switch between hydrophobic character and a hydrophilic character. At first, the C4C5-SAMs (self-assembled monolayer) were established by click reaction between the Si-N3 SAMs and C4C5 in CH3CN (Fig. 6).
Through the SEM (Supplementary Fig. S13), the rough substrate exhibited a regular array of square silicon micro convexes (bright squares) and then the C4C5-modified substrate showed a thin film on the surface. Comparing SEM image of the rough surface before and after modification with C4C5, these results indicated that C4C5 was successfully modified on the silicon surface. From XPS (Supplementary Fig. S14), the concentration of carbon had a significant increase and the concentration of oxygen had an obvious decrease. Moreover, the water-drop profiles showed a different CAs on bare silicon surface. This also proved the above conclusion. The silicon surface was immersed into G solution (1.0 × 10−3 M), after 15 min the silicon wafer was washed by little water to remove the residue G, dried by nitrogen and measured. The results showed in Figure 7a, C4C5 SAMs immersed into G the CA became from superhydrophobic to hydrophilic, which was only 22.8 ± 3.0°, however, when immersed into KClO3 solution (1.0 × 10−3 M) and 18C6 solution (1.0 × 10−3 M) respectively, the wettability of the surface recovered to their original values (150.0 ± 3.0°). Because G interacted with C4C5 and the group of CONH was exposed, which made the surface become hydrophilicity, nevertheless, K+ formed complex with C4C5 and made G released from the space and left the tertiary butyl, which led to the surface become superhydrophility again. 18C6 interacting with K+ didn't change the property of the upper rim of C4C5, so the wettability remained superhydrophobicity (Supplementary Fig. S15).
Upon further treatment of the surface with water, then the silicon was immersed into G, K+ and 18C6 consecutively, the results remained the same as the first time. A cycle experiment (Fig. 7b) reflected a good reversibility for K+-controlled G binding/release switch on the surface. According to above results, we found the experiments were consistent with NMR and Gaussian calculation, which confirmed K+-controlled and binding/release G based on the surface wettability can be established by contact angle measurement.
Discussion
In conclusion, we found that the fluorescent intensity of C4C5 and G increasing/decreased by K+. More interestingly, the application of wettability can provide a general method to realize K+-controlled switch between hydrophobicity and hydrophilicity. It is anticipated that this research can be further extended to fabricate functional surfaces for pesticide control release, intelligent microfluidic and laboratory-on-chip devices
Methods
Materials and instruments
1H NMR and 13C NMR were recorded on Varian Mercury VX400 instrument at ambient temperature with TMS as the internal standard. ESI-MS were recorded on a Finnigan LCQ-Advantage instrument. The static water contact angle was measured at 25°C by means of an OCA 20 contact angle system (Dataphysics, Germany). X-ray photoelectron spectroscopic (XPS) image was gained by PHI Quantera SXM. The scanning electron microscope image was measured at 25°C by means of a JSM-6700F HR-FESEM.
All chemicals were A.R. grade and were purified by standard procedures. Mill-Q water was used to prepare all solutions in this study.
The synthesis of C4C5
Compound 1 (0.5 g, 9.8 mmol) and NaH (0.067 g, 40 mmol) and OTS glycol (0.35 g, 10 mmol) were stirred in the anhydrous THF (70 mL). The mixture reaction was stirred at 50°C for 15 h. When the flask was cooled to room temperature, methanol was added to remove the no reacting NaH. The residue solvent was evaporated by suction filtration to give white powder C4C5 (0.45 g, 73%). 1H NMR (400 MHz, CH3CN): δ 7.22 (4H, s, ArH), 6.95 (4H, s, ArH), 5.50 (4H, s, CH2CCH), 4.48 (4H, d, J = 11.5 Hz, ArCH2Ar), 3.97 (4H, s, OCH2), 3.78 (8H, d, J = 10.7 Hz, OCH2CH2O), 3.68 (4H, s, OCH2CH2O), 3.23 (4H, d, J = 12.3 Hz, ArCH2Ar), 2.57 (2H, s, CCH), 1.27 (18H, s, C(CH3)3), 1.04 (18H, s, C(CH3)3). ESI(+)MS calcd for C58H74O7Na [M+Na]+ 905.5 found: 906.0; calc. for C58H74O7Na [M+Na+H]+ 906.5 found: 907. Anal. Calc. for C58H74O7: C, 78.74; H, 8. 38. Found: C, 78.66; H, 8.26%.
Fluorescence experiments
All experiments of fluorescence were carried out at an excitation wavelength of 288.0 nm using a fluorescence spectrometer and a 1 cm quartz cell. C4C5 solution (1.0 × 10−5 M) was made by dissolving C4C5 in CH3CN (5.0 mL). G solution (1.0 × 10−3 M) was made by dissolving G in CH3CN (5.0 mL) The K+ solution was made at 1.0 × 10−3 M in CH3CN. The fluorescence was carried at 288.0 nm after adding the appropriate volume (20 μL) of C4C5 solution to measure the fluorescence. Then the K+ solution (20 μL) was added to the above mixed solution to measure the fluorescence, which showed a fluorescence recovery after adding K+.
Ultraviolet-visible experiments
All experiments of UV were carried out using a UV spectrometer and a 1 cm quart cell. 1.0 mL1.0 × 10−4 C4C5 interacted with G (100 μL, 1.0 × 10−3 M) and K+ (100 μL, 1.0 × 10−3 M) CH3CN respectively. The liner relationship between intensity of UV at 277.9 nm and different concentration of G, K+ were carried. The host was 1.0 × 10−4 M 2.0 mL CH3CN solution and then different amount of G, K+ were adding into the host solution. The added G, K+ were 0, 20 40, 60, 80, 100, 120, 140, 160, 180, 200 μL, respectively.
DFT computational details
The calculations reported in this article were performed using Gaussian03 program package. The structure of C4C5 and the complex of C4C5, G and K+ have been optimized by going through a cascade process starting from HF/3-21G → HF/6-31G → B3LYP/3-21G → B3LYP/6-31G. The frequency analysis calculations were performed and the absence of imaginary frequencies indicated the low energy minimum of the structures obtained.
Preparation of the Si-N3-modified silicon substrates
Fabrication of the micro–Si interface
A silicon wafer was used directly as the smooth substrate. The structured silicon substrate was fabricated by the combination of photolithography and inductively coupled plasma (ICP) deep etching techniques. The photolithography and ICP techniques were used to obtain the patterned silicon micropillar structure on the silicon wafer. A rough surface introduced geometrical structures with patterned square pillars on a flat silicon wafer, 20 μm high, 9 μm long and with a spacing of 12 μm between the silicon pillars51.
Preparation of the Si-N3 -modified silicon substrates
Silicon substrates cut into 1 × 1 cm2 square pieces were soaked in chromosulfuric acid solution for 30 minutes and then rinsed with double distilled water and dried under a stream of N2 gas. Cleaned wafers were immersed in aqueous NaOH (0.1 mol·L−1) for 6 minutes and subsequently in HNO3 (0.1 mol·L−1) for 12 minutes to generate surface hydroxyl groups. Then the silicon substrates had been washed by an excess of double distilled water and dried under N2 flow. After the silicon substrates had been washed with an excess of water and dried under a stream of N2 flow, they were immersed in a refluxed solution of 5 wt% Si-N3 in dry toluene (10 mL) at 110°C for 6 h. Then they were washed with toluene and chloroform to remove excess Si-N3 and dried under a stream of N2 gas.
The click reaction between Si-N3 and C4C5 on silicon substrates
The silicon surfaces modified Si-N3 were immersed in C4C5 solution in CH3CN at 10−3 M, then the mixture of copper sulfate(10−6 M) and sodium ascorbate (10−7 M) were added into this solution and heated the solution at 75°C for 8 hours. Then the silicon wafer were washed with little CH3CN and dried under a stream of N2 gas.
References
Centeno, J. A. et al. J. Metal Ions in Biology and Medicine (John Libbey Eurotext: Paris, France, 2000).
Togrul, M., Askin, M. & Togrul, H. Synthesis of chiral monoaza-15-crown-5 ethers from a chiral amino alcohol and enantiomeric recognition of potassium and sodium salts of amino acids. Tetrahedron 16, 2771–2777 (2005).
Demirel, N. & Bulut, Y. Synthesis of chiral monoaza-15-crown-5 ethers from a chiral amino alcohol and enantiomeric recognition of potassium and sodium salts of amino acids. Tetrahedron 14, 2633–2637 (2003).
Jiang, Y. et al. Crystal structure and mechanism of a calcium-gated potassium channel. Nature 417, 515–522 (2002).
Salgado, E. N., Radford, R. J. & Tezcan, F. A. Metal-Directed Protein Self-Assembly. Acc. Chem. Res. 43, 661 (2010).
Gomes, C. M. & Wittung-Stafshede, P. Protein Folding and Metal Ions: Mechanisms, Biology and Disease (CRC Press: Boca Raton, FL, 2011).
Vögtle, F. Supramolecular Chemistry (John Wiley and Sons:New York, 1991).
Cram, D. J. & Cram, M. J. Container Molecules and Their Guests (Royal Society of Chemistry: Cambridge, UK, 1994).
Lehn, J. M. Supramolecular Chemistry (VCH Publishers: New York, 1995).
Feringa, B. L. Molecular Switches (VCH-Wiley: Weinheim, Germany, 2001).
Jeon, Y. M., Kim, J., Whang, D. & Kim, K. Molecular Container Assembly Capable of Controlling Binding and Release of Its Guest Molecules: Reversible Encapsulation of Organic Molecules in Sodium Ion Complexed Cucurbituril. J. Am. Chem. Soc. 118, 9790–9791 (1996).
Liu, Y., Flood, H. & Stoddart, J. F. Thermally and Electrochemically Controllable Self-Complexing Molecular Switches. J. Am. Chem. Soc. 126, 9150–9151 (2004).
Sindelar, V., Silvi, S. & Kaifer, A. E. Switching a molecular shuttle on and off: simple, pH-controlled pseudorotaxanesbased on cucurbit[7]uril. Chem. Commun. 20, 2185–2187 (2006).
Huang, F., Switek, K. A. & Gibson, H. W. pH-controlled assembly and disassembly of a cryptand/paraquat[2]pseudorotaxane. Chem. Commun. 31, 3655–3657 (2005).
Kaiser, G. et al. Lithium-Templated Synthesis of a Donor–Acceptor Pseudorotaxane and Catenane. Angew. Chem. Int. Ed. 43, 1959–1962 (2004).
Liu, C. F., Lai, C. C., Peng, S. M. & Chiu, H. S. An Extremely Stable Host–Guest Complex That Functions as a Fluorescence Probe for Calcium Ions. Chem. Eur. J. 12, 4594–4599 (2006).
Chiang, P. T. et al. A Macrocycle/Molecular-Clip Complex that Functions as a Quadruply Controllable Molecular Switch. Chem. Eur. J. 12, 865–876 (2006).
Badjic', J. D. et al. A Molecular Elevator. Science 303, 1845–1849 (2004).
Lin, C. F., Liu, Y. H., Peng, S. M. & Chiu, S. H. Precise Facial Control in Threading Guests into a Molecular Cage and the Formation of a Turtlelike Supramolecular Complex. Angew. Chem. Int. Ed. 45, 3176–3181 (2006).
Harada, A. Cyclodextrin-Based Molecular Machines. Acc. Chem. Res. 34, 456–464 (2001).
Dietrich-Buchecker, C., Jimenez-Molero, M. C., Sartir, V. & Sauvage, J. P. Rotaxanes and catenanes as prototypes of molecular machines and motors. Pure Appl. Chem. 75, 1383–1393 (2003).
Kinbara, K. & Aida, T. Toward Intelligent Molecular Machines: Directed Motions of Biological and Artificial Molecules and Assemblies. Chem. Rev. 105, 1377–1400 (2005).
Kay, E. R., Leigh, D. A. & Zerbetto, F. Synthetic Molecular Motors and Mechanical Machines. Angew. Chem. Int. Ed. 46, 72–191(2007).
Han, Y., Lu, H. Y., Guo, J. B. & Chen, C. F. Synthesis of Triptycene-Derived Macrotricyclic Host Containing Two Dibenzo-[18]-crown-6 Moieties and Its Complexation with Paraquat Derivatives: Li+-Ion-Controlled Binding and Release of the Guests in the Complexes. J. Org. Chem. 77, 2422 (2012).
Sun, R. D., Nakajima, A., Watanabe, T. & Hashimoto, K. Photoinduced Surface Wettability Conversion of ZnO and TiO2 Thin Films. J. Phys. Chem. B. 105, 1984–1990 (2001).
Bico, J., Thiele, U. & Quéré, D. Wetting of textured surfaces. Colloids Surf. A. 206, 41–46 (2002).
Zhang, X., Jiang, L. & Li, Y. X. Polyelectrolyte Multilayer as Matrix for Electrochemical Deposition of Gold Clusters: Toward Super-Hydrophobic Surface. J. Am. Chem. Soc. 126, 3064–3065 (2004).
Mendes, P. Stimuli-responsive surfaces for bio-applications. Chem. Soc. Rev. 37, 2511–2529 (2008).
Li, H., Jiang, L. & Zhu, D. B. Super-“Amphiphobic” Aligned Carbon Nanotube Films. Angew. Chem. Int. Ed. 40, 1742–1746 (2001).
Fu, Q. et al. Reversible Control of Free Energy and Topography of Nanostructured Surfaces. J. Chem. Soc. 126, 8904–8905 (2004).
Feng, N. M. et al. Switchable Wettability Sensor for Ion Pairs Based on Calix[4]azacrown Clicking. Org. Lett. 14, 1958–1961 (2012).
Lankshear, M., Cowley, A. & Beer, P. Cooperative AND receptor for ion-pairs. Chem. Commun. 6, 612–614 (2006).
Kim, S. et al. A Calix[4]arene Strapped Calix[4]pyrrole: An Ion-Pair Receptor Displaying Three Different Cesium Cation Recognition Modes. J. Am. Chem. Soc. 132, 5827–5836 (2010).
Credi, A. et al. Viologen-Calix[6]arene Pseudorotaxanes. Ion-Pair Recognition and Threading/Dethreading Molecular Motions. J. Org. Chem. 69, 5881–5887 (2004).
Massue, J., Quinn, S. J. & Gunnlaugsson, T. Lanthanide Luminescent Displacement Assays: The Sensing of Phosphate Anions Using Eu(III)–Cyclen-Conjugated Gold Nanoparticles in Aqueous Solution. J. Am. Chem. Soc. 130, 6900–6901 (2008).
Yan, B. & Lu, H. Lanthanide-centered covalently bonded hybrids through sulfide linkage: Molecular assembly, physical characterization and photoluminescence. Inorg. Chem. 47, 5601–5611 (2008).
Su, Y., Li, L. & Li, G. Self-assembly and multicolor emission of core/shell structured CaWO4: Na+/Ln3+spheres. Chem. Commun. 34, 4004–4006 (2008).
Shukla, A., Das, A. & vander Boom, M. Electrochemical addressing of the optical properties of a monolayer on a transparent conducting substrate. Angew. Chem. Int. Ed. 44, 3237–3240 (2005).
Desmonts, L., Reinhoudt, D. & Calama, M. Design of fluorescent materials for chemical sensing. Chem. Soc. Rev. 36, 993–1017 (2007).
Bandyopadhyay, K., Liu, H., Liu, S. G. & Echegoyen, L. Self-assembled monolayers of bis-thioctic ester derivatives of oligoethyleneglycols: remarkable selectivity for K+/Na+ recognition. Chem. Commun. 2, 141–142 (2000).
Askawa, M., Iqbal, S., Stoddart, J. F. & Tinker, N. D. Prototype of an Optically Responsive Molecular Switch Based on Pseudorotaxane. Angew. Chem. Int. Ed. 35, 976–978 (1996).
Han, T., Zong, Q. S. & Chen, C. F. Complexation of Triptycene-Based Cylindrical Macrotricyclic Polyether toward Diquaternary Salts: Ion-Controlled Binding and Release of the Guests. J.Org. Chem. 72, 3108–3111 (2007).
Rojas, M. T., Koniger, R., Stoddart, J. K. & Kaifer, A. E. Supported Monolayers Containing Preformed Binding Sites. Synthesis and Interfacial Binding Properties of a Thiolated.beta.-Cyclodextrin Derivative. J. Am. Chem. Soc. 117, 336–343 (1995).
Lee, J. K. & Park, S. M. Electrochemistry of Guest Molecules in Thiolated Cyclodextrin Self-Assembled Monolayers: An Implication for Size-Selective Sensors. J. Phys. Chem. B. 102, 9940–9945 (1998).
Frasconi, M. et al. Ferrocenyl Alkanethiols−Thio β-Cyclodextrin Mixed Self-Assembled Monolayers: Evidence of Ferrocene Electron Shuttling Through the β-Cyclodextrin Cavity. Langmuir. 25, 12937–12944 (2009).
Jaime, C., Prados, P., Nieto, P. M. & Shched, C. Carbon-13NMR chemical shifts. A single rule to determine the conformation of calix[4]arenes. J. Org. Chem. 56, 3372–3376 (1991).
Kim, J. S. & Quang, D. T. Calixarene-derived fluorescent probes. Chem. Rev. 107, 3780–3799 (2007).
Quang, D. T. & Kim, J. S. Fluoro-and chromogenic chemodosimeteres for heavy metal ion detection in solution and biospecimens. Chem. Rev. 110, 6280–6301(2010).
Chen, X. Q., Wang, F., Kim, J. S. & Yoon, J. Fluorescent chemosensors based on spiroring opening of xanthenes and related derivatives. Chem. Rev. 112, 1910–1956 (2102).
Watson, M. et al. A “Clickable” Titanium Surface Platform. Langmuir. 26, 15920–15924 (2010).
Qing, G. Y., Wang, X., Fuchs, H. & Sun, T. L. Nucleotide-Responsive Wettability on a Smart Polymer Surface. J. Am. Chem. Soc. 131, 8370–8371 (2009).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21372092, 21102051), Natural Science Foundation of Hubei Province (2013CFA112) and Self-determined research funds of CCNU from the colleges' basic research and operation of MOE (CCNU13F005,CCNU14Z01001).
Author information
Authors and Affiliations
Contributions
N.M.F., D.M.T., H.T.D. and H.B.L. designed research; L.L. and N.M.F. performed research; N.M.F. synthesized compounds; L.L. and N.M.F. analysed data; L.L. done the calculation; L.L., X.Y.Z. and H.B.L. wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Cation-Induced Pesticide Binding and Release by a Functionalized Calix[4]arene Molecular Host
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Luo, L., Zhang, X., Feng, N. et al. Cation-Induced Pesticide Binding and Release by a Functionalized Calix[4]arene Molecular Host. Sci Rep 5, 8982 (2015). https://doi.org/10.1038/srep08982
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep08982
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.