Abstract
The pentameric serum IgMs are critical to immune defense and surveillance through cytotoxicity against microbes and nascent cancer cells. Ficolins, a group of oligomeric lectins with an overall structure similar to C1q and mannose-binding lectin (MBL) participate in microbe infection and apoptotic cell clearance by activating the complement lectin pathway or a primitive opsonophagocytosis. It remains unknown whether serum IgMs interplay with ficolins in cancer immunosurveillance. Here we report a natural cancer killing of different types of cancer cells by sera from a healthy human population mediated by a novel IgM–H-ficolin complement activation pathway. We demonstrate for the first time that H-ficolin bound to a subset of IgMs in positive human sera and IgM–H-ficolin deposited on cancer cells to activate complement attack in cancer cells. Our data suggest that the IgM–H-ficolin -mediated complement activation pathway may be another defensive strategy for human cancer immunosurveillance.
Similar content being viewed by others
Introduction
Immunoglobulin (Ig) M, the first type of antibody produced in response to primary humoral immune response1, presents as a membrane-bound form on B cells and a secreted form mainly in the serum2. Based on the origin of serum IgMs, they can be divided into natural and immune IgM2. Immune IgM is produced in response to exposure to specific pathogens3, whereas natural IgM (nIgM) pre-existing in neonates and in “antigen-free” healthy adults is mainly produced by innate-like B cells such as B1 and marginal zone B cells in a T cell-independent manner4. Both exist principally as a pentamer and occasionally as a hexamer2. The unique structure makes pentameric IgMs the most potent molecules of the immune system, which is demonstrated by the fact that they have a 1,000-fold greater binding affinity to C1q compared with IgG2. Altogether, serum IgMs play an important role against infections and malignancies predominantly through complement-dependent cytotoxicity (CDC)5.
Ficolins are a group of multimeric lectins made up of single subunits, each of which is composed of a collagen-like domain and a fibrinogen-like domain6,7 and ficolins have a similar overall structure to C1q and MBL8, both of which bind to immunoglobulins, especially IgM, to activate the complement classical or MBL pathway9,10. Three types of ficolins (L-ficolin, H-ficolin and M-ficolin) are present in humans, among which L- and H-ficolin are two kinds of serum ficolins11 and have the ability to activate the complement system in association with MBL-associated serine proteases (MASP-1, MASP-2 and MASP-3) and their truncated proteins, small MBL-associated protein (sMAP) and mannose-binding lectin-associated protein of 44 kDa (MAp44)6. The ficolin-activated complement system activates the lectin pathway6,11, which is similar to the MBL pathway12. It is well known that ficolins play an important role in innate immunity by acting as a pathogen-recognition molecule and participating in the clearance of microbes4,13 and apoptotic cells14. The recent finding that ficolins, especially H-ficolin, collaborate with natural IgG (nIgG) during bacterial infection to launch an immediate effective attack on the bacteria4,13 strengthens the importance of not only ficolins and/or (natural) antibodies but also the interaction between ficolins and (natural) antibodies in immune defense.
Therefore, we reasonably raised a question as to whether serum IgMs and ficolins interact to participate in cancer immunosurveillance and hypothesized that serum antibodies collaborate with ficolins to participate in cancer immune defense. We discovered a correlation between the interaction of H-ficolin with IgM from 1000 healthy human sera samples and cancer cell killing in vitro.
Results
Anti-tumor activity of human sera from a healthy population
Because cancer-specific IgMs were reported to pre-exist in humans15,16, we proposed that human sera from healthy populations should kill different cancer cells without involvement of cellular components. Human sera samples were collected from 1000 healthy donors (Table 1) and stomach cancer KATO III cells were first incubated with the sera. Because aberrant expression of ABO antigens is frequently observed in a variety of cancer cells17,18 and anti-A and anti-B antibodies of human sera are usually IgM type, cytolysis may occur when blood isoantigen to cancer cells meets its corresponding isoantibody in vitro, similar to a specific hemolysic reaction. Because KATO III cells express B antigen, to exclude the cytolysis triggered by incompatibility of anti-B isoantibody and B isoantigen, we examined and confirmed the cytolysis of KATO III cells in matched sera (including sera collected from humans whose blood type was AB and B) by microscopy and electron microscopy (Fig. 1a and 1b). Serum causing greater than 20% cell death was set as positive human serum (PHS) for KATO III cells and that causing 20% or less was designated negative human serum (NHS). Sera from 35.4% of compatible healthy individuals were found to be positive for killing KATO III cancer cells (Fig. 1c and Table 2).
Anti-tumor activity in healthy human sera.
(a) Representative images of typical cytolysis in KATO III cells were taken by optical microscopy and (b) transmission electron microscopy. PHS: positive human serum with a cytolysis percentage >20%. (c) The cancer cell KATO III destruction activity of compatible sera.
To confirm this finding, the cytolysis rates of HT-29 cells expressing blood A antigen, COLO 205 cells expressing blood H antigen and MCF7 cells expressing blood H antigen were then tested with the same set of sera samples (Supplementary Fig. S1A, 1B and 1C). Similarly, compatible sera were found to be able to destroy cancer cells. Although ABO blood group incompatibility between the tested sera and cancer cells expressing A and B antigens caused more cell destruction (Table 2, p < 0.05), compatibility between the tested sera and cancer cells expressing H antigen did not, even among A, AB, B and O sera (Supplementary Table S1). As no A or B antigens are expressed on MCF7 and COLO 205 cell surfaces, these results suggested that epitopes additional to the A and B blood antigens were required for the killing activity. Thus, we believe that compatibility may really reflect the immunity response in individuals; therefore, compatible sera to each cancer cell line were highlighted in the following experiments. For the tested cancer cell lines, there were always certain human populations whose sera were found to be capable of killing them in vitro. The cytolysis percentage of the matched PHS varied among the different cancer cell lines in the order of MCF7 > HT-29 > KATO III > COLO 205. The variation of the pattern of PHS among different cancer cell lines indicated different antigens on these cancer cell lines.
To address whether the cell destruction activity was induced by human leukocyte antigen [HLA, the human version of the major histocompatibility complex, (MHC)] incompatibility, primary tumor cells from colorectal cancer and adjacent normal tissues from 27 patients were examined with ABO-matched PHS for the COLO 205 and HT-29 cell lines (Supplementary Table S2). The tissues were digested with collagenase IV and isolated cells were incubated with PHS to measure the level of cell destruction. Although the levels varied among different patients, the cell destruction against the tumor cells was significantly higher than that from the adjacent tissue in TNM stage I (Supplementary Table S3, p < 0.05), while the killing activity against the tumor cells was not higher than that from normal tissue in clinical stage II and III. PHS samples were capable of distinguishing the primary tumor and adjacent cells in the early stage of colorectal cancer, suggesting that the observed in vitro cell destruction activity was triggered by a factor other than ABO and HLA and was effective and specific for identifying nascent malignant cells. Furthermore, as the primary tumor and adjacent cells were from the same patients, this specificity was likely due to cancer-specific surface epitopes exclusive of ABO or HLA antigens.
Cancer cell destruction activity was complement-dependent
To clarify whether the cancer cell destruction activity of compatible sera was dependent on complement, PHS were heated to 56°C for 30 min to inactivate complement and the cell destruction activity was completely abolished by the heat inactivation (Fig. 2a). The requirement of complement for the cell destruction was further confirmed by immunofluorescence staining of complement attack complex (MAC) using anti-human polyclonal C5b-9 (Fig. 2b). Interestingly, the heat-inactivated PHS regained its activity by addition of NHS (Fig. 2a); after the heat-inactivated PHS that was pre-incubated with cancer cell lines was washed away, PHS-treated cancer cells were still efficiently destructed by NHS. These data indicate that the pre-existence of a specific factor in PHS was capable of interacting with cancer cells and activating complement-dependent cell destruction.
C2-dependent pathway was essential for tumor cell lysing activity.
(a) The cytolysis of four types of cancer cells incubated with PHS, heat-inactivated PHS (heated-PHS) or NHS (negative human serum with a cytolysis percentage ≤20%). (b) MAC detection in KATO III cells. (c) The cytolysis of four types of cancer cells incubated with heated-PHS, C2 depleted human serum (HS-C2) or the purified C2 component (C2). (d) The cytolysis of cancer cells incubated with C1q-blocking antibody and (in) compatible sera. (e) The cytolysis of KATO III cells incubated with PHS plus D-mannose or anti-MBL mAb. (NS indicates p > 0.05, *p < 0.05, **p < 0.01).
Next, C2-depleted human serum and the purified C2 component were used to elucidate a possible complement activation pathway. Considering that sera with whether anti-A or anti-B isoantibody were compatible, MCF7 cells were first tested (Fig. 2c). The C2-depleted human serum (C2-HS) was not active when MCF7 cells were pre-incubated with heat-inactivated PHS (heated-PHS) but regained the greatest activity by addition of the C2-protein (p < 0.01), suggesting the requirement of C2 for MCF7 cell destruction. Next, the requirement of C2 for the destruction of other cancer cells was evidenced by utilizing heat-inactivated PHS and the C2-depleted serum, which was not active but regained activity upon the addition of C2.
To explore whether the cancer cell destruction activity was mediated through the classical pathway or MBL pathway, different amounts of C1q monoclonal antibody (mAb 2204) were pre-mixed with compatible and incompatible PHS and cytolysis of HT-29, KATO III, COLO205, or MCF7 cells was evaluated (Fig. 2d). Unexpectedly, the anti-C1q antibody did not affect the killing of HT-29 and KATO III cells in some compatible sera (p > 0.05) but significantly decreased the killing of HT-29 and KATO III cells in incompatible sera (p < 0.05) and up to 60 mM D-mannose or 60 μg/ml anti-MBL antibody did not affect the destruction activity for some compatible sera in KATO III cells (Fig. 2e), suggesting C1q-initiated classical or MBL-initiated MBL complement activation pathway was not involved in some sera in KATO III cells. In the following experiments, the blood type-matched PHS for which activity was inhibited by neither mAb 2204 nor anti-MBL antibody were analyzed.
H-ficolin was required for the complement activation to destroy cancer cells
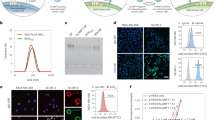
To test whether ficolins are invloved in the cancer cell destruction activity, polyclonal antibodies against H- and L-ficolin were used to block complement activation. Both H- and L-ficolins inhibited lysis of KATO III cells in those matched PHS (Fig. 3a). To exclude the possible cross reaction between H- and L-ficolin with the polyclonal antibodies, deposition of H- and L-ficolin on the KATO III cell surface was examined by pre-incubation with heat-inactivated, matched PHS or NHS. The deposited ficolins on the cell surface were detected with monoclonal antibodies specific to H- or L-ficolin. Positive signals were detected only with the H-ficolin antibody; no signal was detected when the L-ficolin antibody was used (Fig. 3b). These results indicate that H-ficolin specifically deposited on KATO III cancer cell surface, but L-ficolin did not. Notably, no deposition was observed when heat-inactivated, compatible NHS were used. This suggested that only H-ficolin from the population whose sera were matched for “ABO” and positive for killing cancer cells could be deposited on the cancer surface, whereas H-ficolin from the population whose sera were matched for “ABO” and negative could not.
H-ficolin was essential for the tumor cell lysing process.
(a) The cytolysis of KATO III cells by PHS in the presence of the polyclonal antibody against human H- or L- ficolin. (b) Immunofluorescence images of deposition of ficolins on KATO III cells. (c) The H-ficolin-/albumin-depleted positive or negative sera (H/A-PHS or -NHS) and the purified H-ficolin-/albumin (H/A) were analyzed by C. Blue and Western Blotting. (d) The L-ficolin-depleted PHS (L-PHS) was analyzed by C. Blue and Western blotting. (e) The cell lysing activity of H-PHS and L-PHS in KATO III cells. (f) The cell lysing and binding activity of the purified H-ficolin (P-H/A) and H/A-PHS in KATO III cells. (NS indicates p > 0.05, *p < 0.05, **p < 0.01).
Next, immunodepletion was applied to further confirm the essential requirement of H-ficolin. Because H-ficolin is known to interact with acetylated albumin19,20,21, we used Cibacron Blue, a synthetic polycyclic dye that binds human serum albumin with considerable specificity and affinity via electrostatic and/or hydrophobic interactions22,23, to quantitatively deplete human albumin and H-ficolin from those PHS and NHS (Fig. 3c). L-ficolin was depleted from PHS and NHS using an immobilized monoclonal antibody (Fig. 3d). Western blotting and Coomassie Blue (C. Blue) staining showed efficient depletion of H- and L-ficolin. Although the KATO III cell destruction activity was completely abolished by depletion of H-ficolin (Fig. 3e), the activity was not affected by depletion of L-ficolin.
Interestingly, when inactive H-ficolin/albumin-depleted PHS (H/A-PHS) for KATO III cells were mixed with blood type-matched NHS, they were still sufficient to kill KATO III cells in different serum samples (Fig. 3f). Considering that the levels of H-ficolin in PHS and NHS were similar (Fig. 3c and 3d), these data indicate that H-ficolin was not the cancer-specific binding factor; an additional heat-resistant factor in PHS was involved to deposit H-ficolin on the cancer cell surface and initiate H-ficolin-mediated complement activation.
H-ficolin–binding IgM pre-existed in the positive human samples, interacted with cancer cells and initiated H-ficolin–mediated complement activation
We reasoned that the heat-resistant factor that could specifically bind to the cancer cell surface in positive human samples was an immunoglobulin, which provided the specificity and complexity for variable tumor antigens. Using the IgG/albumin removal kit, IgG was first depleted from compatible PHS (IgG/A-depleted PHS) and NHS (IgG/A-depleted NHS) (Fig. 4a). The purified IgG fraction from positive human serum (P-IgG/A) showed no cancer cell destruction activity (p < 0.01), which was not restored by addition of NHS (p > 0.05). The IgG/albumin-depleted fraction, however, was fully active in cancer cell killing (p > 0.05) (Fig. 4b). Thus, IgG in PHS was not involved in the cancer cell destruction activity.
IgM but not IgG was involved in H-ficolin–mediated complement activation pathway.
(a) The purified IgG/albumin (P-IgG/A) and the IgG/albumin-depleted PHS or NHS (IgG/A-depleted PHS or NHS) were stained by C. Blue or analyzed by Western blotting. (b) The cell lysing and binding activity of P-IgG/A and IgG/A-depleted PHS were determined. (c) The IgM-depleted PHS or NHS (IgM-depleted PHS or NHS) and the purified IgM from PHS (P-IgM) or NHS (N-IgM) were analyzed. (d) The cell lysing and binding activity of IgM-depleted PHS and P-IgM. (e) Schematic illustration of the IgM–H-ficolin signal pathway in complement activation. (NS indicates p > 0.05, *p < 0.05, **p < 0.01).
Then, IgM was depleted from compatible PHS using the immobilized antibody against human μ chain. C. Blue staining and Western blotting indicated a complete removal of IgM from PHS and NHS (Fig. 4c). As hypothesized, the IgM-depleted PHS failed to kill KATO III cells alone or even supplemented with NHS (Fig. 4d, p < 0.01), indicating that it lost both its cancer cell destruction activity and the cancer binding factor. Consistently, the purified IgM from PHS (P-IgM) regained its cancer cell killing activity upon addition of NHS (p < 0.01), whereas the purified IgM from NHS (N-IgM) serum did not (p > 0.05). These data clearly demonstrate that IgM from the positive human sera was the heat-resistant factor that specifically bound to the cancer cell surface and initiated H-ficolin–mediated complement activation. Markedly, H-ficolin was co-purified by the anti-human IgM antibody (Fig. 4c), suggesting that the purified IgM specifically interacted with H-ficolin. The interaction between IgM and H-ficolin was consistent with H-ficolin–mediated complement activation (Fig. 4e). In contrast, the IgM purified with MBL-agarose was unable to restore the activity (data not shown), suggesting a difference in the MBL- and H-ficolin–binding IgMs in complement activation against cancer cells.
Discussion
Whether serum IgMs interact with serum ficolins to activate a complement pathway and therefore, play a role in tumor immunosurveillance remains unknown. We demonstrated that sera from a large number of healthy adults had a natural cytotoxicity against different established tumor cell lines and primary tumor cells isolated from cancer patients that was mediated by the interaction of H-ficolin with IgM. To the best of our knowledge, we report here the novel IgM–H-ficolin-mediated complement activation pathway to defend tumor cells in vitro for the first time. We found a complement killing activity dependent of complement component C2 but independent of C1q. Because C2 is an important component of both the classical and the lectin pathways of complement activation24, whereas C1q is the first subcomponent of classical pathway25, we thus excluded the alternative pathway and classical pathway. It is worth noting that the exclusion of the alternative pathway based on the essential role of C2 is limited in the setting of commercial C2-depleted serum. In contrast to the killing of KATO III, COLO 205 and HT-29 cells, killing of MCF7 cells was blocked by anti-C1q antibody in a dose-dependent manner. This is in accordance with an early report in which MCF7 cells were killed through complement-mediated cytolysis that was activated by a human IgM through the classical pathway26,27. Thus, the results in MCF7 cells can be viewed as a control supporting the cancer killing mechanisms of the other cancer cells. Additionally, the MBL pathway is inhibited by 10 mM mannose or 10 μg/ml anti-MBL antibody28, indicating that the traditional MBL pathway might not be involved in KATO III cells for some matched PHS either. Therefore, destruction of KATO III cancer cells by some compatible sera was not mediated through the alternative and classical pathways and was different from the traditional mannose-binding lectin pathway. This phenomenon was also proved in HT-29 cells. Why the IgM–H-ficolin pathway was found only in KATO III and HT-29 cells remains to be further investigated.
We found that the existence of a specific pair of H-ficolin and H-ficolin–binding IgM was specific to two types of cancer cells in humans and mediated complement-dependent cancer cell destruction. This is reasonable because the unique structures of IgMs and lectins underlie their interaction. NIgM with a low affinity requires H-ficolin to recognize undefined antigens. It is likely that once the IgM–H-ficolin complex is formed, either IgM or H-ficolin may activate the downstream complement activation pathway and further research is needed to confirm this mechanism.
Since approximately 20% of total B cells in the peripheral circulation of healthy adults produce natural antibodies4 and IgM antibody from normal human serum cytotoxic for human neuroblastoma cells was defined as nIgM15, importantly, all isolated cancer-specific human monoclonal antibodies were germ-line coded IgM isotypes16. We thus reasonably assumed that H-ficolin–bound IgM cytotoxic to KATO III and HT-29 cancer cells may largely be nIgM. NIgM has several characteristic properties such as low affinity and polyreactivity, which underlie its functions in health and disease2. It has been found to have a cytotoxic activity against human neuroblastoma15 and epithelial cancer cells16 through activation of the complement classical pathway, which is usually triggered by the interaction of the immune complex with C1q, an essential complement component of classical pathway activation. A positive therapeutic effect has been reported when normal human serum positive for the complement activity is transfused into patients with relapsed neuroblastoma29. These studies demonstrate that pre-existing nIgM in healthy individuals performs a cancer immunosurveillance function. We found that the interaction of H-ficolin with IgM activated the complement pathway to kill cancer cells in vitro, suggesting a potential innate immunosurveillance mechanism in the elimination of nascent transformed cancer cells in a healthy population. In fact, H-ficolin interacts with nIgG4,13 and rapidly accumulating data30,31,32,33,34 reveal that adaptive35 and innate immune cells36 as well as key immunologic molecules IFN-γ35,37 and perforin37,38 protect the immunocompetent host from the development of neoplasia.
In conclusion, we demonstrated a novel complement activation pathway mediated by the interaction of cell-free innate immunity molecules nIgM and H-ficolin in tumor cell immunosurveilliance. Our findings suggest that the novel complement activation pathway may be another defensive strategy orchestrating human cancer immunosurveillance and suggest the complexity and diversity of natural immunity against cancer.
Methods
Cells
Human colorectal cancer COLO 205 cell was purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Breast cancer MCF7, gastric cancer KATO III and colorectal cancer HT-29 are routinely used in our laboratory. All cell lines were identified with DNA profiles or provided with certificate (Supplementary Information). COLO 205 and KATO III cells were cultured in RPMI 1640; MCF7 and HT-29 were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA) and 2 mM L-glutamine at 37°C in a humidified atmosphere of 5% CO2.
Sera and antibodies
This study was approved by the Ethics Committee of Xijing Hospital, Fourth Military Medical University (FMMU), China. Written informed consent was obtained from all subjects before blood samples were collected. The methods were carried out in accordance with the approved guidelines. From April 18 to May 3, 2011, sera samples were collected from 1000 healthy people in Xijing Hospital, FMMU. Anti-A and anti-B blood grouping monoclonal antibodies to determine ABO blood types of participants were from Bode Biotechnology Co. (Changchun, China). C2-depleted human complement and the purified complement C2 proteins were purchased from EMD Chemicals (Gibbstown, NJ, USA). Anti-C1q monoclonal antibody (mAb) 2204 was kindly provided by Prof. Daha, Leiden University Medical Center. Polyclonal antibodies and mAb against L- or H-ficolin were from R&D Systems (Minneapolis, MN, USA). Anti-human IgM and IgG were from Invitrogen Life Tech (Carlsbad, CA, USA). FITC-conjugated goat anti-mouse IgG and rabbit anti-goat IgG secondary antibody were from Thermo Scientific (Waltham, MA, USA). D-mannose was from Sigma (St. Louis, MO, USA). Anti-MBL mAb was a gift from Dr. Gregory Stahl, Harvard Medical School (Cambridge, MA, USA).
Human serum killing activity assays
To test serum killing activity, a standard assay was performed as follows: 1 × 104 cells mentioned above were resuspended respectively in 90 μl RPMI 1640 or DMEM without FBS in 96-well plates and then 10 μl human sera (HS) was added and incubated at 37°C in a humidified atmosphere of 5% CO2 for 30 min. Cell lysis was determined by observation of the bubbling lytic structure under an optical microscopy (Leica, Germany) with or without Trypan Blue exclusion. The percentage of cytolysis was calculated by the average numbers of lysed and live cells under three different fields of view. Each experiment was repeated at least three times. Typically, the standard deviation was less than 5% when the total cell number was greater than 200. PHS was set as a cytolysis percentage >20%;NHS was defined as a cytolysis percentage ≤20%. For assessment of morphological change, cancer cells were incubated with 10% PHS in RPMI 1640 or DMEM for 15 min, fixed with 2.5% glutaraldehyde and then observed under transmission electron microscopy (JEM-2000EX, Jeol Ltd., Tokyo, Japan). For the immune inhibition assays, RPMI 1640 or DMEM plus 0, 0.5, 1, 2, 5, 10, or 20 μg/ml C1q-blocking antibody, or 0, 6.25, 12.5, 25, or 50 μg/ml H- or L-Ficolin polyclonal antibody and then 10% PHS was used for cytolysis. For the C2-depletion and rescue assays, the four kinds of cancer cells were pre-mixed with 10% heat-inactivated PHS (sera were heated to 56°C for 30 min to inactivate complement), washed with phosphate-buffered saline (PBS), supplemented with 10% C2-depleted serum or C2-depleted serum plus 10 μg of the purified C2 protein and incubated at 37°C for 30 min.
Depletion of H- and L-ficolins from human sera
Cibacron Blue agarose was used to co-deplete H-ficolin and human serum albumin. Briefly, 0.1 ml of positive or negative human serum was mixed with an equal volume of PBS, loaded on rehydrated SwellGel Disc (ThermoFisher Scientific, Waltham, MA, USA) in a spin column and incubated at room temperature for 5 min. The supernatant was collected and re-applied to the column twice to derive H-ficolin/albumin-depleted sera. The bound albumin and the associated H-ficolin were eluted with PBS + 500 mM NaCl, dialyzed against PBS and concentrated to 0.2 ml using YM-30 filter (Millipore, Billerica, MA, USA). To deplete L-ficolin, 0.1 mg of monoclonal antibody against L-ficolin (R&D Systems, Minneapolis, MN, USA) was immobilized on 0.1 ml of SiMAG/MP-COOH magnetic beads (Chemicell, Berlin, Germany) using EDC and NHS (Pierce, Rockford, IL, USA) following the manufacturer's instruction. Briefly, 0.1 ml of positive human serum were diluted four times with PBS, mixed with L-ficolin-immobilized magnetic beads and incubated at room temperature for 2 hrs in tubes. The supernatants containing the depleted sera were recovered by placing the tubes in the magnetic rack. The bound ficolins were eluted with 0.4 ml of 100 mM glycine (pH 2.8), neutralized with 0.1 ml of 1 M Tris-HCl (pH 8.0), dialyzed against PBS and concentrated to 0.2 ml using a YM-30 filter.
Depletion of IgG and IgM from human sera
To deplete IgG, the IgG/Albumin removal kit (Pierce) with immobilized antibodies against IgG and albumin was used. Briefly, 0.1 ml of PHS was diluted four times with PBS and mixed with 1 ml of the IgG/albumin-immobilized agarose. The depleted sera were collected and the bound IgG and albumin were eluted with 100 mM glycine (pH 2.8) and neutralized with 1 M Tris-HCl (pH 8.0). The depleted sera and purified IgG/albumin were dialyzed against PBS and concentrated to 0.2 ml with a YM-30 filter. To deplete IgM, human μ-chain specific polyclonal antibody-agarose (Abcam, Cambridge, MA, UK) was used. Briefly, 0.1 ml of PHS was diluted with an equal volume of PBS and mixed with 0.5 ml of anti-human IgM agarose at room temperature for 2 hrs. The supernatant was collected as IgM-depleted serum. The bound IgM was eluted with 100 mM glycine (pH 2.8), quickly neutralized with 1 M Tris-HCl (pH 8.0), dialyzed against PBS and concentrated with a YM-30 filter.
Fluorescent antibody labeling
Mouse anti-human ficolin-2 (L-ficolin) and ficolin-3 (H-ficolin) and goat anti-human IgG/IgM mAb were fluorescently labeled with DyLight 549/649 NHS Ester (ThermoFisher Scientific). Then 1 mg of DyLight 549 NHS Ester was resuspended in 1 ml dimethyl formamide (DMF) and divided into 50-μl aliquots before storage at −80°C. Next, 100 μg of antibody was diluted to 0.5 ml with 50 mM of sodium borate buffer (pH 8.5) and dialyzed against the same buffer to remove possible primary amine-containing components. The dialyzed antibody was mixed with one aliquot of the DyLight NHS Ester and incubated at room temperature for 1 hr. The labeling reaction was stopped by addition of 0.1 ml of 100 mM glycine (pH 8.0) and dialyzed against the same buffer.
Protein immunoblotting
Approximately 200 μg proteins from the positive, negative, or the IgG/A or IgM depleted human sera were heated to 95°C for 5 min in 2x protein loading buffer (0.2 M Tris. HCl, pH 6.8, 2% SDS and 40 mM dithiothreitol). All samples were loaded on 5–12% SDS-PAGE gels and transferred to nitrocellulose membranes. The membranes were blocked with 5% non-fat dry milk and incubated with unlabeled or the Dylight 549 fluorescent-labeled primary antibody against human H-ficolin, L-ficolin and human IgG/IgM, with shaking at 4°C overnight. When unlabeled primary antibody was used, FITC-labeled goat anti-mouse IgG or rabbit anti-goat IgG secondary antibody (ThermoFisher Scientific) was added and incubated at room temperature for 1.5 hrs. The membranes were scanned with Typhoon Imager (GE Amersham, Piscataway, NJ, USA).
Cell immunofluorescence
For ficolin deposition assays, KATO III cells were resuspended in serum-free 1640 in 1.5-ml tubes and incubated with 0.2 ml of the heat-inactivated PHS at 37°C for 10 min. After washing with 1640 twice, the cells were incubated with the Dylight 549 fluorescent-labeled human Ficolin-2 and Ficolin-3 mAb at 37°C for 10 min. The cells were washed again, fixed on glass slides with Immu-Mount (Shandon, ThermoFisher Scientific) and observed under a fluorescence microscope (Olympus IX51 with CCD camera). For membrane attack complex (MAC) detection, KATO III cells were resuspended in serum-free 1640, seeded on glass slides in 24-well plates and incubated in PHS for 10 min. Then the cells were washed with PBS, fixed in 4% neutral buffered formaldehyde (Sigma) for 5 min and then incubated with anti-C5b-9 primary antibody (Abcam) and fluorescein-conjugated goat anti-rabbit secondary antibody (Zhongshan Goldenbridge Biotechnology Co., Beijing, China) at room temperature for 1.5 hrs. The cells were observed under confocal microscope under power 400 (Olympus FV1000, Japan).
Statistical analysis
Cellular cytolysis results represent mean ± standard deviation (SD) of at least three experiments. Differences in cellular cytolysis between the two treatment groups for one type of cancer cell were compared using unpaired Student's t-test individually and differences between the case number of negative and positive serum were tested by Chi-Square test. All statistical analyses were performed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA). A p value <0.05 was considered statistically significant.
References
[ Raven, P. & Johnson, G. B. (ed.)] [ The immune system ] [1159] (McGraw-Hill, New York, 2011).
Ehrenstein, M. R. & Notley, C. A. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol 10, 778–786 (2010).
Czajkowsky, D. M. et al. IgM, Fcμ-receptors and malarial immune evasion. J Immunol 184, 4597–603 (2010).
Panda, S., Zhang, J., Tan, N. S., Ho, B. & Ding, J. L. Natural IgG antibodies provide innate protection against ficolin-opsonized bacteria. EMBO J 32, 2905–2919 (2013).
Kuroki, M. et al. Preparation of human IgG and IgM monoclonal antibodies for MK-1/Ep-CAM by using human immunoglobulin gene-transferred mouse and gene cloning of their variable regions. Anticancer Res 25, 3733–3739 (2005).
Matsushita, M. Ficolins in complement activation. Mol Immunol 55, 22–26 (2013).
Matsushita, M. Ficolins: complement-activating lectins involved in innate immunity. J Innate Immun 2, 24–32 (2010).
Lu, J. & Le, Y. Ficolins and the fibrinogen-like domain. Immunobiology 199, 190–199 (1998).
Czajkowsky, D. M. & Shao, Z. The human IgM pentamer is a mushroom-shaped molecule with a flexural bias. Proc Natl Acad Sci U S A 106, 14960–14965 (2009).
McMullen, M. E. et al. Mannose-binding lectin binds IgM to activate the lectin complement pathway in vitro and in vivo. Immunobiology 21, 759–766 (2006).
Matsushita, M. et al. Activation of the lectin complement pathway by H-ficolin (Hakata antigen). J Immunol 168, 3502–3506 (2002).
Ren, Y., Ding, Q. & Zhang, X. Ficolins and infectious diseases. Virol Sin 29, 25–32 (2014).
Panda, S., Zhang, J., Yang, L., Anand, G. S. & Ding, J. L. Molecular interaction between natural IgG and ficolin-mechanistic insights on adaptive-innate immune crosstalk. Sci Rep 4, 3675 (2014).
Kuraya, M., Ming, Z., Liu, X., Matsushita, M. & Fujita, T. Specific binding of L-ficolin and H-ficolin to apoptotic cells leads to complement activation. Immunobiology 209, 689–697 (2005).
Ollert, M. W. et al. Normal human serum contains a natural IgM antibody cytotoxic for human neuroblastoma cells. Proc Natl Acad Sci U S A 93, 4498–4503 (1996).
Brändlein, S. et al. Natural IgM antibodies and immunosurveillance mechanisms against epithelial cancer cells in humans. Cancer Res 63, 7995–8005 (2003).
Cooper, H. S., Malecha, M. J., Bass, C., Fagel, P. L. & Steplewski, Z. Expression of blood group antigens H-2, Le(y) and sialylated-Le(a) in human colorectal carcinoma. An immunohistochemical study using double-labeling techniques. Am J Pathol 138, 103–110 (1991).
Pour, P. M. et al. Expression of blood group-related antigens ABH, Lewis A, Lewis B, Lewis X, Lewis Y and CA 19–9 in pancreatic cancer cells in comparison with the patient's blood group type. Cancer Res 48, 5422–5426 (1988).
Angal, S. & Dean, P. D. The effect of matrix on the binding of albumin to immobilized Cibacron Blue. Biochem J 167, 301–303 (1977).
Thomsen, T., Schlosser, A., Holmskov, U. & Sorensen, G. L. Ficolins and FIBCD1: soluble and membrane bound pattern recognition molecules with acetyl group selectivity. Mol Immunol 48, 369–381 (2011).
Zacho, R. M., Jensen, L., Terp, R., Jensenius, J. C. & Thiel, S. Studies of the pattern recognition molecule H-ficolin: specificity and purification. J Biol Chem 287, 8071–8081 (2012).
Andac, C. A., Andac, M. & Denizli A. Predicting the binding properties of cibacron blue F3GA in affinity separation systems. Int J Biol Macromol 41, 430–438 (2007).
Subramanian, S. Dye-ligand affinity chromatography: the interaction of Cibacron Blue F3GA with proteins and enzymes. CRC Crit Rev Biochem 16, 169–205 (1984).
Martini, P. G. et al. Recombinant human complement component C2 produced in a human cell line restores the classical complement pathway activity in-vitro: an alternative treatment for C2 deficiency diseases. BMC Immunol 11, 43 (2010).
Nayak, A., Pednekar, L., Reid, K. B. & Kishore, U. Complement and non-complement activating functions of C1q: a prototypical innate immune molecule. Innate Immun 18, 350–363 (2012).
Aihara, K., Yamada, K., Murakami, H., Nomura, Y. & Omura, H. Production of human-human hybridomas secreting monoclonal antibodies reactive to breast cancer cell lines. In Vitro Cell Dev Biol 24, 959–962 (1988).
Tamaki, Y. et al. human monoclonal antibody derived from axillary lymph nodes of a breast cancer patient. Hybridoma 8, 293–302 (1989).
Jordan, J. E., Montalto, M. C. & Stahl, G. L. Inhibition of mannose-binding lectin reduces postischemic myocardial reperfusion injury. Circulation 104, 1413–1418 (2001).
Erttmann, R. Treatment of neuroblastoma with human natural antibodies. Autoimmun Rev 7, 496–500 (2008).
Dunn, G. P., Old, L. J. & Schreiber, R. D. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 21, 137–148 (2004).
Smyth, M. J., Dunn, G. P. & Schreiber, R. D. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol 90, 1–50 (2006).
Vesely, M. D., Kershaw, M. H., Schreiber, R. D. & Smyth, M. J. Natural innate and adaptive immunity to cancer. Annu Rev Immunol 29, 235–271 (2011).
Swann, J. B. & Smyth, M. J. Immune surveillance of tumors. J Clin Invest 117, 137–146 (2007).
Fridman, W. H., Mlecnik, B., Bindea, G., Pagès, F. & Galon, J. Immunosurveillance in human non-viral cancers. Curr Opin Immunol 23, 272–278 (2011).
Shankaran, V. et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410, 1107–1111 (2001).
O'Sullivan, T. et al. Cancer immunoediting by the innate immune system in the absence of adaptive immunity. J Exp Med 209, 1869–1882 (2012).
Street, S. E., Cretney, E. & Smy, M. J. Perforin and interferon-gamma activities independently control tumor initiation, growth and metastasis. Blood 97, 192–197 (2001).
Smyth, M. J. et al. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med 192, 755–760 (2000).
Acknowledgements
This work was jointly supported by the Susan G. Komen Foundation for a Cure (No. BCTR0707230) and the 985 program of Shandong University to Dr. J.H.Y., the National Natural Science Foundation of China (No. 30901357; No.81200323; No. 81272276) and the National Key Basic Research Program (No.2010CB933902) to Dr. J.H.Y., Z.Y., X.Y.L. and C.X.L. This is the result of work partially supported with resources and the use of facilities at VA Boston Healthcare System. The authors thank Medjaden Bioscience company for English proofreading.
Author information
Authors and Affiliations
Contributions
J.H.Y. designed and conceived the experiments; X.Y.L. and C.X.L. conducted the immunodepletion and western blot assay; X.Y.L. and C.L.L. conducted cellular cytolysis and immunofluorescence experiments; F.L., X.A., J.B.S. and Y.H.G. contributed to clinical sample collection; Q.C.Z., Z.Y. and J.H.Y. provided professional advices. X.Y.L. and J.H.Y. wrote and all authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Lei, X., Liu, C., Azadzoi, K. et al. A novel IgM–H-Ficolin complement pathway to attack allogenic cancer cells in vitro. Sci Rep 5, 7824 (2015). https://doi.org/10.1038/srep07824
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07824
This article is cited by
-
Mesangial C4d deposition is independently associated with poor renal survival in patients with primary focal segmental glomerulosclerosis
Clinical and Experimental Nephrology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.