Abstract
SIRT1 could protect degenerative human NP cells against apoptosis and there were extensive and intimate connection between apoptosis and autophagy. Up to now, the role of autophagy in the process of human IVD degeneration is unclear. We sought to explore the relationship between autophagy and human IVD degeneration and to understand whether autophagy is involved in the protective effect of SIRT1 against apoptosis in NP cells. Our results showed that the autophagosomes number, the mRNA level of LC3 and Beclin-1, the protein expression of LC3-II/I and Beclin-1, decreased in NP from DDD. Resveratrol could increase the protein expression of LC3-II/I and Beclin-1 and reduce apoptosis in degenerative NP cells. In contrast, the protein levels of LC3-II/I and Beclin-1 were down-regulated and apoptosis level was significantly up-regulated in treatment with nicotinamide or SIRT1-siRNA transfection. Further analysis identified that the expression of cleaved Caspase3 and apoptosis incidence significantly increased with the pretreatment of bafilomycin A, whether resveratrol was added or not. These suggested that autophagy may play an important role in IVD degeneration and SIRT1 protected degenerative human NP cells against apoptosis via promoting autophagy. These findings would aid in the development of novel therapeutic approaches for degenerative disc disease treatment.
Similar content being viewed by others
Introduction
More than half the population will experience significant low back pain (LBP) during their life1 and LBP has caused a significant social and economic problem2. Although the pathogenesis of LBP is poorly understood, many studies have produced evidence that the intervertebral disc (IVD) degeneration is a major cause of LBP3,4,5,6. IVD consists of nucleus pulposus (NP), annulus fibrosus (AF) and cartilage end plates (CEP). Many studies demonstrated that the excessive apoptosis of NP cells which are capable of producing cartilage-specific extracellular matrix (ECM) components is one of the most evident cellular and biochemical changes in degenerative IVD7,8,9,10,11,12. The decreased number of NP cells caused by excessive apoptosis, leads to that the synthesis of ECM decreased. Finally, excessive reduction of the ECM results in IVD degeneration. Therefore, excessive apoptosis of NP cells has been believed to contribute to the degradation of ECM and plays an important role in the process of IVD degeneration. Consequently, inhibition of apoptosis of NP cells may decrease the degradation of ECM and postpone the progression of the IVD degeneration.
The silent information regulation 2 (Sir2), an NAD-dependent deacetylase, is linked to the regulation of life span. The activity of Sir2 can extend the life span of model organisms such as yeast and flies13. Recent studies showed that silent information regulation 2 homolog-1 (SIRT1), the closest relative of yeast Sir2 in mammalian cells, is a longevity gene which can inhibit apoptosis and enhance cell survival in a variety of cell systems under calorie restriction14,15. Our previous studies showed that SIRT1 could inhibit apoptosis of degenerative human disc NP cells16, however, the specific mechanisms of this protective effect are not fully understood.
Macroautophagy (abbreviated as “autophagy”) is a conserved cellular process that eliminates long-lived proteins and damaged organelles and proteins and recycles cytoplasmic components17. It has been demonstrated that autophagy plays an important role in cell growth, survival, differentiation and homeostasis18. There was also evidence for the protective role of autophagy against apoptosis19. Recent reports showed that autophagy increased in the pathological process of IVD degeneration in rat NP20,21. However, the cell types in NP are different between adult humans and rats: chondrocytic NP cells in adult humans but notochordal cells in rats22. Hence results obtained from animals which retain notochordal cells well into adulthood, may have little relevance at all to the situation of adult humans. Up to now, the role of autophagy in the process of human IVD degeneration has not been reported. We sought to explore the relationship between autophagy and human IVD degeneration, furthermore, understand whether autophagy is involved in the protective effect of SIRT1 against apoptosis in degenerative human disc NP cells.
Results
Less autophagosomes in NP of patients with DDD compared with those in NP of patients with LVF
Transmission electron microscope (TEM) was used to identify autophagosomes in NP. Double-or multiple-membraned autophagosomes were observed both in NP of patients with degenerative disc disease (DDD) and lumbar vertebral fracture (LVF). Visual differences suggested less autophagosomes in NP of patients with DDD than in NP of patients with LVF (Figure 1).
Autophagosomes were detected by TEM in NP cells from patients with LVF and DDD.
(A) Autophagosomes were detected by TEM (30,000×) in NP cells from LVF (black arrow). Double-limiting membrane could be observed in some autophagosomes. (B) One autophagosome was detected by TEM (50,000×) in NP cells from DDD (black arrow).
Low Beclin-1 and LC3 expression in NP of patients with DDD compared with that in NP of patients with LVF
In order to further confirm the relationship between autophagy and IVD degeneration, real-time reverse transcription-polymerase chain reaction (RT-PCR) was used to measure the mRNA expression of Beclin-1, microtubule-associated protein 1 light chain 3 (LC3) of NP from DDD and LVF, western blotting was used to measure the protein levels of Beclin-1 and the ratio of LC3-II/I. real time RT-PCR results show that the mRNA expressions of Beclin-1 and LC3 of NP from DDD were lower than those of NP from LVF, respectively (Figure 2). Western blot showed that the expression of Beclin-1protein of NP from DDD was lower than that of NP from LVF, the ratio of LC3-II/I protein of NP from DDD was also lower than that of NP from LVF (Figure 3). The expression of SIRT1 mRNA and protein of NP from DDD were remarkably decreased compared with those of NP from LVF (Figure 2, 3).
Immunohistochemistry results show that SIRT1-postive cells in NP from DDD were significantly decreased compared with those in NP from LVF (Figure 4A). Conversely, Caspase3-postive cells were increased in NP from DDD compared with those in NP from LVF (Figure 4B). Both the number of Collagen II-positive cells and the intensity of the staining were remarkably decreased in NP from DDD (Figure 4C).
Immunohistochemistry analysis of SIRT1, Caspase3 and Collegan II expression in human NP from patients with LVF and DDD.
(A) SIRT1 levels are decreased in NPs from patients with DDD (n = 3, 200×, *P<0.05). (B) Caspase3 levels are elevated in NPs from patients with DDD (n = 3, 200×, *P<0.05). (C) Collegan II levels are decreased in NPs from patients with DDD (n = 3, 200×, *P<0.05). (D) Apoptotic cells in the NPs from LVF and DDD were detected by TUNEL. The percentages of apoptotic cells are elevated in NPs from patients with DDD (n = 3, 400×, *P<0.05).
Apoptosis of NP specimens from LVF and DDD was detected by TUNEL assay. We found that the TUNEL-labeled apoptotic cells in NP from DDD were significant more than those in NP from LVF (Figure 4D).
SIRT1 enhances autophagy and inhibits apoptosis on degenerative human NP cells
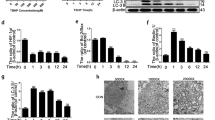
Resveratrol (8 μM) or nicotinamide (12 mM) was used as activator or inhibitor of SIRT1 to treat degenerative human NP cells. Meanwhile, endogenous SIRT1 of degenerative human NP cells was silenced by siRNA transfection. Western blotting was used to measure the effects of up- or down-regulation of SIRT1.Western blotting results showed that the level of SIRT1 protein expression was increased after treatment with resveratrol and it was reduced after treatment with nicotinamide or siRNA transfection for SIRT1. Moreover, the level of SIRT1 protein expression was reduced more obviously after siRNA transfection for SIRT1 compared with that after treatment with nicotinamide (Figure 5A).
SIRT1 enhances autophagy and inhibits apoptosis in degenerative human NP cells.
(A) SIRT1 protein levels were detected by western blot. SIRT1 expression was significantly elevated in degenerative human NP cells which were treated with resveratrol (8 μM) for 48 h. Conversely, SIRT1 expression was reduced in NP cells treated with nicotinamide (12 μM) or SIRT1-siRNA for 48 h. Protein expression levels are normalized against β-actin (*P<0.05 versus control). (B) Autophagy related-protein expressions were assessed by Western blot. Autophagy related-protein expression was significantly elevated in degenerative human NP cells which were treated with resveratrol (8 μM) for 48 h. Conversely, autophagy related-protein expression was reduced in NP cells treated with nicotinamide (12 μM) or SIRT1-siRNA for 48 h. Protein expression levels are normalized against β-actin (*P<0.05 versus control). (C) The apoptosis incidence of degenerative human NP cells evaluated by flow cytometry. The apoptosis incidence decreased significantly in NP cells treated with resveratrol (8 μM) and increased significantly in NP cells treated with nicotinamide (12 μM) or SIRT1-siRNA for 48 h (*P<0.05 versus control).
Western blotting results revealed that the protein expression of both LC3-II/I and Beclin-1 increased significantly in NP cells with treatment of resveratrol and decreased in cells with treatment of nicotinamide or SIRT1-siRNA, indicating that SIRT1 administration enhanced autophagy activation (Figure 5B).
To confirm the role of SIRT1 in the regulation of apoptosis of degenerative human NP cells, cells were treated with resveratrol (8 μM), nicotinamide (12 mM) or SIRT1-siRNA respectively and then the apoptotic incidence was detected by flow cytometry. Apoptotic incidence was decreased in resveratrol-treated cells and increased in nicotinamide and SIRT1-siRNA treated cells. Moreover, the apoptotic incidence was decreased more obviously in SIRT1-siRNA treated cells compared with that in resveratrol-treated cells (Figure 5C).
SIRT1 inhibits apoptosis of degenerative human NP cells by promoting autophagy
In order to investigate whether the SIRT1 inhibits apoptosis was mediated by autophagy, degenerative human NP cells were pretreated with 100 nM bafilomycin A for 3 h and then cultured with or without resveratrol (8 μM) for 48 h. Western blotting results revealed that LC3-II/I was significant higher in NP cells with treatment of both resveratrol and bafilomycin A than those treated with resveratrol alone (P<0.05) and it was also higher in NP cells with treatment of both resveratrol and bafilomycin A than those treated with bafilomycin A alone but without difference in statistics (P>0.05). Meanwhile, the expression of cleaved Caspase3 protein increased in NP cells with bafilomycin A in combination with or without resveratrol, as compared with those treated with resveratrol alone (Figure 6A). Further results of flow cytometry also showed that the apoptotic incidence increased significantly in NP cells with bafilomycin A pretreatment independently whether resveratrol was added or not (Figure 6B). All the results indicated that resveratrol failed to rescue the degenerative human NP cells pretreated with bafilomycin A from apoptosis.
SIRT1 inhibits apoptosis of degenerative human NP cells by promoting autophagy.
(A) LC3-II/I and cleaved caspase3 protein detected by western blot. LC3-II/I enhanced in human degenerative NP cells treated with bafilomycin A (100 nM) in combination with resveratrol (8 μM) or not. The levels of cleaved caspase3 decreased in NP cells treated with resveratrol (8 μM) and increased in NP cells treated with bafilomycin A (100 nM) in combination with resveratrol (8 μM) or not (*P<0.05 versus control, #P<0.05 versus resveratrol). (B) The apoptosis incidence of degenerative human NP cells evaluated by flow cytometry. The apoptosis incidence increased significantly in NP cells with bafilomycin A (100 nM) treatment independently whether resveratrol (8 μM) was added or not (*P<0.05 versus control, #P<0.05 versus resveratrol).
Discussion
Autophagy is an essential, conserved lysosomal degradation pathway and it occurs at a basal rate in most cells, maintaining cytoplasmic homeostasis by eliminating protein aggregates and damaged organelles23. Recent studies provided compelling evidences that at least in model organisms autophagy protects against diverse pathologies, such as, neurodegeneration, heart diseases, infections, cancer and aging24. Beatriz Caramés et al25 found that autophagy may be a protective or homeostatic mechanism in human normal cartilage. Since disc NP cells have the same morphology and avascular supply as chondrocytes and the incidence of DDD increases rapidly with age, we speculated that there may be an inherent relationship between autophagy and the IVD degeneration. In our study, we observed changes in SIRT1, Caspase3, Collagen II and apoptosis levels in the degenerative NP and these results were consistent with the findings of our previous studies16. In this study, we confirmed that, for the first time, autophagy took place in human NP cells as shown by evidence from TEM. To our knowledge, this is the first report of autophagy in human NP cells. By TEM, visual differences suggested less autophagosomes in NP of patients with DDD than those in NP of patients with LVF. Moreover, the mRNA expressions of LC3 and Beclin-1reduced and the protein expressions of LC3-II/I and Beclin-1 also reduced in NP from DDD compared with those in NP from LVF. These results showed that autophagy may play an important role in IVD degeneration. These observations in human NP were similar with those in different tissues25,26,27. However, the conflicting observations of autophagic activity were reported in animal IVD degeneration models20,21. The main possible explanation can account for the differences in cell population, tissue composition, disc and spine anatomy, development, physiology and mechanical properties, between animal species and humans22. Of particular importance, is the difference in cells which populate the central NP. There are no notochordal cells present in the adult human nucleus pulposus, but most other species retain them throughout much of their adult lives28. Hence results obtained from animals which retain notochordal cells well into adulthood, may have little relevance at all to the situation of adult humans. In our study, we compared DDD patients with patients undergoing surgery for lumbar vertebral fracture (LVF), which they presume are otherwise normal and the LVF patients have an average age of 22 years old, whilst the mean age of the DDD group is 56. It is difficult to determine whether any differences we watched were due to degeneration, or simply ageing (or a combination) because the age is different in two groups. In fact, there was limitation for samples collection, in clinical work, relatively normal NP could only be obtained from young patients with LVF and it is scarcely possible to find relatively normal NP in donors whose age match with patients of DDD. In order to eliminate the affection of age, in our study, the degeneration grade of IVD was classified according to the Pfirrmann classification and grouping was determined by degeneration degree of IVD but not age. Many reports29,30 and our early study16 also adopted this grouping method to determine any differences between normal and degenerative IVD. However, we still consider that age may be a factor results in different changes in two groups.
SIRT1 have been demonstrated to play an important role in apoptosis and aging14,15,31,32,33,34. We have also affirmed that SIRT1 could inhibit apoptosis of degenerative human disc NP cells in our early study16. Recent studies reported that SIRT1 activates autophagy35,36,37 and autophagy inhibits apoptosis38,39. Therefore, these have led to the hypothesis that there may be some inherent relationships among SIRT1 activity, autophagic activity and apoptosis of human NP cells. Our results showed that SIRT1 expression and autophagic activity decreased in NP from DDD and apoptosis of human NP cells changed conversely. In order to further confirm whether autophagy is involved in the protective effect of SIRT1 against apoptosis in human NP cells, we next employed cell experiments with degenerative human NP cells. Treatment with resveratrol, a natural activator of SIRT1, led to an increased protein expression of LC3-II/I and Beclin-1 and a reduced apoptosis in degenerative human NP cells. In contrast, protein levels of LC3-II/I and Beclin-1 were down-regulated and apoptosis levels were significantly up-regulated in treatment with nicotinamide or SIRT1-siRNA transfection. These observations implied that SIRT1 could promote autophagy and inhibit apoptosis in human NP cells. Then, bafilomycin A, a vacuolar ATPase inhibitor that causes an increase in lysosomal/vacuolar pH and ultimately blocks fusion of autophagosomes with lysosome, was used to inhibit autophagy40 to see whether SIRT1 protected against apoptosis of human NP cells by promoting autophagy. We found that LC3-II/I was significant higher in NP cells with the treatment of resveratrol and bafilomycin A than that treated with resveratrol alone (P<0.05) and it was also higher in NP cells with the treatment of resveratrol and bafilomycin A than that treated with bafilomycin A alone, however, without significant difference (P>0.05). Yue-Hua Yang et al41 described similar results in their report. The possible explanations may be that the effect of resveratrol inducing autophagosome formation was weakened due to bafilomycin A blocking autophagic flux. In other words, when fusion of autophagosomes with lysosomes was blocked by bafilomycin A, there were more autophagosomes in NP cells so that resveratrol could not induce more autophagosomes formation. We will further explore the specific mechanisms in the future. Meanwhile, our data showed that both expression of cleaved Caspase3 protein and apoptosis incidence significantly increased with bafilomycin A treatment, whether resveratrol was added or not. These results confirmed that autophagy is necessary in SIRT1 protected against apoptosis of degenerative human NP cells. This was in consistent with previous studies in neurodegenerative disorders42, diabetic cardiomyopathy43 and atherosclerosis44.
The drawback of this study is that resveratrol (activator of SIRT1) was substituted for SIRT1 plasmid transfection because of extremely low transfection efficiency of lipofection. It is no doubt that more safe and efficient SIRT1 plasmid transfection manner (for example, nucleofector technology) should be chosen for more credible experiment results.
We confirmed that SIRT1 inhibits apoptosis of degenerative human NP cells by promoting autophagy. In fact, there are complex networks between SIRT1 and autophagy45,46 and between autophagy and apoptosis47,48. To elucidate exactly the mechanisms involve SIRT1, autophagy and apoptosis in human NP cells, we need further investigation of some key factors, for example, forkhead box class O (FoxO) and p53 pathways (between SIRT1 and autophagy)45,46, p62 and Beclin-1 pathways (between autophagy and apoptosis)47,48.
In conclusion, our results revealed that autophagy may play an important role in IVD degeneration and SIRT1 protected degenerative human NP cells against apoptosis via promotion of autophagy. These findings would aid in the development of novel therapeutic approaches for degenerative disc disease (DDD) treatment.
Methods
Patients
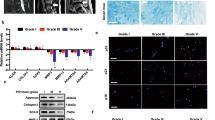
All the subjects were lumbar spine surgery patients admitted to The First Affiliated Hospital of Chongqing Medical University (Chongqing, China) from February 2013 to May 2014. The relatively normal human intervertebral disc (IVD) tissues as control were obtained from 3 male patients (aged 19, 22 and 25 years old, respectively) and 1 female patient (aged 22 years old) with lumbar vertebral fracture (LVF) undergoing posterior discectomy, spinal fusion, decompression and stability within 24 h of trauma, without formerly documented clinical history of LBP (Figure 7A). The degenerative human IVD tissues were obtained from 25 patients (12 females and 13 males, mean age, 56; 45–68) with degenerative disc disease (DDD) during discectomy and intervertebral fusion surgery (Figure 7B). The IVD tissues were harvested under sterile conditions and immediately sent to the laboratory (within 30 min after being harvested). Complete culture medium with serum at 4°C was used as transport medium. The NP tissues were carefully isolated from IVD tissues by a scalpel microscopically under sterile condition.
Representative MRI of patients.
(A) Patient with lumbar LVF undergoing posterior discectomy, spinal fusion, decompression and stability within 24 h of trauma, without formerly documented clinical history of LBP. The red arrow indicates the experimental material position. The L1 vertebrae is fractured and dislocated and the disc of T12/L1 is classified as Grade I according to the Pfirrmann classification. (B) Patient with DDD undergoing discectomy and intervertebral fusion surgery. The red arrow indicates the experimental material position. The disc of L4/5 is herniated and classified as Grade IV according to the Pfirrmann classification.
Routine magnetic resonance imaging (MRI) scan of spine was performed for all the patients prior to surgery. The degeneration grade of IVD was classified according to the Pfirrmann classification49. The lumbar discs of all patients with DDD were classified as Grades III-IV and the discs of 4 patients with LVF were classified as Grade I.
The study complied with the Declaration of Helsinki and with approval from the Ethics Committee of Chongqing Medical University and informed consent of all the patients involved in our study was obtained.
Chemicals and antibodies
Chemicals and antibodies were purchased as follows: resveratrol (Sigma, R5010) dissolved in 100% dimethyl sulfoxide (DMSO; Sigma, D2650) to a concentration of 40 mM, nicotinamide (Sigma, 72340) dissolved in phosphate-buffered saline (PBS) to a concentration of 200 mM, bafilomycin A (Sigma, B1793) dissolved in DMSO to a concentration of 5 μM, 0.25% trypsin solution (Sigma, 59429C), 0.2% type II collagenase (Sigma, V900892), Dulbecco's modified Eagle's medium and Ham's F-12 medium (DMEM/F12, 1:1; Gibco, 11320-033), fetal bovine serum (FBS; Gibco, 10099-141), penicillin-streptomycin (Sigma, G4664), rabbit anti-SIRT1 (Epitomics, ab32441), rabbit anti-Caspase3 (Epitomics, ab32351), rabbit anti-Collagen II (Abcam, ab34712), rabbit anti-LC3 (Cell Signaling Technology, 4599), mouse anti-Beclin-1 (Abcam, ab114071), mouse anti-β-actin (Beyotime, AA128), goat anti-rabbit IgG-HRP (Beyotime, A0208), goat anti-mouse IgG-HRP (Beyotime, A0216).
Transmission electron microscopy (TEM)
NP samples from 3 DDD patients (total number of DDD patients is 25) and 3 LVF (total number of LVF patients is 4) patients were selected randomly to finish TEM detecting. The human NP tissues were fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) for 1 day at room temperature. The tissues were then washed 3 times with 0.1 M phosphate-buffered saline (PBS; pH 7.4). Next, The tissues were fixed in 2% osmium tetroxide and block-stained with 2% uranyl acetate and then were washed again 3 times with 0.1 M PBS (pH 7.4). The specimens were embedded in epoxy resin following the dehydration in an ascending series of ethanol (50% ethanol for 20 min, 70% ethanol for 20 min, 90% ethanol for 20 min and 100% ethanol for 20 min thrice). At last, the specimens were cut into 60 nm sections and the sections were stained with uranyl acetate and lead citrate. Ultrathin sections were examined with a TEM (Hitachi-7500, Japan).
Immunohistochemistry
NP samples from 3 DDD patients (total number of DDD patients is 25) and 3 LVF (total number of LVF patients is 4) patients were selected randomly to finish immunohistochemistry detecting. Human NP specimens were embedded in paraffin after fixation with 4% paraformaldehyde for 24 h and then sectioned (4 μm). The sections were deparaffinized with xylene and rehydrated through graded ethanol to distilled water. Afterwards, the sections were treated with 3% H2O2 for 15 min at room temperature to eliminate endogenous peroxidases activity, incubated with trypsin for 30 min at 37°C to retrieve the antigen and blocked with normal goat serum for 15 min at room temperature. Next, the sections were incubated with rabbit anti-SIRT1 (1:100), rabbit anti-Caspase3 (1:50) and rabbit anti-Collagen II (1:100) primary antibodies overnight at 4°C. Then, the sections were incubated with secondary antibody goat anti-rabbit IgG-HRP (1:5000) and counterstained with hematoxylin.
TUNEL
NP samples from 3 DDD patients (total number of DDD patients is 25) and 3 LVF (total number of LVF patients is 4) patients were selected randomly to finish TUNEL detecting. A terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay kit (Roche, 40306ES20) that enzymatically labels DNA strand breaks was used to assess the apoptotic cells of human NP. First, the NP sections were incubated with 3% H2O2 for 10 min at room temperature to quench endogenous peroxidase. Next, the sections were washed three times with PBS. Then, the TUNEL reaction solution (50 μl) was added and the sections were incubated in a humidified atmosphere for 1 h at 37°C. Apoptotic NP cells were quantified by counting TUNEL-labeled apoptotic cells and the results were expressed as counts of TUNEL-labeled apoptotic cells.
Isolation and culture of human degenerative NP cells
The human NP tissues were carefully isolated from degenerative IVD tissues by a scalpel microscopically under sterile condition. Then, they were washed with PBS twice and cut into 1 mm3 fragments. The fragments of NP tissues were digested in 0.25% trypsin solution for 30 min, following 3–4 h in 0.2% type II collagenase at 37°C. Tissue debris was removed by passing through a 200-μm filter and then the NP cells were resuspended in DMEM/F12 containing 15% FBS and 1% penicillin-streptomycin at 37°C in a humidified atmosphere containing 5% CO2. When the cells grew to confluence of 80–90%, they were digested by 0.25% trypsin solution and subcultured in culture flasks. The third generation of NP cells was used for all experiments.
siRNA transfection
Double-stranded small interfering RNA (siRNA) for human SIRT1 gene silencing was designed and synthesized by Invitrogen (Invitrogen, USA) to suppress the expression of endogenous SIRT1 of degenerative human NP cells. Sequences of the SIRT1-siRNA were as follows: sense 5′-CCAAGCAGCUAAGAGUAAUTT-3′, antisense 5′- AUUACUCUUAGCUGCUUGGTT-3′. Cells were seeded in 6 wells plate for 24 h and were transfected with SIRT1 or negative control siRNA duplexes using PepMute siRNA Transfection Reagent (SignaGen, SL100566) according to the manufacturer's instructions. After further various treatments, cells were harvested for flow cytometry and protein extracts were used for Western blot experiments.
Flow cytometry
Apoptotic incidence was detected by Annexin V/PI double-staining flow cytometry. Briefly, degenerative human NP cells following different treatments were harvested, washed with PBS twice and resuspended in binding buffer. The cells were incubated with Annexin V solution and PI in the dark for 10–15 min at room temperature and then the fluorescence intensities of Annexin V/PI-stained cells were analyzed by flow cytometry within 1 h. Apoptotic cells, including those show Annexin V+/PI− and Annexin V+/PI+ were counted and represented as a percentage of the total cell count.
RNA extraction and real-time RT-PCR
NP samples from 3 DDD patients (total number of DDD patients is 25) and 3 LVF (total number of LVF patients is 4) patients were selected randomly to finish real-time RT-PCR detecting. The total RNA was extracted from human NP tissues using TRIZOL® Reagent (Invitrogen, 15596-018) according to the manufacturer's instruction. An ultraviolet spectrophotometer (Olympus, Japan) was used to measure the purity and concentration of RNA. Next, the isolated RNA samples were used to synthesized cDNA using PrimeScript® RT reagent Kit With gDNA Eraser (Takara, RR047Q). The expression of SIRT1, LC3 and Beclin-1 human genes was determined by Real-time quantitative RT-PCR using SYBR® Green Real-time PCR Master Mix (TOYOBO, QPK-201) and an ABI Prism 7500 Fast sequence detection system (Applied Biosystems, USA). The reaction conditions were as follows: one cycle at 95°C for 3 min, followed by 40 cycles at 95°C for 15 s, respective annealing temperature for 20 s and an elongation phase at 72°C for 20 s. The optimal concentrations of primers and templates used in each reaction were established according to the standard curve created before the reaction and corresponding to the nearly 100% efficiency of the reaction. The fold-change in gene expression relative to the control was calculated by 2-ΔΔCT. Primers for human genes: 5′- CCAGAACATAGACACGCTGGAAC-3′ and 5′-CTCCTCGTACAGCTTCACAGTCA-3′ for SIRT1; 5′-GTGAGTGTGTCCACGCCCAT-3′ and 5′-AGGTTTCCTGGGAGGCGTAG-3′ for LC3; 5′-TTGGCACAATCAATAACTTCAGGC-3′ and 5′- CCGTAAGGAACAAGTCGGTATCTC-3′ for Beclin-1; 5′-CTTTGGTATCGTGGAAGGACTC-3′ and 5′-GTAGAGGCAGGGGATGATGTTCT-3′ for GAPDH. All the primers were synthesized by TaKaRa (TaKaRa, China).
Western blot analysis
NP samples from 3 DDD patients (total number of DDD patients is 25) and 3 LVF (total number of LVF patients is 4) patients were selected randomly to finish western blot analysis. The protein of human NP tissues was extracted using Tissue Protein Extraction Kit (ComWin Biotechnology, CW0891) according to the manufacturer's instruction. The degenerative human NP cells were washed in ice-cold PBS (PH = 7.5) and lysed using RIPA Lysis Buffer (Beyotime, P0013B). Protein concentration was measured by Enhanced BCA Protein Assay Kit (Beyotime, P0010S) and protein samples were mixed with 5× sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) sample loading buffer, boiled for 5 min. After electrophoresed in 6–12% SDS-PAGE gels, protein samples were transferred to polyvinylidene difluoride membrane (PVDF, 0.45 or 0.22 μm). The membranes were blocked with 5% nonfat dry milk in tris-buffered saline (TBST) for 1 h and incubated with primary antibodies rabbit anti-SIRT1 (1:2000), rabbit anti-LC3 (1:1000), mouse anti-Beclin-1 (1:1000), rabbit anti-Caspase3 (1:1000) and mouse anti-β-actin (1:500) overnight at 4°C. After washed three times for 10 min in TBST, the membranes were incubated in secondary antibody goat anti-rabbit IgG-HRP (1:5000) and goat anti-mouse IgG-HRP (1:5000) for 1 h. Finally, the membranes were treated with ECL plus reagent (Invitrogen, WP20005) and the results were analyzed by the software.
Statistical analysis
Data were expressed as mean ± SD (standard deviation) for at least three independent experiments. SPSS 13 statistical software program (SPSS Inc., IL, USA) was used for statistical analyses. Statistical differences were measured with Student's t-test for comparison between two groups or an analysis of variance (ANOVA) followed by the Turkey's t-test for comparison of multiple groups and p<0.05 was considered statistically significant.
References
McBeth, J. & Jones, K. Epidemiology of chronic musculoskeletal pain. Best Pract Res Clin Rheumatol 21, 403–425, 10.1016/j.berh.2007.03.003 (2007).
Joud, A., Petersson, I. F. & Englund, M. Low back pain: epidemiology of consultations. Arthritis Care Res 64, 1084–1088, 10.1002/acr.21642 (2012).
Barrick, W. T. et al. Anterior lumbar fusion improves discogenic pain at levels of prior posterolateral fusion. Spine 25, 853–857 (2000).
Kellgren, J. H. The anatomical source of back pain. Rheumatol Rehabil 16, 3–12 (1977).
Kuslich, S. D., Ulstrom, C. L. & Michael, C. J. The tissue origin of low back pain and sciatica: a report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Orthop Clin North Am 22, 181–187 (1991).
Luoma, K. et al. Low back pain in relation to lumbar disc degeneration. Spine 25, 487–492 (2000).
Gruber, H. E. & Hanley, E. N., Jr Analysis of aging and degeneration of the human intervertebral disc. Comparison of surgical specimens with normal controls. Spine 23, 751–757 (1998).
Gruber, H. E. & Hanley, E. N., Jr Biologic strategies for the therapy of intervertebral disc degeneration. Expert Opin Biol Ther 3, 1209–1214, 10.1517/14712598.3.8.1209 (2003).
Park, J. B., Chang, H. & Kim, K. W. Expression of Fas ligand and apoptosis of disc cells in herniated lumbar disc tissue. Spine 26, 618–621 (2001).
Park, J. B., Kim, K. W., Han, C. W. & Chang, H. Expression of Fas receptor on disc cells in herniated lumbar disc tissue. Spine 26, 142–146 (2001).
Zhao, C. Q., Jiang, L. S. & Dai, L. Y. Programmed cell death in intervertebral disc degeneration. Apoptosis 11, 2079–2088, 10.1007/s10495-006-0290-7 (2006).
Zhao, C. Q., Wang, L. M., Jiang, L. S. & Dai, L. Y. The cell biology of intervertebral disc aging and degeneration. Ageing Res Rev 6, 247–261, 10.1016/j.arr.2007.08.001 (2007).
Guarente, L. & Picard, F. Calorie restriction--the SIR2 connection. Cell 120, 473–482, 10.1016/j.cell.2005.01.029 (2005).
Cohen, H. Y. et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science (New York, N.Y.) 305, 390–392, 10.1126/science.1099196 (2004).
Saunders, L. R. & Verdin, E. Sirtuins: critical regulators at the crossroads between cancer and aging. Oncogene 26, 5489–5504, 10.1038/sj.onc.1210616 (2007).
Wang, D. et al. SIRT1 inhibits apoptosis of degenerative human disc nucleus pulposus cells through activation of Akt pathway. Age (Dordrecht, Netherlands) 35, 1741–1753, 10.1007/s11357-012-9474-y (2013).
Shintani, T. & Klionsky, D. J. Autophagy in health and disease: a double-edged sword. Science (New York, N.Y.) 306, 990–995, 10.1126/science.1099993 (2004).
Yang, Z. & Klionsky, D. J. Eaten alive: a history of macroautophagy. Nat Cell Biol 12, 814–822, 10.1038/ncb0910-814 (2010).
Thorburn, A. Apoptosis and autophagy: regulatory connections between two supposedly different processes. Apoptosis 13, 1–9, 10.1007/s10495-007-0154-9 (2008).
Ye, W. et al. Age-related increases of macroautophagy and chaperone-mediated autophagy in rat nucleus pulposus. Connect Tissue Res 52, 472–478, 10.3109/03008207.2011.564336 (2011).
Ye, W. et al. Increased macroautophagy in the pathological process of intervertebral disc degeneration in rats. Connect Tissue Res 54, 22–28, 10.3109/03008207.2012.715702 (2013).
Alini, M. et al. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J 17, 2–19, 10.1007/s00586-007-0414-y (2008).
Ravikumar, B. et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev 90, 1383–1435, 10.1152/physrev.00030.2009 (2010).
Levine, B. & Kroemer, G. Autophagy in the pathogenesis of disease. Cell 132, 27–42, 10.1016/j.cell.2007.12.018 (2008).
Carames, B., Taniguchi, N., Otsuki, S., Blanco, F. J. & Lotz, M. Autophagy is a protective mechanism in normal cartilage and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum 62, 791–801, 10.1002/art.27305 (2010).
Stupina, A. S., Terman, A. K., Kvitnitskaia-Ryzhova, T., Mezhiborskaia, N. A. & Zherebitskii, V. A. The age-related characteristics of autophagocytosis in different tissues of laboratory animals. Tsitol Genet 28, 15–20 (1994).
Taneike, M. et al. Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy 6, 600–606, 10.4161/auto.6.5.11947 (2010).
Hunter, C. J., Matyas, J. R. & Duncan, N. A. Cytomorphology of notochordal and chondrocytic cells from the nucleus pulposus: a species comparison. J Anat 205, 357–362, 10.1111/j.0021-8782.2004.00352.x (2004).
Liang, W. et al. Differential expression of extracellular-signal-regulated kinase 5 (ERK5) in normal and degenerated human nucleus pulposus tissues and cells. Biochem Biophys Res Commun 449, 466–470, 10.1016/j.bbrc.2014.05.042 (2014).
Xu, H., Xiong, S., Wang, H., Zhang, M. & Yu, Y. The evidence and the possible significance of autophagy in degeneration model of human cervical end-plate cartilage. Exp Ther Med 7, 537–542, 10.3892/etm.2013.1465 (2014).
Alcendor, R. R. et al. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circulation research 100, 1512–1521, 10.1161/01.RES.0000267723.65696.4a (2007).
Araki, T., Sasaki, Y. & Milbrandt, J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science (New York, N.Y.) 305, 1010–1013, 10.1126/science.1098014 (2004).
Gagarina, V. et al. SirT1 enhances survival of human osteoarthritic chondrocytes by repressing protein tyrosine phosphatase 1B and activating the insulin-like growth factor receptor pathway. Arthritis Rheum 62, 1383–1392, 10.1002/art.27369 (2010).
Takayama, K. et al. SIRT1 regulation of apoptosis of human chondrocytes. Arthritis Rheum 60, 2731–2740, 10.1002/art.24864 (2009).
Guo, H., Chen, Y., Liao, L. & Wu, W. Resveratrol protects HUVECs from oxidized-LDL induced oxidative damage by autophagy upregulation via the AMPK/SIRT1 pathway. Cardiovasc Drugs Ther 27, 189–198, 10.1007/s10557-013-6442-4 (2013).
Lee, I. H. et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A 105, 3374–3379, 10.1073/pnas.0712145105 (2008).
Ou, X., Lee, M. R., Huang, X., Messina-Graham, S. & Broxmeyer, H. E. SIRT1 positively regulates autophagy and mitochondria function in embryonic stem cells under oxidative stress. Stem cells (Dayton, Ohio) 32, 1183–1194, 10.1002/stem.1641 (2014).
Zeng, R., Chen, Y., Zhao, S. & Cui, G. H. Autophagy counteracts apoptosis in human multiple myeloma cells exposed to oridonin in vitro via regulating intracellular ROS and SIRT1. Acta Pharmacol Sin 33, 91–100, 10.1038/aps.2011.143 (2012).
Zou, X. et al. ER stress-mediated autophagy protects against LPS-induced apoptosis in HL-1 cardiomyocytes. Exp Physiol 99, 1348–1358, 10.1113/expphysiol.2014.079012 (2014).
Klionsky, D. J. et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8, 445–544 (2012).
Yang, Y. H. et al. Estradiol inhibits osteoblast apoptosis via promotion of autophagy through the ER-ERK-mTOR pathway. Apoptosis 18, 1363–1375, 10.1007/s10495-013-0867-x (2013).
Jeong, J. K., Moon, M. H., Lee, Y. J., Seol, J. W. & Park, S. Y. Autophagy induced by the class III histone deacetylase Sirt1 prevents prion peptide neurotoxicity. Neurobiol Aging 34, 146–156, 10.1016/j.neurobiolaging.2012.04.002 (2013).
Wang, B. et al. Resveratrol-enhanced autophagic flux ameliorates myocardial oxidative stress injury in diabetic mice. J Cell Mol Med 18,1599–1611, 10.1111/jcmm.12312 (2014).
Liu, B., Zhang, B., Guo, R., Li, S. & Xu, Y. Enhancement in efferocytosis of oxidized low-density lipoprotein-induced apoptotic RAW264.7 cells through Sirt1-mediated autophagy. Int J Mol Med 33, 523–533, 10.3892/ijmm.2013.1609 (2014).
Ng, F. & Tang, B. L. Sirtuins' modulation of autophagy. J Cell Physiol 228, 2262–2270, 10.1002/jcp.24399 (2013).
Salminen, A. & Kaarniranta, K. SIRT1: regulation of longevity via autophagy. Cell Signal 21, 1356–1360, 10.1016/j.cellsig.2009.02.014 (2009).
Gump, J. M. & Thorburn, A. Autophagy and apoptosis: what is the connection? Trends Cell Biol 21, 387–392, 10.1016/j.tcb.2011.03.007 (2011).
Rubinstein, A. D. & Kimchi, A. Life in the balance - a mechanistic view of the crosstalk between autophagy and apoptosis. J Cell Sci 125, 5259–5268, 10.1242/jcs.115865 (2012).
Pfirrmann, C. W., Metzdorf, A., Zanetti, M., Hodler, J. & Boos, N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 26, 1873–1878 (2001).
Acknowledgements
We would like to acknowledge the service provided by Chongqing Key Laboratory of Ophthalmology. This study was supported by grants from the National Natural Science Foundation of China (81171751, 81372003) to Z.H.
Author information
Authors and Affiliations
Contributions
W.J., Z.H. J.H. and X.Z. designed research; W.J. performed experiments; W.J., J.S., X.Z., J.F., W.D., W.S., D.W. and L.L. analyzed data; and W.J., Q.Q., B.L. and Y.L. wrote the manuscript. All authors discussed the results and reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Jiang, W., Zhang, X., Hao, J. et al. SIRT1 protects against apoptosis by promoting autophagy in degenerative human disc nucleus pulposus cells. Sci Rep 4, 7456 (2014). https://doi.org/10.1038/srep07456
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07456
This article is cited by
-
Melatonin Attenuates Arsenic-Induced Neurotoxicity in Rats Through the Regulation of miR-34a/miR-144 in Sirt1/Nrf2 Pathway
Biological Trace Element Research (2024)
-
Fighting age-related orthopedic diseases: focusing on ferroptosis
Bone Research (2023)
-
Sinapic acid ameliorates paracetamol-induced acute liver injury through targeting oxidative stress and inflammation
Molecular Biology Reports (2022)
-
MiR-200a-3p Aggravates DOX-Induced Cardiotoxicity by Targeting PEG3 Through SIRT1/NF-κB Signal Pathway
Cardiovascular Toxicology (2021)
-
miR-573 regulates cell proliferation and apoptosis by targeting Bax in nucleus pulposus cells
Cellular & Molecular Biology Letters (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.