Abstract
To explore the importance of soil microbial community composition on explaining the difference in heterotrophic soil respiration (Rh) across forests, a field investigation was conducted on Rh and soil physiochemical and microbial properties in four subtropical forests in southern China. We observed that Rh differed significantly among forests, being 2.48 ± 0.23, 2.31 ± 0.21, 1.83 ± 0.08 and 1.56 ± 0.15 μmol m−2 s−1 in the climax evergreen broadleaf forest (BF), the mixed conifer and broadleaf forest (CF), the conifer plantation (CP) and the native broadleaved species plantation (BP), respectively. Both linear mixed effect model and variance decomposition analysis indicated that soil microbial community composition derived from phospholipid fatty acids (PLFAs) was not the first-order explanatory variable for the Rh variance across the forests, with the explanatory power being 15.7%. Contrastingly, vegetational attributes such as root biomass (22.6%) and soil substrate availability (18.6%) were more important for explaining the observed Rh variance. Our results therefore suggest that vegetation attributes and soil carbon pool size, rather than soil microbial community composition, should be preferentially considered to understand the spatial Rh variance across the subtropical forests in southern China.
Similar content being viewed by others
Introduction
Heterotrophic soil respiration (Rh), often used to approximate the rate of soil organic matter (SOM) decomposition, is regulated by numerous factors such as climate and chemical recalcitrance of its components to decay1,2,3. Although having been acknowledged to play a critical role in the process of SOM decomposition4,5,6, the importance of soil microorganisms as a determinant of Rh variances across study sites has rarely been carefully examined3,5,7, probably because of the huge diversity and functional redundancy of microbial communities and the metabolic flexibility of individual microbial species4. Due to the lack of empirical evidence, soil microorganisms have been included only in exceptionally few ecosystem or soil process models and even then treated merely as a “black box”5,8,9, although the existing models involving SOM mineralization are highly diverse with very different complexity levels10. Explicit representation of microorganisms was recommended as a future component of models, but the optimal level of detail remains to be defined3.
Also in southern China, few studies have explored whether the spatial variations of soil microbial community composition affected the Rh variances across Chinese subtropical forests, despite that this region has been identified as a substantial carbon sink11,12. The mechanisms controlling CO2 fluxes are not deeply understood. In particular, the roles of soil microorganisms on spatial dynamics of heterotrophic soil respiration remain unclear in this area, raising extra uncertainty to estimate the change of carbon source-sink relationship for these subtropical forests under the future environmental changes.
Furthermore, carbon fractions of surface soil in these subtropical forests could change in response to environmental changes13. To better understand the soil carbon cycle in this area, it is urgent to determine if soil microbial community composition is one of the main factors controlling the Rh variances across these forests, due to the potential shifts of soil microbial community as a result of changed environmental conditions and substrate supply14,15,16. In this study, we conducted field measurements on heterotrophic soil respiration and soil physicochemical and microbial properties across four forests in southern China to explore whether soil microbial community composition was relevant to varied Rh across forests. Such observations were expected to provide empirical supports for predicting the future forest carbon cycling more exactly in this region.
Results
Heterotrophic soil respiration and vegetational and soil properties across the four forests
Heterotrophic soil respiration rate was significantly higher in the two natural forests compared to the two plantations (P = 0.002, Fig. 1a), being 2.48 ± 0.23, 2.31 ± 0.21, 1.83 ± 0.08 and 1.56 ± 0.15 μmol m−2 s−1 in BF, CF, CP and BP, respectively. No statistically significant difference existed between the two young plantations (P = 0.14) or between the two natural forests (P = 0.51). Because soil carbon stock was significantly higher in the climax forest than in the other three sites (Table 1), we also tested the differences in heterotrophic respiration rate per unit of total organic carbon (Rh/TOC) or readily oxidizable organic carbon (Rh/ROC). These two indexes of relative decomposition capacity tended to be higher in the mixed forest than in the other three forests studied (Fig. 1b). We therefore assessed whether the higher respiration rates in climax forest or the higher relative decomposition capacity in mixed forest could be attributed to compositional differences of soil microbial community. Moreover, much higher litterfall production and root biomass were observed in the two natural forests than in the two plantations (Table 1).
Heterotrophic soil respiration rate (a) and relative decomposition capacity (b) in the four forests.
Columns represent average values and error bars represent standard errors (n = 3). Different letters above bars indicate significant differences at P<0.05 level. The BF stands for climax evergreen broadleaf forest, CF for mixed conifer and broadleaf forest, CP for conifer plantation and BP for native broadleaved species plantation. Relative decomposition capacity was defined as the ratio of the heterotrophic respiration rate over total organic carbon (Rh/TOC) and as the ratio of the heterotrophic respiration rate over readily-oxidizable organic carbon (Rh/ROC).
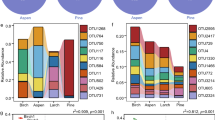
Microbial biomass carbon (MBC) was the highest at the climax forest (P = 0.011) whereas it was not significantly different among the other three sites (P>0.05). The PCA on phospholipid fatty acids (PLFAs) showed that, for our single sampling event, only the soil microbial structure in the climax forest was isolated from the others (Fig. 2a). When pooling the fatty acids into different microbial functional groups, we further observed that BF contained significantly higher contents of Gram-positive bacterial PLFAs than CP and also significantly higher actinomycetal PLFAs content than CP and BP (P<0.05, Fig. 2b). When looking at the ratios of PLFAs for different microbial functional groups, however, we did not observe significant variation among the four forests (P>0.05, Fig. 2c).
Scores of the first two principal components of PLFA profile (a), contents of PLFA biomarkers for different microbial groups (b) and ratios of PLFA content between different microbial groups (c) in the four forests.
Columns and triangles represent average values and error bars give standard errors (n = 3). For each microbial group, different letters above bars indicate significant differences at P<0.05 level among forests. In the figure, G+ stands for Gram-positive bacterial PLFAs, G- for Gram-negative bacterial PLFAs, B for total bacterial PLFAs, A for actinomycetal PLFA and F for fungal PLFA. The abbreviations for the four forests are the same with that in Fig. 1.
Explanatory variables for the Rh variances across forests
For differences in heterotrophic respiration rate and the relative decomposition capacities across the forests, neither of them was attributed to the variation of soil microbial community composition (P>0.05, Table 2). However, soil carbon stocks (TOC) or decomposability (ROC/TOC) induced significant or marginally significant effects on heterotrophic respiration rate (Table 2). Moreover, the results of variance decomposition (Table 3) showed that root biomass explained the most of the Rh variance among forests (22.6%), followed by SAP_PC2 (18.6%; the second principal component of the dataset including soil abiotic properties), PLFA_PC2 (15.7%) and litterfall production (12.6%). As well, SAP_PC2, a principal component closely related to soil carbon pool size indexes including TOC, ROC and NROC (non-readily oxidizable organic carbon), explained the most of the total variances of the two relative decomposition capacity indexes Rh/TOC (35.1%) and Rh/ROC (30.5%).
Discussion
In the present study, Rh was significantly higher in the two older natural forests than in the two younger plantations. This could be attributed to different age-related stand and soil properties across the four forests, e.g., litterfall inputs and soil substrate supply. Likewise, Saiz et al.17 observed that soil CO2 efflux rate at trenched plots across a Sitka spruce chronosequence was the highest in the forest with the largest annual litter inputs and pool of easily decomposable organic matter. The substrate-mediated heterotrophic respiration has been recorded in previous studies18,19,20. Consistently, much higher litterfall production and root biomass mean more supplies of nutrients from both litterfall decomposition and root excretions in the two natural forests, comparing with the two plantations in this study. Therefore, forest type and soil substrate supply seem to be of major importance to explain the Rh differences across forests, by controlling organic matter inputs and recalcitrance to decay. This is supported by both results of linear mixed effect model and variance decomposition analysis, which presented that vegetational attributes and soil substrate supplies together explained most of the cross-site Rh variance in this study.
Soil microbial biomass and community composition were also significantly different across the studied forests, indicated by fumigation-extracted MBC and the result of PCA on the profile of PLFA biomarkers. However, our results of linear mixed effect model suggested that neither of soil microbial biomass and community structure was the first-order variable to explain the Rh pattern across the forest sites. The absence of soil microbial communities controlling on Rh implies a subordinate effect of PLFA-derived soil microbial communities on the Rh variance across forests. This is verified by the insignificantly different ratios of PFLA biomarkers for different microbial groups among the four forests, in spite of the significantly different Rh. The observation was also confirmed by results of variance decomposition that vegetational and soil properties explained greater proportions of the Rh variance across forests than soil microbial communities. Moreover, soil microbial biomass and community structure are greatly affected by substrate supply5,21 and the latter is mainly determined by standing vegetation by means of litter input and root excretions. In consideration of complex interactions among environmental variables in natural ecosystems, modifications of changed microbial community composition on Rh could have confounded with that of changes in other preferable variables22, e.g., forest type and soil substrate supply21. Likewise, Wang and colleagues observed that Rh was determined to a greater extent by substrate supply than by microbial biomass carbon across thirty soils, which were collected from three states of Australia23. The Rh variance may be mainly determined by soil microbial communities only when substrate supply is sufficient and soil microorganisms in different soils have similar decomposability to substrate23.
An alternative, but not mutually exclusive, explanation is that PLFAs are not sufficiently discriminative to detect the differences in microbial community structure that may control the decomposition pattern across the study sites. By short term laboratory incubation, for example, Cleveland et al.5 found that increased Rh induced by the additions of dissolved organic matter (DOM) coincided with a profound raise in the abundances of some opportunistic soil bacterial groups. Fierer et al.24 also observed that SOC mineralization rate was significantly correlated with the abundances of several dominant bacterial groups across ecosystems in North America. Comparing with the present study, the two aforementioned studies employed molecular biology techniques with higher identifying capacity, i.e., 16S ribosomal DNA analysis, to further identify bacterial phylogenetic groups and observed synchronous changes in Rh and soil bacterial community composition. Moreover, soil microbial communities among the studied forests may not vary enough to explore the relationship with the Rh variance. In the present study, the PLFA ratios of different microbial functional groups, indicating soil microbial community structure to some extent, did not show significant differences among these forests, although BF was isolated from the other three by PCA on PLFAs profile. Obviously, further studies using advanced molecular analyses (e.g., quantitative polymerase chain reaction [qPCR] and 16S ribosomal DNA) and including more forest types might help confirming the role of soil microbial community composition in the heterotrophic soil respiration process in the forests of southern China.
Nevertheless, it must be noted that we conducted this study aiming to test the explanatory capacity of soil microbial communities for the spatial variations in Rh across forests and used only a single sampling event when Rh was at its maximum, since we assumed microorganisms at this period were the most active and therefore the most representative to study the role of activated community structure within a year. Hence, we cannot rule out the potential importance of soil microbial communities in heterotrophic soil respiration processes, as soil microbial community composition may play a crucial role in the temporal variation of Rh25,26, as well as in the Rh responses to environmental changes27. Moreover, microbial extracellular enzyme activities, representing soil microbial community functions to a great extent, are frequently observed to influence SOM decomposition28,29, but changes in soil microbial enzyme activities are not always accompanied with variations in soil microbial community composition and vice versa30,31. Further studies remain needed to test whether microbial community composition and enzyme activities play a dominant role in explaining the temporal variation of heterotrophic decomposition processes.
In summary, vegetational attributes such as root biomass and litter production and soil carbon pool size such as TOC and ROC played the dominant role in determining the variance of Rh across the four forests. Soil microbial community composition identified by PLFAs was not a major determinant of the Rh differences across the forests included in our study. These results suggest that a good representation of vegetational attributes and soil carbon pool size appears to be more important to reproduce the observed spatial pattern of heterotrophic soil respiration with a model, whereas an explicit representation of soil microbial community composition might be only alternative.
Methods
Site description
This study was conducted in four subtropical forests in southern China, including two natural forests: one climax evergreen broadleaf forest (BF, more than 400 years old) and one mixed conifer and broadleaf forest (CF, about 110 years old) and two plantation forests: one conifer plantation (CP, 26 years old) and one native broadleaved species plantation (BP, 26 years old; see details in Table 1). Three 10 × 10 m2 replicate quadrats were established in each forest in November 2009 and each quadrat included three trenched plots with a size of 1 × 1 m2. In the trenches, double nylon net (100 meshes) was used to prevent roots from penetrating in and meanwhile get allow lateral transfers of water and solutes. A polyvinyl chloride collar of 20 cm in diameter was installed in each plot, with the upper 2 cm letting above ground for Rh measurements.
Field measurements
In July 2011 when soil microorganisms were the most active, heterotrophic soil respiration rate, together with soil temperature (ST5) and moisture (SM5) at 5 cm depth, was recorded in the field by a Li-cor 8100 Auto Soil CO2 Flux System connecting with temperature and moisture sensors (Li-Cor Biosciences, NE, USA). Before recording, litterfall within the measurement collars was removed by hands carefully. Surface soil at 0–20 cm depth was then collected once per plot by an auger with 4 cm of inner diameter and samples in the same quadrat were mixed completely to form a composite sample for further analysis. Finally, twelve soil samples were analyzed for soil microbial community composition (via phospholipid fatty acids analysis; PLFAs) and soil physicochemical properties.
Laboratory analyses
Microbial biomass carbon was analyzed with the fumigation extraction method32, in which carbon content of unfumigated soil samples was considered as an estimate for dissolved organic carbon (DOC). ROC was determined using wet oxidization with 333 mM KMnO433. TOC was analyzed by the Walkley-Black method and total nitrogen concentration (TN) by the micro-Kjeldahl method34. The PLFAs analysis was conducted following Bossio & Scow35, in which lipids 15:0, a15:0, i15:0, i16:0, a17:0, i17:0 were considered as Gram-positive bacterial biomarkers (G+), cy17:0 and cy19:0 as Gram-negative bacterial biomarkers (G-), 18:2ω6,9c as fungal biomarker (F) and 10ME18:0 as actinomycetal biomarker (A). The ratios of G+ to G- PLFAs, fungal to bacterial PLFAs (G+ plus G-), actinomycetal to fungal PLFA and actinomycetal to bacterial PLFAs were also used to indicate soil microbial community structure.
Statistical analyses
One-way analysis of variance (ANOVA) with Student-Newman-Keuls post-hoc multiple comparisons was used to test significant differences in heterotrophic soil respiration rate and other soil and microbial properties among forests and principal components analysis (PCA) was conducted on microbial community composition based on PLFA biomarker contents of the entire microbial fatty acids profile. A linear mixed effect model using the sites as random effect to take into account the non-independency of the replicates was employed to determine the significant explanatory variables for different Rh. Considering the limitation of number of sample in our case, all the soil indexes were separated into five groups when tested in the linear mixed effect model, including environmental variables (ST5, SM5 and pH), soil nutrients (DOC, ROC, TOC, TN, ROC/TOC and TOC/TN), PLFA contents of microbial groups (MBC, total PLFAs, G+, G-, bacteria, fungi and actinomycetes), ratios of the PLFA biomarker for different microbial groups to the total PLFA content (G+ PLFAs ratio, G- PLFAs ratio, bacterial PLFAs ratio, fungal PLFAs ratio and actinomycetal PLFAs ratio) and soil microbial community structure (G+/G-, F/B, A/F and A/B). We also employed variance decomposition analysis to explore the explanation capacity of vegetational attributes and soil physiochemical and microbial properties on the total variance of Rh across forests, by using calc.relimp function in the “relaimpo” package in R. The PCA was first conducted on soil abiotic properties (SAP) for extracting principal components (PCs). The first four PCs of SAP and PLFA profiles, explaining more than 90% of the total variances of the SAP and PLFA datasets accordingly, were used in further analysis. Because of non-normality and heteroscedasticity, the data were rank transformed before analyses if necessary. Significant level was set at P<0.05. The PCA and linear mixed effect model analyses were also conducted in R software (version 2.15.2) and ANOVA in SPSS 16.0 for windows (SPSS Inc., Chicago, US).
References
von Lützow, M. et al. Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions - review. Eur. J. Soil Sci. 57, 426–445 (2006).
Shen, W. J., Reynolds, J. F. & Hui, D. F. Responses of dryland soil respiration and soil carbon pool size to abrupt vs. gradual and individual vs. combined changes in soil temperature, precipitation and atmospheric [CO2]: a simulation analysis. Global Change Biol. 15, 2274–2294 (2009).
Schmidt, M. W. I. et al. Persistence of soil organic matter as an ecosystem property. Nature 478, 49–56 (2011).
Swift, M. J., Heal, O. W. & Anderson, J. M. Studies in ecology (5) [Anderson, D., Greig-Smith, P. & Pitelka, F. (ed.)] [66–112] University of California Press, 1979.
Cleveland, C. C., Nemergut, D. R., Schmidt, S. K. & Townsend, A. R. Increases in soil respiration following labile carbon additions linked to rapid shifts in soil microbial community composition. Biogeochemistry 82, 229–240 (2007).
Garcia-Pausas, J. & Paterson, E. Microbial community abundance and structure are determinants of soil organic matter mineralisation in the presence of labile carbon. Soil Biol. Biochem. 43, 1705–1713 (2011).
Waldrop, M. P., Balser, T. C. & Firestone, M. K. Linking microbial community composition to function in a tropical soil. Soil Biol. Biochem. 32, 1837–1846 (2000).
Friedlingstein, P. et al. Climate-carbon cycle feedback analysis: Results from the C4MIP model intercomparison. J. Climate 19, 3337–3353 (2006).
Manzoni, S., Taylor, P., Richter, A., Porporato, A. & Ågren, G. I. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol. 196, 79–91 (2012).
Manzoni, S. & Porporato, A. Soil carbon and nitrogen mineralization: theory and models across scales. Soil Biol. Biochem. 41, 1355–1379 (2009).
Zhou, G. et al. Old-growth forests can accumulate carbon in soils. Science 314, 1417–1417 (2006).
Piao, S. et al. The carbon balance of terrestrial ecosystems in China. Nature 458, 1009–1013 (2009).
Chen, X. M., Liu, J. X., Deng, Q., Yan, J. H. & Zhang, D. Q. Effects of elevated CO2 and nitrogen addition on soil organic carbon fractions in a subtropical forest. Plant Soil 357, 25–34 (2012).
Janssens, I. A. et al. Reduction of forest soil respiration in response to nitrogen deposition. Nature Geoscience 3, 315–322 (2010).
Guenet, B. et al. The impact of long-term CO2 enrichment and moisture levels on soil microbial community structure and enzyme activities. Geoderma 170, 331–336 (2012).
Liu, L., Gundersen, P., Zhang, T. & Mo, J. M. Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China. Soil Biol. Biochem. 44, 31–38 (2012).
Saiz, G. et al. Stand age-related effects on soil respiration in a first rotation Sitka spruce chronosequence in central Ireland. Global Change Biol. 12, 1007–1020 (2006).
Curiel Yuste, J. et al. Microbial soil respiration and its dependency on carbon inputs, soil temperature and moisture. Global Change Biol. 13, 2018–2035 (2007).
Fanin, N., Hattenschwiler, S., Barantal, S., Schimann, H. & Fromin, N. Does variability in litter quality determine soil microbial respiration in an Amazonian rainforest? Soil Biol. Biochem. 43, 1014–1022 (2011).
Luan, J., Liu, S., Wang, J., Zhu, X. & Shi, Z. Rhizospheric and heterotrophic respiration of a warm-temperate oak chronosequence in China. Soil Biol. Biochem. 43, 503–512 (2011).
Fierer, N., Strickland, M. S., Liptzin, D., Bradford, M. A. & Cleveland, C. C. Global patterns in belowground communities. Ecology Letters 12, 1238–1249 (2009).
Degens, B. P. Decreases in microbial functional diversity do not result in corresponding changes in decomposition under different moisture conditions. Soil Biol. Biochem. 30, 1989–2000 (1998).
Wang, W. J., Dalal, R. C., Moody, P. W. & Smith, C. J. Relationships of soil respiration to microbial biomass, substrate availability and clay content. Soil Biol. Biochem. 35, 273–284 (2003).
Fierer, N., Bradford, M. A. & Jackson, R. B. Toward an ecological classification of soil bacteria. Ecology 88, 1354–1364 (2007).
Treseder, K. K. et al. Interating microbial ecology into ecosystem models: challenges and priorities. Biogeochemistry 109, 7–18 (2012).
Wieder, W. R., Bonan, G. B. & Allison, S. D. Global soil carbon projections are improved by modelling microbial processes. Nature Clim. Change 3, 909–912 (2013).
Wei, H. et al. Thermal acclimation of organic matter decomposition in an artificial forest soil is related to shifts in microbial community structure. Soil Biol. Biochem. 71, 1–12 (2014).
Sinsabaugh, R. L., Gallo, M. E., Lauber, C., Waldrop, M. P. & Zak, D. R. Extracellular enzyme activities and soil organic matter dynamics for northern hardwood forests receiving simulated nitrogen deposition. Biogeochemistry 75, 201–215 (2005).
Bell, C., Stromberger, M. & Wallenstein, M. New insights into enzymes in the environment. Biogeochemistry 117, 1–4 (2014).
Allison, S. D. & Martiny, B. H. Resistance, resilience and redundancy in microbial communities. Proc. Natl. Acad. Sci. USA 105, 11512–11519 (2008).
Purahong, W. et al. Uncoupling of microbial community structure and function in decomposing litter across beech forest ecosystems in Central Europe. Sci. Rep. 4, 7014; 10.1038/srep07014 (2014).
Vance, E. D., Brookes, P. C. & Jenkinson, D. An extraction method for measuring soil microbial biomass. C. Soil Biol. Biochem. 19, 703–707 (1987).
Blair, G. J., Lefrog, R. D. B. & Lisle, L. Soil carbon fractions based on their degree of oxidation and the development of a carbon management index for agricultural systems. Aust. J. Agr. Res. 46, 1459–1466 (1995).
Liu, G. S. Soil physicochemical analysis and description of soil profiles. China Normative Publishing House, Beijing, 1996 (in Chinese).
Bossio, D. A. & Scow, K. M. Impacts of carbon and flooding on soil microbial communities: phospholipid fatty acid profiles and substrate utilization patterns. Microbial Ecol. 35, 265–278 (1998).
Huang, W., Liu, J., Zhou, G., Zhang, D. & Deng, Q. Effects of precipitation on soil acid phosphatase activity in three successional forests in Southern China. Biogeosciences 8, 1901–1910 (2011).
Zhou, C., Zhou, G., Zhang, D., Wang, Y. & Liu, S. CO2 efflux from different forest soils and impact factors in Dinghu Mountain, China. Sci. China Ser. D 48, 198–206 (2005).
Fang, H. et al. 13C abundance, water-soluble and microbial biomass carbon as potential indicators of soil organic carbon dynamics in subtropical forests at different successional stages and subject to different nitrogen loads. Plant Soil 320, 243–254 (2009).
Fu, S., Lin, Y., Rao, X. & Liu, S. Investigation and research dataset of stand properties in Chinese forest ecosystems - Heshan National Field Reseach station of Forest Ecosystem (1998-2008). China Agriculture Press, Beijing, 2011 (in Chinese).
Acknowledgements
We thank Mr. Yongbiao Lin and Mr. Xingquan Rao for their help on field work. Dr. Guojun Lin is also acknowledged for his help on statistical analysis. Financial supports came from the National Natural Science Foundation of China (NSFC-31290222, 31130011, 31425005), the Major State Basic Research Development Program (973 Planning Program 2011CB403206) and the Natural Science Foundation of Guangdong province (S2012020011084).
Author information
Authors and Affiliations
Contributions
H. W. and G. X. contributed equally to this study. W. S. conceived the study. H. W. and G. X. carried out the field measurements and laboratory analyses. H. W. and B. G. conducted statistical analyses. H.W., G. X., B. G., I. J. and W. S. contributed to manuscript writing and revisions.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Wei, H., Xiao, G., Guenet, B. et al. Soil microbial community composition does not predominantly determine the variance of heterotrophic soil respiration across four subtropical forests. Sci Rep 5, 7854 (2015). https://doi.org/10.1038/srep07854
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07854
This article is cited by
-
Carbonated waste valorisation from a peat bog exploitation in the treatment of arsenic-polluted waters
International Journal of Environmental Science and Technology (2022)
-
Differential linkages between soil respiration components and microbial community structures under long-term forest conversion
Journal of Soils and Sediments (2022)
-
Different plant covers change soil respiration and its sources in subtropics
Biology and Fertility of Soils (2017)
-
Invariant community structure of soil bacteria in subtropical coniferous and broadleaved forests
Scientific Reports (2016)
-
Spatial variability of soil respiration in a 64-year-old longleaf pine forest
Plant and Soil (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.