Abstract
Atypically-shaped cardiomyocytes (ACMs) constitute a novel subpopulation of beating heart cells found in the cultures of cardiac myocyte-removed crude fraction cells obtained from adult mouse cardiac ventricles. Although ~500 beating ACMs are observed under microscope in the cell cultures obtained from the hearts of either male or female mice, the origin of these cells in cardiac tissue has yet to be elucidated due to the lack of exclusive markers. In the present study, we demonstrate the efficacy of cellular prion protein (PrP) as a surface marker of ACMs. Cells expressing PrP at the plasma membrane in the culture of the crude fraction cells were found to develop into beating ACMs by themselves or fuse with each other to become larger multinuclear beating ACMs. Combining PrP with a cardiac-specific contractile protein cardiac troponin T (cTnT) allowed us to identify native ACMs in the mouse cardiac ventricles as either clustered or solitary cells. PrP- and cTnT-marked cells were also found in the adult, even aged, human cardiac ventricles. These findings suggest that interstitial cells marked by PrP and cTnT, native ACMs, exhibit life-long survival in the cardiac ventricles of both mice and humans.
Similar content being viewed by others
Introduction
The functional heart comprises heterogeneous cell lineages, in addition to cardiomyocytes, such as vascular smooth muscle cells, endothelial cells and fibroblasts. Since the discovery of cardiac stem or progenitor cells in the adult mammalian heart1,2,3, a number of studies of the efficacy of manipulating these cells to differentiate into functional cardiomyocytes have been reported in mice4,5,6,7,8 and humans9,10,11,12 (for reviews13,14,15). Generally, cardiac stem cells are identified based on their expression of stem cell markers, such as stem cell antigen-1 (Sca-1)2,6, stem cell factor receptor (c-kit)1,4,5,7,10,11 and insulin gene enhancer protein Islet1 (Isl-1)16, or the ability to efflux fluorescent dye17, thus allowing for the isolation of these cells to grow and differentiate into cardiomyocytes in vitro and/or in vivo transplantation experiments14,15.
We have previously discovered a novel subpopulation of heart cells, distinct from the cardiac stem cells, that spontaneously develop into beating cardiomyocytes in the culture of cardiomyocyte-removed crude fraction cells obtained from the adult mouse cardiac ventricles18,19,20. We have defined these beating cells as atypically-shaped cardiomyocytes (ACMs) based on their peculiar morphology, displaying the cell shapes far different from those of cardiomyocytes. Usually, ~500 beating ACMs were found under microscope in the culture of the crude fraction obtained from an adult mouse heart. These cells do not appreciably proliferate even during the prolonged culture. Although ACMs are isolated from cardiac ventricular tissues, the protein expression patterns detected by immunocytochemical experiments appear to be a mixture of those observed in atrial and ventricular myocytes and pacemaker cells, including pacemaker channel hyperpolarization-activated cyclic nucleotide-gated channel 4 (HCN4), gap junction protein connexin 43 (Cx43), atrial natriuretic peptide (ANP) and T-type Ca2+ channel Cav3.218,19. However, the localization of “native” ACMs in the heart has yet to be elucidated due to the lack of exclusive surface marker protein.
In this study, cellular prion protein (PrP) was found to serve as a surface marker for ACMs that enabled us to identify these cells within various types of non-myocytes in the culture. PrP-expressing small cells were found not only to develop into beating ACMs by themselves but also to fuse with each other to become larger multinuclear beating ACMs in the culture. In combination with cardiac specific contractile protein cardiac troponin T (cTnT), PrP was demonstrated to specifically identify native ACMs in the interstitial spaces among ventricular myocytes in the adult mouse hearts. We also found the presence of the interstitial cells co-expressing PrP and cTnT in the adult, even aged, human cardiac ventricles. Our results suggest that the PrP and cTnT-marked interstitial cells, native ACMs, survive in the cardiac ventricles for a life-long period in humans as well as in mice.
Results
Morphological characterization of ACMs
Beating ACMs can be found in cultures of cardiomyocyte-removed crude fraction cells (Fig. 1a and Supplementary Movie S1). These cells exhibit peculiar morphological characteristics, such as a high degree of branching with many projections, multiple nuclei, surface bulge(s) and organized sarcomeric structures characterized by the expression of cardiac-specific α-actinin (ACTN, Fig. 1b, c). ACMs usually possess plural numbers of nuclei; ~76% of these cells were multiple nuclear cells (Fig. 1c, d). Unlike normal cardiomyocytes, the multinuclear ACMs were found to contain predominantly two or three, sometimes more than four nuclei and usually possess bulge(s) on the cell surface; ~43% of these cells contain bulge(s) (Fig. 1d). Furthermore, three-dimensional (3D) images of DAPI staining and ACTN immunostaining in the ACMs showed that the cell body and bulge each contained nuclei and sarcomeric structures (Supplementary Movie S2). To examine the origin of bulge(s) on the surface of ACMs, it needs to determine appropriate surface marker proteins for identifying ACMs.
Characterization of atypically-shaped cardiomyocytes (ACMs).
(a) Phase contrast (PC) image of a beating ACMs cultured for 12 days. See Supplementary Movie S1 for the synchronous beating of the cell body and fused small cell (arrow). Bar, 50 μm. (b) Confocal laser scanning microscopy of immunostaining for α-actinin (ACTN, red), DAPI staining for nuclei (blue) and a differential interference contrast (DIC) image of an ACM with a surface bulge (arrowhead) cultured for 7 days. See Supplementary Movie S2 for the three-dimensional (3D) structures of sarcomeres and locations of nuclei. Bar, 50 μm. (c) Immunostaining for ACTN, DAPI-staining and DIC images of representative ACMs with single (upper left), double (upper right), triple (lower left) or quadruple (lower right) nuclei. Cultured for 7–9 days. The arrows indicate nuclei. Bar, 50 μm. (d) Number of ACMs and nuclei, for the total population (black) and classified according to the absence (white) or presence (gray) of bulges. Data for 1,302 cells cultured for 5–7 days obtained from 52 hearts.
ACMs express cellular prion protein (PrP)
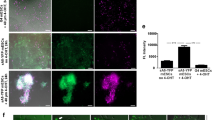
Recently, a cellular prion protein (PrP), also known as CD230, has been reported to serve as a surface marker for isolating nascent cardiomyocytes and cardiac progenitors and cardiogenic populations in differentiating embryonic stem (ES) cells21. PrP is a highly conserved glycoprotein anchored to the cell membrane by glycosylphosphatidylinositol that constitutively cycles between the plasma membrane and endocytic cellular components, being most abundantly expressed in the brain, although it has been detected in many non-neural tissues22,23,24,25. To examine whether ACMs express PrP, immunostaining analyses were performed using well-characterized anti-PrP-antibody21.
Figure 2a and b demonstrate the specificity of the monoclonal anti-PrP antibody (Bertin Pharma Co. clone SAF83)21 in the mouse tissue using lymphocytes (Fig. 2a) and liver cells (Fig. 2b) as PrP-positive and -negative controls, respectively. Consistent with the previous literature23,24, strong immunofluorescent signals of PrP were detected in the lymphocytes accumulated in the blastic centers of the splenic white pulp (Fig. 2a), but not liver cells (Fig. 2b), thus suggesting that the anti-PrP antibody (clone SAF83) used in the present experiments recognizes PrP in mice.
Expression of prion protein (PrP) in the ACMs.
(a, b) Specificity of the anti-PrP antibody for the mouse tissues. Confocal laser scanning microscopy of PrP immunostaining (green), DAPI staining (blue) and DIC images of the mouse spleen (a) and liver (b). PrP was expressed in the lymphocytes in the blastic center of the splenic white pulp (a), but not the liver cells (b), in the mouse tissue. Bar, 25 μm. (c) Immunostaining for PrP and a DIC image in ACMs with a bulge (arrowhead) cultured for 3 days. The middle and right panels focus on the cell body and bulge, respectively. Both the cell body and bulge were beating synchronously prior to fixation. Bar, 25 μm. (d) Immunostaining for PrP in isolated ventricular myocytes. Bar, 50 μm. (e) Fluorescent intensity determined on the image of ACMs (left) or ventricular myocytes (right) obtained from the line profile across each cell (white lines in the inserts). (f) Immunostaining for PrP in living ACMs pre-incubated with PrP antibody prior to culture. The cardiomyocyte-removed crude fraction cells were incubated with PrP antibody, washed out, cultured in the absence of the antibody and visualized with fluorescent-labeled secondary antibody just before observation. Cultured for 2 (bar, 25 μm) or 3 (bar, 50 μm) days. (g) Double immunostaining for PrP (green) and cardiac troponin T (cTnT, red) in the cardiomyocyte-removed crude fraction cells cultured for 7 days. An ACM is identified as a PrP+cTnT+ cell in the culture. Bar, 50 μm.
Figure 2c demonstrates that PrP is strongly expressed at the plasma membrane of ACMs in both the cell body and bulge and is also present in the intracellular space to some extent. In contrast, the fluorescent signals of PrP in isolated ventricular myocytes were found to be weak and instead detected in the intracellular space (Fig. 2d). Figure 2e demonstrates representative data of the line profiles of the fluorescent intensity of the immunostained PrP across the cells in the ACM and isolated ventricular myocytes. The relative fluorescent signals of PrP in the plasma membrane in the ACMs and ventricular myocytes were calculated to be 0.61 ± 0.05 and 0.16 ± 0.02 (mean ± s.e.m., n = 5), respectively, which indicates that ~60% of the immunosignals of PrP in the ACMs were detected in the plasma membrane.
Evidence that isolated small cells grew in size and developed into large beating ACMs within several days of culture18,20 indicates the upregulation of protein synthesis during this period. We next examined whether ACMs newly synthesize PrP during culture or already express this protein since these cells are resident in the heart. The cardiomyocyte-removed crude fraction cells were therefore incubated with anti-PrP antibody prior to plating and cultured in the absence of the antibody. The PrP-antibodies bound to the cell surface were then labeled with Alexa Fluor 488-conjugated anti-mouse IgG antibody. Two days after the culture, most of the cells fluorescently labeled at the plasma membrane were rounded in shape without elongation or beating (Fig. 2f, left). At three days of culture, some of the PrP-labeled cells began to change their cell shape and start beating. The fluorescent signals of PrP were observed on punctate staining due to enlargement of the surface membrane during the culture (Fig. 2f, right). These observations thus suggest that the “native” ACMs resident in the heart endogenously express PrP specifically at the plasma membrane and also continue to produce this protein during culture.
Sorting of PrP-expressing cells (PrP+ cells) with flow cytometry also identified PrP as a surface marker for the native ACMs; however, this method is not practical at the present state due to the extremely low yield of cells preserving the ability to develop into beating ACMs (7.7 ± 1.2 cells from two hearts, n = 3) (Supplementary Fig. S1). Finally, the PrP+ cells in the culture of cardiomyocyte-removed crude fraction cells were confirmed to be ACMs based on their co-expression of cardiac specific contractile protein cardiac troponin T (cTnT) (Fig. 2g).
PrP+ cells become bulge(s) of beating ACMs
To examine how isolated ACMs develop into beating cells, the same cultured cell images were acquired every few hours. Figure 3a (72 h) demonstrates two rounded cells: one cell appeared to be stacked on the other cell that tightly adhered to the bottom of the dish. In addition, the upper cell was attached to the lower cell with a small area of contact and moved along the slope when the culture dish was tilted. Neither of these cells was observed to beat at that time. The lower cell gradually grew in size and changed its shape, similar to that observed in the typical developmental process of ACMs, whereas the upper cell stacked on the lower cell did not show further appreciable morphological changes and the two cells were detected to beat synchronously at that time (Fig. 3a, 96 h). Most components of the upper cell were then absorbed into the lower cell, so that the remaining portion of the upper cell was identified as a surface bulge (arrowhead) of the lower cell, resulting in the presence of two nuclei in this “mature” ACM (Fig. 3a, 145 h). These observations thus suggest that the fusion occurs within the same type of PrP+ cells (Fig. 3b).
Formation of bulge(s) of ACMs in culture.
(a) PC images of living ACMs cultured for 72–145 h. The cartoons show the side (left) and top (middle and right) views of the cells. Two fused cells were synchronously beating after 96 h of culture. The arrowhead indicates a bulge. Bar, 50 μm. (b) Confocal laser scanning microscopy of PrP immunostaining and DAPI staining in the cells shown in (a). Fixed at 192 h. The arrowhead indicates a bulge. Bar, 50 μm.
Figure 4a and b demonstrate the time-dependent changes in the beating ACMs cultured for 72–168 hours. The small cells were observed to migrate towards the triple-nuclear beating ACMs with a bulge (Fig. 4a, 72 and 81 h). Interestingly, both the surface bulge and fused cells were progressively absorbed into the cell body and moved within the cell to reconstruct a larger ACM (Fig. 4a, 100-168 h). Both the targeted cell and fused small cell were confirmed to express PrP at the plasma membrane (Fig. 4c), further supporting that such cell fusion occurs within the same type of PrP+ cell. If cell division of beating ACMs results in the attachment of small surface cells to the beating ACMs (Fig. 4a and b, 81 h, asterisks), two nuclei should be present near each other. However, nuclei were observed within the small surface cells, not close in place, while the beating ACM possess five nuclei in total (Fig. 4b, c), indicating that fusion rather than cell division is likely to occur in these cells. The fact that many of the multinuclear ACMs contained an odd number of nuclei also supports the concept that ACMs can fuse with each other. These observations presumably explain the formation of the surface bulges and multiple nuclei observed in the ACMs, that is, the fusion of native ACMs with each other plays a role in the formation of a larger and more complex shape of multinuclear ACMs, even though cell division may contribute to multinucleation in the early stage, immediately following isolation of the cells in the culture.
Formation of multinuclear ACMs in the culture.
(a) PC images of living beating ACMs cultured for 72–168 h. The arrow indicates the edge of the imprinted grid on the culture dish. The asterisk indicates small cells migrating towards beating ACMs. The arrowhead indicates a bulge. Bar, 50 μm. (b) Light microscopic images of ACMs at 81, 100 and 168 h shown in (a) with higher magnification. The cartoons show the top views of the cells. The asterisk indicates small cells migrating towards beating ACMs. The arrowhead indicates a bulge. Bar, 50 μm. (c). Confocal laser scanning microscopy of the PrP immunostaining and DAPI staining in the cells shown in (a). Fixed at 192 h. The arrows indicate nuclei. Bar, 50 μm.
The more interesting behavior of ACMs was the separation of the cells (Fig. 5 and Supplementary Fig. S2). Two ACMs connected at the edge of the cell protrusion exhibiting synchronous beating migrated in different directions while tearing the plasma membrane. Following complete separation, the two cells were beating at their individual rates. These observations also indicate that each component of the cell assembly originated from different native ACMs.
Separation of two connected beating ACMs in culture.
PC images of living ACMs, cells 1 and 2, connected at the edge of the plasma membrane (arrows) and beating synchronously (126 h). The cells migrated in different directions while tearing the plasma membrane (175 h). The completely separated cells 1 and 2 were beating at individual rates (217 h). Bar, 50 μm. Images with shorter intervals are shown in Supplementary Fig. S2.
If fusion of the ACMs occurs after isolation and plating, it is expected that it would be difficult for the dispersed PrP+ cells to meet their fusion partners in the culture dish. Therefore, these cells may attach or adhere to each other, even after enzymatic digestion, suggesting that several native ACMs form clusters in the heart. On the other hand, the fact that ~24% of the ACMs contained only a single nucleus (Fig. 1d) indicates that some of the native ACMs may also exist as solitary single cells.
Presence of PrP+cTnT+ cells in mouse cardiac ventricles
We next examined when PrP-positive ACMs start to express cTnT in cultures of cardiomyocyte-removed crude fraction cells. As shown in Fig. 6a, some of the solitary and clustered cells cultured on day 1 (< ~20 h) were observed to express both PrP and cTnT, although these cells remained small and rounded in shape and no morphological characteristics were yet observed in the ACMs. The PrP- and cTnT-co-expressing cells cultured for two to three days gradually began to change in shape, exhibiting a peculiar morphology. These observations that PrP-positive ACMs express cTnT, at least when the cells adhere to the bottom of the dish, suggest the possibility that PrP-expressing native ACMs already express cTnT when the cells are resident in the heart.
Immunostaining for PrP and cTnT in the early culture of ACMs and the cardiac ventricles in mouse.
(a) Confocal laser scanning microscopy of PrP (green) and cTnT (red) double immunostaining, DAPI staining (blue) and DIC images of cardiac myocyte-removed crude fraction cells cultured for one (< ~20 h), two or three days. The arrowheads indicate PrP+cTnT+ cells. Bar, 50 μm. (b, c) Double immunostaining for PrP (green) and cTnT (red) in the mouse cardiac ventricular tissues. Representative images show PrP+cTnT+ small cells (arrowheads) as either clustered (b) or solitary (c) states. Bar, 10 μm. (d) Double immunostaining for PrP (green) and neuronal cell marker neurofilament light subunit (Nf-L, red). String-shaped PrP+ cells (or part of the cell) were found to co-express Nf-L. The arrow indicates PrP+Nf-L+ cells. Bar, 10 μm.
To examine the localization of the native ACMs in the heart, immunohistochemical analyses were performed using a combination of PrP and cTnT as marker proteins. Small PrP+ cells co-expressing cTnT were detected in the interstitial space among cardiomyocytes in mouse cardiac ventricular tissue, thus indicating the presence of native ACMs in the heart (Fig. 6b, c and Supplementary Fig. S3). The PrP+cTnT+ cells were found to exist as either clustered (Fig. 6b and Supplementary Fig. S3a, b) or solitary (Fig. 6c and Supplementary Fig. S3c, d) cells. Depending on the orientation of cutting of the tissue sections, the clustered PrP+cTnT+ cells were observed to be rounded in shape and located in spaces between myocyte bundles (DIC images of Fig. 6b and Supplementary Fig. S3a, b), while the solitary single cells were packed into narrower spaces adjacent to ventricular myocytes (DIC images of Fig. 6c and Supplementary Fig. S3c, d) with some located along the plasma membrane in the longitudinal direction of cardiomyocytes (Fig. 6c). Although the epicardium has been reported to contribute to the formation of the cardiomyocyte lineages26,27, PrP+cTnT+ cells were not specifically observed in the epicardium or endocardium, but rather detected in various places in the myocardium.
Since it is well known that PrP is expressed in neurons23,24, double-immunostaining for PrP and the neuronal cell marker neurofilament light subunit (Nf-L) was performed. Long and slender PrP+ cells crawling across cardiomyocytes were found to co-express Nf-L (Fig. 6d), indicating that these string-shaped PrP+ cells in this experiment were thought to be neurons.
We next examined whether the PrP+cTnT+ cells detected in the mouse heart express cardiac myocyte transcription factor Nkx2.5, a conserved homeobox gene expressed starting in the cardiac crescent stage of heart development and maintained in the cardiomyocyte lineage throughout the lifespan28. Consequently, immunostaining analyses demonstrated that most of the interstitial PrP+cTnT+ cells co-expressed Nkx2.5 in the nuclear area, although the strength of the signals varied (Fig. 7a, b, arrowheads). In contrast, immunofluorescent signals of Nkx2.5 were not detected in the interstitial cells without an expression of PrP or cTnT (Fig. 7a, b, arrows). We further examined whether the PrP+cTnT+ cells in the mouse heart express the fibroblast marker ER-TR7 (TR7)29. Accordingly, neither PrP+cTnT+ interstitial cells with a small rounded shaped (Fig. 7c, arrowhead) nor elongated fibroblast-like cells (Fig. 7d, arrowhead) were observed to co-express TR7. Meanwhile, in the culture of the cardiac myocyte-removed crude fraction cells, immunofluorescent signals of TR7 were observed in the cTnT-negative cells (Fig. 7e, arrow), but not cTnT-positive ACMs (Fig. 7e, arrowheads). There are some limitations in directly identifying native ACMs in cardiac tissues, as ACMs can be identified based on their features of a peculiar morphology and spontaneous beating in cultures of crude fraction cells. However, in the present state, the interstitial PrP+cTnT+ cells observed in the cardiac ventricle in the mouse heart are thought to most likely be native ACMs.
Immunostaining for PrP, cTnT and Nkx2.5 or TR7 in the mouse cardiac ventricles.
(a, b) Confocal laser scanning microscopy of triple immunostaining for PrP (green), cTnT (red) and Nkx2.5 (blue) in the mouse cardiac ventricular tissues. Panels labeled with Nkx2.5 and Merged are merged with DIC images. The arrowhead and arrow indicate PrP+cTnT+Nkx2.5+ and PrP−cTnT−Nkx2.5− cells, respectively. Bar, 10 μm. (c, d) Triple immunostaining for PrP (green), cTnT (red) and TR-7 (blue) in the mouse cardiac ventricular tissues. Panels for TR7 and Merged are merged with DIC images. The arrowheads indicate PrP+cTnT+TR7− cells. Bar, 10 μm. (e) Double immunostaining for ACTN (green) and TR7 (red), DAPI staining (blue) and DIC images of ACMs cultured for 6 days. A merged image is shown. The arrowhead and arrow indicate ACTN+TR7− ACMs and ACTN−TR7+ cells, respectively. Bar, 50 μm.
Presence of PrP+cTnT+ cells in human cardiac ventricles
We next examined whether native ACMs are resident in the normal adult human heart using immunostaining for PrP and cTnT. Figure 8a demonstrates the specificity of the monoclonal anti-PrP antibody (Bertin Pharma Co. clone SAF54) in human tissues using lymphocytes and segmented neutrophils (mature granulocytes) as PrP-positive and -negative controls, respectively. Consistent with the previous literature24, immunofluorescent signals of PrP were strongly observed in the lymphocytes (Fig. 8a), but not segmented neutrophils (Fig. 8a, arrows), in the splenic white pulp. These data thus suggest that the anti-PrP antibody (clone SAF54) used in the present experiments recognizes PrP in human tissues.
Presence of PrP+cTnT+ cells in the human cardiac ventricles.
(a) Specificity of the anti-PrP antibody for the human tissues. Confocal laser scanning microscopy of PrP immunostaining (green), DAPI staining (blue) and DIC images of the human spleen. PrP was expressed in the lymphocytes, but not segmented neutrophils (arrows), in the human splenic white pulp. Bar, 25 μm. Enlarged image of a segmented neutrophil without an expression of PrP marked by a square is shown in the lower left of each panel (bar, 10 μm). (b–e) Double immunostaining for PrP (green) and cTnT (red), DAPI staining (blue) and DIC images of the human left cardiac ventricular tissue specimens. The healthy portions of the myocardium were selected for observation. Representative images obtained from each human heart specimen. The arrowhead indicates PrP+cTnT+ cells. Bar, 10 μm. (b) Female, 85 years; (c) Male, 70 years; (d) Female, 51 years; (e) Female, 41 years of age.
Double-immunostaining for PrP and cTnT was performed using the human heart tissue specimens obtained from four patients, 85, 70, 51 and 41 years of age, who had not directly died from heart failure. Consequently, interstitial PrP+cTnT+ cells were found in the healthy portions of the cardiac ventricles obtained from all heart tissue specimens, both males and females (Fig. 8b–e), similar to those observed in the mice hearts (Fig. 6b, c and Supplementary Fig. S3). It is noted that the PrP+cTnT+ cells were detected in the normal cardiac ventricles obtained from the aged heart tissue specimen (from patient 85 years of age) (Fig. 8b). The human PrP+cTnT+ cells were usually observed as solitary single cells (Fig. 8b–d), although they were sometimes as clustered small cells (Fig. 8e). Almost all cTnT-positive cells in the interstitial spaces in the human heart tissue specimens used in the current experiments were found to be PrP-positive. These results suggest that PrP+cTnT+ cells, presumably native ACMs, could survive for a life-long period in the human cardiac ventricles.
Discussion
In the present study, we demonstrate that PrP and cTnT-marked interstitial cells from mouse cardiac ventricles not only develop into beating cells by themselves but also fuse with each other to become larger multinuclear beating cells. We also show that these cells are present in the adult human hearts.
Since the discovery of ACMs in the culture of cardiac myocyte-removed fraction cells, the bulge(s) on the cell surface has been considered to be a morphological characteristic of these cells, in addition to their beating activity and features of dendritic shapes18,19,20. The bulge(s) are thought to be the same kind of ACM, or part of cells, due to the fact that the bulge(s) synchronously beat with the cell body, express ACTN and usually contain nuclei. The presence of bulge(s) on the cell surface of ACMs thus suggests that these cells can bud from the cell body following nuclear division or fuse with each other, which is not likely to occur in mature ventricular myocytes.
Migrating cells are characterized by an elongated cell shape or rounded amoeboid morphology and cell protrusions are a typical structure present in the majority of mesenchymal cell types that play a role in migration, differentiation and intercellular communication30,31,32. In the present study, the methylcellulose-based semisolid culture medium supplied the ACMs with an environment to allow for a high degree of heterogeneity in cellular morphology and protrusive activity (Figs. 1–5). The detection of two ACMs connected with protrusions and beating synchronously (Fig. 5, 126 h, 175 h and Supplementary Fig. S2, 126–196 h) indicates that cell protrusion plays a role in intercellular communication between these cells, although the cells are able to beat independently following complete separation (Fig. 5, 217 h and Supplementary Fig. S2, 217 h, 240 h).
There is a possibility that other types of cells differentiate and transform into native ACMs in the heart. However, a previous study19 demonstrated that the number of beating ACMs in culture is highest in neonates then significantly decreases at 5 weeks of age (~20% and ~26% of those observed in neonates and day-8 mice, respectively) and subsequently reaches a plateau in adulthood. Meanwhile, beating ACMs obtained from the aged mouse heart express fetal cardiac gene products ANP and Cav3.2, which are known to be expressed at no or very low levels in the adult cardiac ventricles under physiological conditions33,34,35,36. These data suggest that ACMs are residual cells in the fetal heart that preserve the expression of fetal cardiac gene products. Moreover, evidence showing that ACMs do not require additional hormonal or chemical stimulation for development into beating cells and PrP+cTnT+ cells resident in the heart expressing Nkx2.5 (Fig. 7a, b) supports the view that native ACMs have already stepped into the cardiomyogenic lineage.
Gap junction protein connexin 43 (Cx43)37 is abundantly expressed in beating ACMs in culture, which enables isolated ACMs to form a gap junction structure with the adjacent cells, thus augmenting intercellular electrical coupling18,19. In the present study, immunofluorescent signals of Cx43 were not detected in the PrP+cTnT+ cells with small rounded (Supplementary Fig. S4a) or elongated (Supplementary Fig. S4b) shapes in the mouse heart. Double-immunostaining with PrP and Cx43 also demonstrated that immunosignals of Cx43 were not observed in either PrP+ cells adjacent to ventricular myocytes (Supplementary Fig. S4c) existing as solitary cells (Supplementary Fig. S4d) or forming clusters (Supplementary Fig. S4e). Meanwhile, the expression of Cx43 in the ventricular myocytes was primarily observed at the cell periphery, as expected. These observations suggest that native ACMs are not likely to interact with ventricular myocytes via gap junction proteins in the heart under physiological conditions. It is therefore probable that the protein expression of Cx43 is enhanced after the start of the spontaneous development of ACMs in the culture.
PrP has recently been considered to maintain the pluripotency and/or capacity for differentiation of stem cells during embryogenesis38, including that involving neural39 and cardiogenic21 cell lineages, to adult tissues, such as hematopoietic cells22 and skeletal muscle precursor cells40. PrP+ cells sorted from the E8 pool in mouse embryos have been shown to abundantly express cardiac markers21, suggesting the possibility that the PrP+cTnT+ native ACMs detected in the heart originate from embryogenic cells of that or later stages. Mammalian cardiac tissue mostly arises from mesodermal progenitors with two distinct embryonic origins, the first heart field (FHF) and the second heart field (SHF). The left ventricle is largely derived from FHF cells and sparsely populated by SHF cells, whereas a large portion of the right ventricle is derived from SHF cells41. Recently, HCN4 has been shown to be a useful marker for isolating FHF cells from mouse cardiomyogenic cell lineages and human ES cells42,43. However, the number of beating ACMs obtained from the right and left ventricles was 33.7 ± 5.8 and 452.7 ± 36.3 (mean ± s.e.m., n = 3, cultured at 6 days), respectively, indicating that native ACMs are also resident in the right ventricles, although the number is low. Future studies will need to determine the origin of native ACMs in the fetal heart.
Evidence showing that the number of beating ACMs reaches a plateau in adulthood in the postnatal mouse heart19 and that PrP+cTnT+ cells are detected in human heart tissues derived from patients over 80 years of age (Fig. 8) suggests that native ACMs stay in the heart until advanced stages, without appreciably differentiating into normal cardiomyocytes, under physiological conditions. We usually counted ~500 beating ACMs per heart after several days of culture, which is thought to be lower than the actual number of ACMs resident in the heart. This underestimation of the cell number may due to the loss of a number of native ACMs following repeated centrifugation during the isolation procedure, as the isolated ventricular fraction contains heterogeneous small cells, including native ACMs, co-precipitated with cardiomyocytes20, and/or to the reduced ability to develop into beating cells during the preparation procedures.
It is expected that the quiescence of native ACMs under physiological conditions makes it possible for these cells to stay within the normal heart highly organized to generate rhythmic impulses to induce rhythmic contractions of cardiomyocytes. On the other hand, ACMs preserve their ability to develop into beating cells under culture conditions, even immature and incomplete cardiomyocytes. It may therefore be possible in the future to identify certain culture conditions to develop native ACMs into more mature cardiomyocytes following the complete isolation of these cells.
The trigger of starting the spontaneous development of native ACMs into beating cells remains unclear, at least with respect to the isolation of the cells and culture under three-dimensional conditions using methylcellulose-based semisolid culture medium. In the present and previous studies18,19,20, we found that sufficient isolation of native ACMs from neighboring ventricular myocytes is required to yield beating cells in culture, and, indeed, insufficient enzymatic digestion results in the failure to obtain beating ACMs from the mouse heart. In the normal mouse or human heart, PrP+cTnT+ native ACMs were usually observed as solitary or clustered cells (Figs 6 and 8 and Supplementary Fig. S3), but not multinucleated cells. These observations also suggest that the fusion or multinucleation of ACMs occurs after the isolation procedures. These data may lead to the view that the independence of native ACMs from the microenvironments due to the death of the neighboring ventricular myocytes stimulates the spontaneous development of these cells into beating cardiomyocytes under pathophysiological conditions. It would be of interest to explore the nature of ACMs in infarct areas and/or border zones in which adjacent cardiomyocytes die in diseased human hearts in the future study.
Our findings are thus considered to provide a view concerning the presence of quiescent but still functionally viable cells in the normal adult organ that originate from the fetal stage tissues.
Methods
Animal experiments
All experimental protocols were approved by the Institutional Review Board of the Shiga University of Medical Science Animal Care and Use Committee (Approval no. 2012-6-6). The methods were carried out in accordance with the approved guidelines. Adult C57BL/6J mice were purchased from Charles River Japan.
Human tissue specimens
Formalin-fixed, paraffin-embedded human heart tissue specimens were obtained from archival autopsy samples according to the guidelines of the Japan Society of Pathology. All protocols were approved by the Shiga University of Medical Science Ethics Committee (Approval no. 26–65). The methods were carried out in accordance with the approved guidelines. Informed consent for the use of tissue samples for the experiments was obtained from all subjects. Cardiac tissues fixed within 2 h after death were selected from 4 patients (1 male, 3 females, 41–85 years of age) who had died of cancer or respiratory failure.
Solutions
Tyrode solution contained (in mM): 140 NaCl, 5.4 KCl, 1.8 CaCl2, 0.5 MgCl2, 0.33 NaH2PO4, 5.5 glucose and 5.0 Hepes (pH adjusted to 7.4 with NaOH). The cell isolation buffer contained (in mM): 130 NaCl, 5.4 KCl, 0.5 MgCl2, 0.33 NaH2PO4, 22 glucose, 50 μU mL−1 bovine insulin and 25 Hepes (pH adjusted to 7.4 with NaOH). The cell isolation buffer was used to isolate both ventricular myocytes and ACMs. The cell suspension buffer contained Tyrode solution supplemented with 0.2 mg mL−1 bovine serum albumin (BSA), 100 μg mL−1 penicillin, 0.1 mg mL−1 streptomycin and 15 μg mL−1 phenol red. The semisolid culture medium consisted of an 80:20 mixture of methylcellulose-based medium MethoCult® M3231 (StemCell Technologies) and Iscove's modified Dulbecco's medium (IMDM). The final composition of the cell culture medium was 1% methylcellulose, 30% fetal bovine serum (FBS), 1% BSA, 2 mM L-glutamine and 0.1 mM 2-mercaptoethanol in IMDM.
Antibodies
The primary antibodies were: mouse monoclonal anti-prion protein (anti-PrP, Bertin Pharma Co. clone SAF8321 for mouse and SAF54 for human samples, 1:100) and anti-α-actinin (anti-ACTN, Sigma, A7811, clone EA-53, 1:400), rabbit monoclonal anti-neurofilament light subunit (anti-Nf-L, Cell Signaling, clone C28E10, 1:100), rabbit polyclonal anti-Nkx2.5 (Santa Cruz, sc-14033, 1:50)44 and anti-connexin 43 (anti-Cx43, Cell Signaling, #3512, 1:100), rat monoclonal anti-fibroblast protein (anti-ER-TR7, Santa Cruz, sc-73355, 1:100)29 and goat polyclonal anti-cardiac troponin T (anti-cTnT, Santa Cruz, sc-8121, 1:50) antibodies.
Cell preparation and culture
The method used for the isolation and culture of cardiomyocyte-removed crude fraction cells has been previously described18. Briefly, mice of 8-16 weeks of age, either males or females, with a body weight of 20–30 g were killed via the i.p. injection of an overdose of sodium pentobarbital (>300 mg kg−1) with heparin (8,000 U kg−1). The heart was quickly excised, placed in ice-chilled Tyrode solution, then cannulated via the ascending aorta and perfused in a retrograde fashion with Tyrode solution for 3 min to discharge blood, followed by the infusion of cell isolation buffer supplemented with 0.4 mM EGTA for 3 min at 37°C. The heart was then perfused with an enzyme solution containing 0.1% collagenase (Class II, Worthington Biochemical), 0.006% trypsin (Sigma), 0.006% protease (Sigma) and 0.3 mM CaCl2 in the cell isolation buffer for ~7–8 min at 37°C. Both ventricles were cut out at the level of 2/3 from the ventricular apex. The atrial tissue-free ventricular tissues were further digested with the enzyme solution, the CaCl2 level was increased to 0.7 mM. The mixture was supplemented with 2 mg mL−1 of BSA, with intermittent pipetting for ~5–6 min at 37°C and washed by centrifugation at 14 × g (300 rpm) for 3 min. The cells were then suspended in the cell isolation buffer supplemented with 1.2 mM CaCl2 and 2 mg mL−1 BSA and incubated for 10 min at 37°C and centrifuged at 14 × g for 3 min to sediment the ventricular myocyte fraction. The supernatant fraction was then filtered through a 40-μm mesh filter and further centrifuged at 150 × g (1,000 rpm) for 5 min. The pelleted cells of the final centrifugation were obtained as cardiomyocyte-removed crude fraction cells. The crude fraction cells resuspended in 600 μL cell suspension buffer were added to the semisolid culture medium at a dilution of 1:10, vortexed for 2 s to disperse the cells, plated in high-quality plastic dishes μ-Dishes (ibidi) and maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2. Beating ACMs were identified in the culture of the cardiomyocyte-removed crude fraction cells after ~3 days.
Microscopy
Phase contrast images of ACMs were acquired using an inverted microscope DIAPHOT 300 (Nikon) and a charged-coupled device camera RETIGA 2000R (QImaging) and then analyzed with the Image Pro Plus software program (MediaCybernetics). The sample size of each acquired image was 1,600 × 11,200 pixels (588 × 442 μm with a × 20 objective).
Immunostaining
For the immunocytochemical experiments, the cells cultured on μ-Dishes were fixed with 4% paraformaldehyde and permeabilized/blocked with 0.2% Triton X-100 and 10% FBS in PBS. The permeabilized cells were then labeled with antibodies and probed with an AlexaFluor® 488-or 568-conjugated secondary antibody (Molecular Probes-Invitrogen). The nuclei were stained with 2 μg mL−1 4′,6′-diamino-2-phenylindole (DAPI).
The relative fluorescent signals in the plasma membrane and cytoplasm were determined on each image based on the intensity obtained from line profiles across each cell (Fig. 2e). The fluorescent level in the plasma membrane was calculated according to the amplitude of the fluorescent intensity in the plasma membrane (Ipm) and average cytoplasmic fluorescent intensity (Icy), where, Ipm is the intensity at the edge of the cells, with a ~0.5-μm distance from the edge and Icy is the average intensity of the central 30% area (15% distance to both sides from the central mark). The relative fluorescent signal in the plasma membrane was defined as the amplitude of the fluorescent signals in the plasma membrane (Ipm) divided by the intracellular fluorescent intensity (Ipm + Icy).
For the immunohistochemical experiments, the mouse heart was perfused in a retrograde fashion at 37°C with Tyrode solution for 3 min to discharge blood and followed by cell isolation buffer for 3 min. The heart was then fixed with 4% paraformaldehyde for 3 days at 4°C and embedded in paraffin. The 3-μm paraffin sections were deparaffinized with xylene, rehydrated by a series of 100-70% ethanol, retrieved with antigen retrieving solution ImmunoSaver (Nisshin EM Co. 1:200 dilution) for 45 min at 98°C and blocked with 0.2% Triton X-100 and 10% FBS in PBS for 1 h. The sections were subsequently incubated with the primary antibody for overnight at 4°C and then probed with the secondary antibodies for 2–3 h at room temperature. The probed sections were mounted with glass cover slips in a mounting medium Vectashield (Vector Lab.).
The fluorescent signals were analyzed using a confocal laser scanning system C1si (Nikon) on an Eclipse TE2000-E inverted microscope (Nikon). Each image acquired by C1si was 1,024 × 1,024 pixels (636.5 × 636.5 μm with a × 20 objective or 318.25 × 318.25 μm with a × 40 objective) in size. The data for the mouse tissues reflect representative images obtained from three experiments and the data for the human tissues indicate representative images obtained from three paraffin sections of each specimen.
Flow cytometry and culture of the sorted cardiomyocyte-removed crude fraction cells
The mouse monoclonal anti-PrP antibody was labeled with AlexaFluor®488-conjugated goat anti-mouse IgG1 using a Zenon® mouse IgG labeling kit (Molecular Probes-Invitrogen) just before use. The crude fraction cells obtained from two hearts were collected and suspended in 200 μL of the cell suspension buffer and then stained with 1 μg AlexaFluor®488-labeled anti-PrP antibody for 30 min at 4°C. An aliquot of the cells was incubated with the same isotype as the fluorescent-labeled anti-PrP antibody under the same conditions and used as a control. The stained cells were washed and resuspended in 500 μL of cell suspension buffer, then subjected to cell sorting using a FACSAria cell sorter (Becton Dickinson) at 4°C. The flow cytometric data were analyzed using the FlowJo software package (Treestar).
The sorted cells in the P4 and P6 fractions were collected via the centrifugation and resuspended in fresh cell suspension buffer, mixed with semisolid culture medium in a 1:10 proportion and cultured in μ-Dishes.
References
Beltrami, A. P. et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114, 763–776 (2003).
Oh, H. et al. Cardiac progenitor cells from adult myocardium: homing, differentiation and fusion after infarction. Proc. Natl. Acad. Sci. USA 100, 12313–12318 (2003).
Matsuura, K. et al. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J. Biol. Chem. 279, 11384–11391 (2004).
Urbanek, K. et al. Stem cell niches in the adult mouse heart. Proc. Natl. Acad. Sci. USA 103, 9226–9231 (2006).
Tallini, Y. N. et al. c-kit expression identifies cardiovascular precursors in the neonatal heart. Proc. Natl. Acad. Sci. USA 106, 1808–1813 (2009).
Takamiya, M., Haider, K. H. & Ashraf, M. Identification and characterization of a novel multipotent sub-population of Sca-1+ cardiac progenitor cells for myocardial regeneration. PLoS One 6, e25265 (2011).
Jesty, S. A. et al. c-kit+ precursors support postinfarction myogenesis in the neonatal, but not adult, heart. Proc. Natl. Acad. Sci. USA 109, 13380–13385 (2012).
Malliaras, K. et al. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO Mol. Med. 5, 191–209 (2013).
Messina, E. et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ. Res. 95, 911–921 (2004).
Pouly, J. et al. Cardiac stem cells in the real world. J. Thorac. Cardiovasc. Surg. 135, 673–678 (2008).
Ferreira-Martins, J. et al. Spontaneous calcium oscillations regulate human cardiac progenitor cell growth. Circ. Res. 105, 764–774 (2009).
de Boer, T. P. et al. Human cardiomyocyte progenitor cell-derived cardiomyocytes display a maturated electrical phenotype. J. Mol. Cell Cardiol. 48, 254–260 (2010).
Leri, A., Kajstura, J. & Anversa, P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol. Rev. 85, 1373–1416 (2005).
Barile, L., Messina, E., Giacomello, A. & Marban, E. Endogenous cardiac stem cells. Prog. Cardiovasc. Dis. 50, 31–48 (2007).
Laflamme, M. A. & Murry, C. E. Heart regeneration. Nature 473, 326–335 (2011).
Moretti, A. et al. Multipotent embryonic isl+ progenitor cells lead to cardiac, smooth muscle and endothelial cell diversification. Cell 127, 1151–1165 (2006).
Martin, C. M. et al. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev. Biol. 265, 262–275 (2004).
Omatsu-Kanbe, M. & Matsuura, H. A novel type of self-beating cardiomyocytes in adult mouse ventricles. Biochem. Biophys. Res. Commun. 381, 361–366 (2009).
Omatsu-Kanbe, M., Yamamoto, T., Mori, Y. & Matsuura, H. Self-beating atypically shaped cardiomyocytes survive a long-term postnatal development while preserving the expression of fetal cardiac genes in mice. J. Histochem. Cytochem. 58, 543–551 (2010).
Omatsu-Kanbe, M. & Matsuura, H. Ischemic survival and constitutively active autophagy in self-beating atypically-shaped cardiomyocytes (ACMs): characterization of a new subpopulation of heart cells. J. Physiol. Sci. 63, 17–29 (2013).
Hidaka, K. et al. The cellular prion protein identifies bipotential cardiomyogenic progenitors. Circ. Res. 106, 111–119 (2010).
Liu, T. et al. Normal cellular prion protein is preferentially expressed on subpopulations of murine hemopoietic cells. J. Immunol. 166, 3733–3742 (2001).
Ford, M. J., Burton, L. J., Morris, R. J. & Hall, S. M. Selective expression of prion protein in peripheral tissues of the adult mouse. Neurosci. 113, 177–192 (2002).
Linden, R. et al. Physiology of the prion protein. Physiol. Rev. 88, 673–728 (2008).
Zomosa-Signoret, V., Arnaud, J. D., Fontes, P., Alvarez-Martinez, M. T. & Liautard, J. P. Physiological role of the cellular prion protein. Vet. Res. 39, 9 (2008).
Cai, C. L. et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature 454, 104–108 (2008).
Zhou, B. et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 454, 109–113 (2008).
Harvey, R. P. NK-2 homeobox genes and heart development. Dev. Biol. 178, 203–216 (1996).
Li, Y. et al. Clusterin induces CXCR4 expression and migration of cardiac progenitor cells. Exp. Cell Res. 316, 3435–3442 (2010).
Friedl, P. & Wolf, K. Plasticity of cell migration: a multiscale tuning model. J. Cell Biol. 188, 11–19 (2010).
Bergert, M., Chandradoss, S. D., Desai, R. A. & Paluch, E. Cell mechanics control rapid transitions between blebs and lamellipodia during migration. Proc. Natl. Acad. Sci. USA 109, 14434–14439 (2012).
Prockop, D. J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276, 71–74 (1997).
Bloch, K. D., Seidman, J. G., Naftilan, J. D., Fallon, J. T. & Seidman, C. E. Neonatal atria and ventricles secrete atrial natriuretic factor via tissue-specific secretory pathways. Cell 47, 695–702 (1986).
Cameron, V. A. & Ellmers, L. J. Natriuretic peptides during development of the fetal heart and circulation. Endocrinol. 144, 2191–2194 (2003).
Perez-Reyes, E. Molecular physiology of low-voltage-activated T-type calcium channels. Physiol. Rev. 83, 117–161 (2003).
Chiang, C. S. et al. The Cav3.2 T-type Ca2+ channel is required for pressure overload-induced cardiac hypertrophy in mice. Circ. Res. 104, 522–530 (2009).
Verheijck, E. E. et al. Electrophysiological features of the mouse sinoatrial node in relation to connexin distribution. Cardiovasc. Res. 52, 40–50 (2001).
Lopes, M. H. & Santos, T. G. Prion potency in stem cells biology. Prion 6, 142–146 (2012).
Peralta, O. A., Huckle, W. R. & Eyestone, W. H. Expression and knockdown of cellular prion protein (PrPC) in differentiating mouse embryonic stem cells. Differentiation 81, 68–77 (2011).
Stella, R., Massimino, M. L., Sandri, M., Sorgato, M. C. & Bertoli, A. Cellular prion protein promotes regeneration of adult muscle tissue. Mol. Cell Biol. 30, 4864–4876 (2010).
Srivastava, D. Making or breaking the heart: from lineage determination to morphogenesis. Cell 126, 1037–1048 (2006).
Garcia-Frigola, C., Shi, Y. & Evans, S. M. Expression of the hyperpolarization-activated cyclic nucleotide-gated cation channel HCN4 during mouse heart development. Gene Expr. Patterns 3, 777–783 (2003).
Spater, D. et al. A HCN4+ cardiomyogenic progenitor derived from the first heart field and human pluripotent stem cells. Nat. Cell Biol. 15, 1098–1106 (2013).
Nagel, S., Kaufmann, M., Drexler, H. G. & MacLeod, R. A. The cardiac homeobox gene NKX2-5 is deregulated by juxtaposition with BCL11B in pediatric T-ALL cell lines via a novel t(5;14)(q35.1;q32.2). Cancer Res. 63, 5329–5334 (2003).
Acknowledgements
We thank T. Yamamoto and Y. Mori for their help with preparing the paraffin sections and flow cytometric experiments. This work was supported by a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (25460286 to M.O.-K. and 25460287 to H.M.)
Author information
Authors and Affiliations
Contributions
M.O.-K. designed the experiments and wrote the manuscript. M.O.-K., H.M. Y.N. and N.N. performed the experiments. H.S. provided the human tissue specimens and advised on morphological study.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Supplementary Information
Movie S1
Supplementary Information
Movie S2
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Omatsu-Kanbe, M., Nishino, Y., Nozuchi, N. et al. Prion protein- and cardiac troponin T-marked interstitial cells from the adult myocardium spontaneously develop into beating cardiomyocytes. Sci Rep 4, 7301 (2014). https://doi.org/10.1038/srep07301
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07301
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.