Abstract
Members of the Toll-like receptor (TLR) family serve as pathogen sensors and participate in local autoimmune responses. This study found a correlation between glomerular injury and TLR expression by analysing BXSB/MpJ-Yaa (BXSB-Yaa) lupus model mice. In isolated glomeruli, the mRNA expression of several TLRs was higher in BXSB-Yaa mice than in healthy control BXSB mice. In particular, the expression of Tlr8 and its downstream cytokines was markedly increased. In mouse kidneys, TLR8 protein and mRNA localized to podocytes and TLR8 protein expression in the glomerulus was higher in BXSB-Yaa mice than in BXSB mice. In BXSB-Yaa mice, the glomerular levels of Tlr8 mRNA negatively correlated with the glomerular levels of podocyte functional markers (Nphs1, Nphs2, and Synpo) and positively correlated with urinary albumin levels. Furthermore, the glomerular and serum levels of miR-21, a putative microRNA ligand of TLR8, were higher in BXSB-Yaa mice than in BXSB mice. The urinary levels of Tlr8 mRNA were also higher in BXSB-Yaa mice than in BXSB mice. In conclusion, the overexpression of TLR8 correlates with the progression of podocyte injury in glomerulonephritis. Thus, altered levels of urinary Tlr8 mRNA might reflect podocyte injury.
Similar content being viewed by others
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by autoantibody production and immune complex deposition that result in tissue inflammation and damage1. SLE-related glomerulonephritis (GN), also known as lupus nephritis (LN), is one of the most common and severe complications of SLE because of the risk of cardiovascular disease and end-stage renal disease2.
NZB, (NZB × NZW) F1 hybrid, BXSB/MpJ-Yaa (BXSB-Yaa) and MRL/MpJ-lpr mice are commonly used as spontaneous SLE models3. These strains develop systemic autoimmune diseases characterized by increased serum autoantibody levels and vasculitis, in addition to GN that is similar to human LN3. Recently, we described the pathological interactions between the immune-associated genes on chromosome 1 and the genetic locus on chromosome Y in the glomerular pathogenesis of BXSB-Yaa mice4. BXSB-Yaa mice carry a genetic mutation located on the Y chromosome, namely, Y-linked autoimmune acceleration (Yaa). The severity of GN is greater in males than in females because of the Yaa mutation3,4,5,6. The Yaa mutation is a translocation from the telomeric end of the X chromosome to the Y chromosome. The duplicated segment plays a crucial role in the activation of auto-reactive B cells, thereby contributing to the Yaa-mediated enhancement of the autoimmune phenotype in male BXSB-Yaa mice7. The Yaa locus contains several immune-associated genes, including Toll-like receptor (TLR) family members7.
TLRs are expressed on the plasma membrane or intracellular vesicular membrane of hematopoietic and non-hematopoietic cells8. They have been characterized as innate immune sensors that recognize danger signals arising from pathogen-associated molecular patterns (PAMPs), including flagellin, lipopolysaccharide (LPS) and nucleic acids derived from bacteria, mycobacteria, mycoplasma, fungi and viruses8. Previous studies have identified 12 members of the TLR family in mice (TLR1–9 and TLR11–13) and 10 in humans (TLR1–10)8. When activated by their own pathogenic ligands, TLRs enhance inflammatory cytokine expression mainly through the NF-κΒ pathway to provide host defence8.
Interactions between TLRs and their endogenous ligands have been shown to play important roles in the pathogenesis of non-infectious injury9,10,11,12. Mersmann et al. have suggested that endogenous high-mobility group box 1 (HMGB1) contributes to myocardial injury through the activation of TLR2 signalling11. Shichita et al. have demonstrated a pathological interaction between endogenous peroxiredoxin and TLR2 or TLR4 on macrophages in ischemic brain injury12. Endogenous TLR ligands are called danger-associated molecular patterns (DAMPs) and are thought to be danger signals that relay the presence of tissue injury to immune cells or local intrinsic cells, thereby inducing local tissue inflammation and damage9,10,11,12.
Experimental and clinical studies have shown that TLRs expressed in intrinsic renal cells are involved in the pathogenesis of several kidney diseases13,14,15,16,17,18. In particular, TLR4, TLR5 and TLR11 in tubular epithelial cells play an important role in the pathogenesis of urinary tract infections and sepsis-induced renal failure14,15,16. The activation of TLR2 or TLR4 by DAMPs in tubular epithelial cells contributes to the progression of kidney ischemia-reperfusion injury and subsequent renal fibrosis17,18. This suggests that activation of the TLR signalling pathway plays a crucial role in renal tubulointerstitial injury in various pathological conditions. However, little is known about the involvement of TLRs in glomerular diseases. In the present study, we focused on LN and found marked upregulation of Tlr8 and its downstream cytokines in the glomeruli of BXSB-Yaa mice.
Results
Clinical parameters of BXSB-Yaa mice
With regard to the clinical index of the systemic autoimmune condition, serum anti-double-strand DNA (dsDNA) antibody levels were higher in BXSB-Yaa mice than in BXSB/MpJ-Yaa+ (BXSB) mice at 2 and 4 months of age (Table 1). There were no differences in the indices of renal function, including serum blood urea nitrogen (sBUN) and serum creatinine (sCre), between the strains at any age. However, urinary albumin-to-creatinine ratio (uACR) levels, which serve as an index of glomerular dysfunction, were higher in BXSB-Yaa mice than in BXSB mice at 4 months of age (Table 1).
Glomerular histopathology in BXSB-Yaa mice
Glomerular histopathology was examined in kidney sections stained with periodic acid-Schiff (PAS) (Fig. 1a–d) or periodic acid methenamine silver (PAM) (Fig. 1e–h) at 2 and 4 months of age. No glomerular lesions were observed at any age in BXSB mice (Fig. 1 a, b, e and f) or at 2 months in BXSB-Yaa mice (Fig. 1c and g). In contrast, at 4 months of age, BXSB-Yaa mice developed GN, which was characterized by glomerular hypertrophy, increases in mesangial cell number and the mesangial matrix, thickening of the glomerular basement membrane (GBM) and spike-like structures on the GBM (Fig. 1d and h).
Glomerular histopathology of BXSB-Yaa mice.
(a–d) Histopathology of glomeruli in periodic acid-Schiff (PAS)-stained sections from BXSB and BXSB-Yaa mice. In BXSB mice (a and b), there are no histological differences at the ages of 2 and 4 months. In BXSB-Yaa mice (c and d), mesangial matrix expansion and mesangial cell proliferation are clearly observed at 4 months, but not at 2 months. (e–h) Histopathology of glomeruli in periodic acid methenamine silver (PAM)-stained sections from BXSB and BXSB-Yaa mice. In BXSB mice (e and f), there are no histological differences at 2 and 4 months. In BXSB-Yaa mice (g and h), glomerular hypertrophy, wrinkling of the glomerular basement membrane (GBM) and spike-like structures of the GBM (inset, arrows) are clearly observed at 4 months (h). Bars = 50 μm.
Glomerular expression of TLR family members and activation of TLR-mediated signalling in BXSB-Yaa mice
To determine which TLR members are associated with GN pathogenesis, we first examined the expression of 12 TLR family genes in the isolated glomeruli of BXSB-Yaa mice at 4 months of age (Fig. 2a). The glomerular expression of Tlr1, 2, 7, 8, 9 and 13 was higher in BXSB-Yaa mice than in BXSB mice. In particular, Tlr8 expression increased markedly (108-fold, P < 0.001). Semi-quantitative RT-PCR analysis (Fig. 2b) showed that glomerular Tlr8 expression was higher in BXSB-Yaa mice than in BXSB mice at 2 and 4 months of age and that the Tlr8 band intensity was stronger at 4 months than at 2 months in the glomeruli of BXSB-Yaa mice. An increase in glomerular Tlr8 expression was also observed in B6.MRLc1(68-81) mice, which comprise an autoimmune GN model that we established previously19 (Supplementary Fig. 1). Because of these findings, we focused on TLR8 in subsequent analyses.
mRNA expression of TLR family members and their downstream factors in the glomeruli of BXSB-Yaa mice.
(a) Relative mRNA expression of TLR family genes in isolated glomeruli from BXSB-Yaa and BXSB mice at 4 months. The expression levels were normalized to the levels of Actb. Values are the mean ± s.e. *, significantly different from control BXSB mice (Mann-Whitney U-test, P < 0.05); n ≥ 4. (b) RT-PCR analysis of Tlr8 and Actb mRNA expression in the glomeruli and spleen of BXSB and BXSB-Yaa mice at 4 months of age. n = 2. (c) Relative mRNA expression of Nfkb, Il1b, Il6, Tgfb, Tnfa and Ifnb1 in isolated glomeruli from BXSB-Yaa and BXSB mice at 4 months of age. The expression levels were normalized to the levels of Actb. The values are the mean ± s.e. *, significantly different from control BXSB-Yaa mice (Mann-Whitney U-test, P < 0.05); n ≥ 4.
Figure 2c shows the glomerular expression of inflammatory mediators induced by the activation of TLRs13. The glomerular expression of inflammatory cytokines in the NF-κB pathway, including interleukin 1 beta (Il1b), Il6 and tumour necrosis factor (Tnfa), was higher in BXSB-Yaa mice than in BXSB mice at 4 months of age. In contrast, there were no differences between the strains in the expression of Nfkb, transforming growth factor beta (Tgfb) and interferon beta 1 (Ifnb1).
Localization of TLR8 in mouse and human kidneys
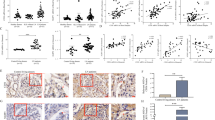
In immunofluorescence analysis, synaptopodin, a podocyte marker, was detected in the podocyte regions of all examined mice (Figs. 3a, d and g). However, synaptopodin immunoreactivity was weaker in BXSB-Yaa mice (Fig. 3d) than in BXSB and C57BL/6 mice (Fig. 3a and g) at 4 months of age. TLR8 was observed along the glomerular capillary rete, especially in podocyte regions (Fig. 3b, e and h). The immunoreactivity was stronger in BXSB-Yaa mice (Fig. 3e) than in the other two strains (Fig. 3b and h) at 4 months of age. TLR8 co-localized with synaptopodin in the glomeruli of all examined strains (Fig. 3c, f and i). Although synaptopodin positivity was lower in BXSB-Yaa mice (Fig. 3d), we still detected co-localization of synaptopodin with TLR8 (Fig. 3f). As observed in mice, TLR8 co-localized with synaptopodin in healthy human kidneys (Fig. 3j, k and l).
Localization of TLR8 protein and mRNA in the kidneys of mice and humans.
(a–l) Immunofluorescence of synaptopodin (a, d, g and j) and TLR8 (b, e, h and k) in the glomeruli of BXSB (a–c), BXSB-Yaa (d–f) and C57BL/6 (g–i) mice and humans (j–l). Merged images are shown (c), (f), (i) and (l). Synaptopodin immunoreactivity (green) co-localized with that of TLR8 (red) (c, f, i and l). TLR8 positivity in BXSB-Yaa mice (e) is stronger than that in BXSB (b) and C57BL/6 (h) mice. In the human glomerulus (j–l), TLR8 immunoreactivity co-localizes with synaptopodin immunoreactivity, as in the mouse glomerulus. (m and n) In situ hybridization for Tlr8 mRNA. Positive reactions are observed in the glomeruli of BXSB-Yaa cells, especially in podocyte regions (n, arrow). In contrast, no positive reaction is observed in the BXSB glomerulus (m). Bars = 50 μm.
In in situ hybridization analysis of Tlr8 mRNA, signal was not detected in the glomeruli of BXSB mice (Fig. 3m). In contrast, signal localized to the podocyte region in the glomeruli of BXSB-Yaa mice (Fig. 3n).
Correlation between podocyte injury and Tlr8 mRNA expression in BXSB-Yaa mice
The correlations between indices of podocyte injury and Tlr8 mRNA expression in isolated glomeruli were analysed in BXSB-Yaa mice at 4 months of age (Table 2). Glomerular Tlr8 mRNA levels positively correlated with uACR levels, a functional index of glomerular injury, in BXSB-Yaa mice (Spearman's test, P < 0.01). Furthermore, glomerular Tlr8 mRNA levels negatively correlated with the glomerular mRNA levels of podocyte functional markers, including nephrin (Nphs1), podocin (Nphs2) and synaptopodin (Synpo), in BXSB-Yaa mice.
Glomerular and serum levels of a putative endogenous ligand of TLR8 in BXSB-Yaa mice
A recent study reported that microRNAs, particularly miR-21, act as ligands for TLR820. We next examined the glomerular and serum levels of miR-21. miR-21 levels were higher in BXSB-Yaa mice than in BXSB mice at 4 months of age (Fig. 4).
Glomerular and serum levels of miR-21.
The relative glomerular and serum expression of miR-21 in BXSB and BXSB-Yaa mice at 4 months of age. Values are the mean ± s.e. Data are presented as the fold increase vs. BXSB in the same samples. *, significantly different from control BXSB mice (Mann-Whitney U-test, P < 0.05); n = 3.
Detection of Tlr8 mRNA in the urine of BXSB-Yaa mice
The urinary Tlr8 mRNA levels of BXSB-Yaa mice and control mice were determined (Fig. 5). The urinary Tlr8 levels were higher in BXSB-Yaa mice than in BXSB mice at 4 months of age. On the other hand, we detected no difference between serum Tlr8 levels in BXSB-Yaa mice and BXSB mice (Supplementary Fig. 2).
Discussion
Among glomerular cells, TLRs are expressed by mesangial cells (TLR2–4), endothelial cells (TLR2, 4, 9) and podocytes (TLR1–6, 8, 9)13,21. Pawar et al. have demonstrated that the administration of poly (I:C), the ligand for TLR3, aggravates autoimmune GN in MRL/MpJ-lpr mice, whereas this ligand does not alter anti-DNA autoantibody levels and does not induce B cell activation22. According to Fu et al., anti-GBM antibody-treated mice develop mild GN, but when the treatment is coupled with specific TLR ligands, including peptidoglycan (TLR2), poly (I:C) (TLR3), LPS (TLR4), or flagellin (TLR5), the treated mice developed GN of greater severity associated with the activation of the NF-κΒ pathway23. Thus, several in vivo studies suggest that TLRs and their exogenous ligands have pathogenic roles in GN13,22,23.
In the present study, we demonstrated that TLRs, including Tlr1, 2, 7, 8, 9, and 13 and their downstream factors (Il1b, Il6, and Tnfa) were upregulated through the NF-κΒ pathway in the glomeruli of autoimmune GN mice. From these findings, we concluded that the TLR-mediated NF-κΒ pathway plays an important role in the pathogenesis of autoimmune GN. Importantly, the mRNA expression of TLR family members was induced by major cytokines such as interferon gamma (IFNγ) and TNFα in both inflammatory cells and tissue-intrinsic cells17,24. Experimental and clinical studies have shown that IFNγ and TNFα are upregulated in the serum and kidneys of SLE patients and SLE-prone mice25,26. Indeed, we detected higher levels of glomerular Tnfa and Ifng in BXSB-Yaa mice than in control mice (Fig. 2c). Collectively, these results indicate that local cytokines increased local Tlr8 expression, especially in podocytes. A previous study showed that Tlr8 is broadly expressed on myeloid dendritic cells, monocytes, differentiated macrophages and CD4+ regulatory T cells27. Podocytes might have differential sensitivity to Tlr8-inducing cytokines when compared to immunocompetent cells and this might contribute to the overexpression of glomerular Tlr8. Thereby, Tlr8 overexpression in podocytes would enhance the cells' responsiveness to their own ligands. These processes might aggravate the pathological conditions of autoimmune GN.
In a previous study, we found that the BXSB-type genome causes SLE-like symptoms and subsequent GN without involvement of Yaa and that Yaa accelerates disease progression4. Recently, the Yaa mutation was characterized as a translocation from the telomeric end of the X chromosome to the Y chromosome7. The duplicated segment contains at least 19 genes, including Tlr7 and Tlr87. We observed local overexpression of Tlr7 and Tlr8 in BXSB-Yaa glomeruli; expression of Tlr8 was markedly increased. Importantly, TLR8 mRNA and protein localized to podocytes in BXSB-Yaa mice. Furthermore, the B6.MRLc1(68-81) lupus-prone strain also showed increased glomerular Tlr8 expression with age. These results suggest that the Yaa mutation in addition to the autoimmunity-prone genetic background caused glomerular TLR8 overexpression in BXSB-Yaa mice. Although Gurkan et al. have reported that Tlr8 mRNA is expressed in a mouse immortalized podocyte cell line21, our present study demonstrates for the first time that TLR8 protein is expressed by podocytes in vivo.
In contrast to TLR8, TLR7 is mainly expressed on infiltrating inflammatory cells, not on renal intrinsic cells, under physiological and pathological conditions13. In addition to TLR8, podocytes reportedly express several other members of the TLR family, as described above and recent studies have indicated a pathological correlation between the TLR-mediated NF-κB pathway in podocytes and podocyte injury in vitro28,29. Banas et al. have shown that TLR4 in podocytes interacts with the innate immune system to mediate glomerular injury28. Moreover, Machida et al. have suggested that TLR9 expression in podocytes is associated with glomerular disease in vivo29. Because of their unique localization in the glomerulus, podocytes are continuously exposed to various plasma solutes containing TLR ligands such as PAMPs and DAMPs. Therefore, podocytes might contribute to renal immunosurveillance by the TLR-mediated immune system.
TLR8 localizes in the endosomal membrane and recognizes single-stranded RNA and short, double-stranded RNA from microbial organisms, leading to the production of a variety of NF-κB-mediated cytokines8. Several studies have shown that TLR8 also recognizes endogenous miRNAs8. Although previous studies have demonstrated that secreted miRNAs, which are generally secreted within exosomes, can regulate gene expression in recipient cells via canonical binding to their target mRNAs30, Fabbri et al. have shown that exosomal miR-21 and miR-29a can function as ligands for TLR820. SLE patients show elevated serum levels of miRNAs, including miR-211,31. Furthermore, Pan et al. have shown that miR-21 is overexpressed in CD4+ T cells from patients with lupus and MRL/MpJ-lpr mice32. In the present study, we demonstrated that serum and glomerular miR-21 is overexpressed in autoimmune GN models. These findings indicate that an NF-κB-mediated pathway initiated by the interaction between TLR8 and endogenous ligands, including miR-21, correlates with the pathogenesis of autoimmune GN.
We also found that the glomerular expression of Tlr8 correlated with the expression of podocyte functional markers and with uACR. These results suggest that the TLR8-mediated pathway closely correlates with podocyte injury. IL-1β, one of the most important cytokines in the NF-κB pathway, is involved in kidney injury; in the glomerulus, IL-1β is mainly produced by podocytes33,34. Furthermore, recent studies have shown that various inflammatory factors, including IL-1β, induce podocyte injury by reducing the production of podocyte functional markers, especially nephrin35,36. We found a strong correlation between the glomerular expression of TLR8-mediated cytokines, including Il1b and the expression of podocyte functional markers (Supplementary Table 1). Furthermore, our previous study showed that T cells and B cells infiltrate the BXSB-Yaa glomerulus as the disease progresses6. These findings indicate that factors downstream of TLR8, such as IL-1β, directly contribute to podocyte injury and promote the glomerular recruitment of leukocytes in autoimmune GN pathogenesis.
The urinary expression of Tlr8 mRNA was higher in BXSB-Yaa mice than in control mice. A recent study has suggested that glomerular injury in proteinuric renal diseases is strongly associated with the effacement of podocytes caused by disruption of foot processes and/or the slit diaphragm; podocyte mRNA is detected in the urine of patients with renal disease37. We observed TLR8 expression in human and murine podocytes. Therefore, altered urinary levels of Tlr8 mRNA might indicate podocyte injury in autoimmune GN.
In conclusion, we showed that members of the TLR family and the TLR8-mediated pathway in particular correlate with podocyte injury in murine autoimmune GN, suggesting that TLR8 is a novel therapeutic and diagnostic target for mouse and human glomerular diseases.
Methods
Ethics statement
All animal experiments was approved by the Institutional Animal Care and Use Committee, which convenes at the Graduate School of Veterinary Medicine, Hokkaido University (approval No. 13-0032). The investigators adhered to the Guide for the Care and Use of Laboratory Animals of Hokkaido University, Graduate School of Veterinary Medicine (approved by the Association for the Assessment and Accreditation of Laboratory Animal Care International).
Animals
Male BXSB-Yaa mice and BXSB mice, which carry the C57BL/6-type Y chromosome on a BXSB-Yaa background, were purchased from Japan SLC Inc. (Shizuoka, Japan) and assigned to an autoimmune GN model group or a healthy control group at 2–4 months of age. All mice were maintained under specific pathogen-free conditions. The animals were anesthetized (60 mg/kg pentobarbital sodium, administered intraperitoneally) and urine was collected by bladder puncture. After urine collection, the mice were euthanized by exsanguination from the carotid artery and the serum, kidneys and spleen were collected.
Sample preparation
The kidneys were fixed in 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB; pH 7.4) at 4°C for histopathological analysis. PFA-fixed paraffin sections (2-μm-thick) were then prepared and used for PAS staining, PAM staining, or immunofluorescence. For in situ hybridization, a portion of the kidneys was embedded in Tissue-Tek OCT Compound (Sakura Finetechnical, Tokyo, Japan). The splenic tissue was stored in RNAlater solution (Life Technologies, Carlsbad, CA, USA) for total RNA isolation.
Human samples
Normal kidney tissues from autopsied humans without renal disease were obtained from KAC Inc. (Tokyo, Japan). The kidney samples were used for immunofluorescence analysis of TLR8.
Glomerular isolation
Murine glomeruli were isolated as previously described6. Briefly, 40 mL of Hank's balanced salt solution (HBSS) containing 8 × 107 Dynabeads (Life Technologies) was perfused from the left ventricle. The kidneys were removed and digested with collagenase A (1 mg/mL; Roche, Basel, Switzerland) and deoxyribonuclease I (100 U/mL; Life Technologies) in HBSS at 37°C for 30 min. The digested tissue was gently pressed through a 100-μm cell strainer (BD Falcon, Franklin Lakes, NJ, USA) using a flattened pestle and the cell suspension was centrifuged at 200 × g for 5 min. The cell pellet was resuspended in 2 mL of HBSS. Finally, glomeruli containing Dynabeads were collected using a magnetic particle concentrator (Life Technologies). The collected glomeruli were used for total RNA isolation.
Serological and urinary analysis
To evaluate the systemic autoimmune condition, serum levels of anti-dsDNA antibody were measured using the mouse anti-dsDNA Ig (Total A+G+M) ELISA kit (Alpha Diagnostic International, San Antonio, TX, USA). To evaluate renal function, sBUN and sCre levels in all animals were measured using a Fuji Dri-Chem 7000v instrument (Fujifilm, Tokyo, Japan). The uACR was determined using Albuwell M and the Creatinine Companion assay (Exocell, Philadelphia, PA, USA).
In situ hybridization
cRNA probes for Tlr8 were synthesized in the presence of digoxigenin (DIG)-labelled UTP using a DIG RNA Labelling Kit in accordance with the manufacturer's protocol (Roche Diagnostics, Mannheim, Germany). The primer pairs for each probe are shown in Table 3 (product size: 758 bp). Cryosections (6 μm) were treated with acetylation solution and digested with proteinase K. The sections were incubated with a prehybridization solution and then with a hybridization buffer containing 50% formamide, 10 mM Tris-HCl (pH 7.6), 200 mg/mL RNA, 1× Denhardt's solution (0.02% bovine serum albumin, 0.02% polyvinylpyrrolidone and 0.02% Ficoll PM400; Sigma-Aldrich, St. Louis, MO, USA), 10% dextran sulphate, 600 mM NaCl, 0.25% SDS, 1 mM EDTA (pH 8.0) and a sense or antisense RNA probe (final concentration, 0.2 μg/mL) for 24 h at 58°C. After washes in saline sodium citrate buffer, the sections were then incubated with 0.2% polyclonal sheep anti-digoxigenin Fab fragments conjugated to alkaline phosphatase (1: 400; Nucleic Acid Detection Kit, Roche Diagnostics) for 24 h at room temperature. The signal was detected by incubating the sections with a colour substrate solution (Roche Diagnostics) containing nitro blue tetrazolium/X-phosphate in a solution composed of 100 mM Tris-HCl (pH 9.5), 100 mM NaCl and 50 mM MgCl2 in a dark room overnight at room temperature.
Immunofluorescence
In deparaffinized sections, antigen retrieval was performed in 10 mM citrate buffer at 105°C for 20 min. The sections were washed and blocked with 5% normal donkey serum for 60 min at room temperature. The sections were then incubated with rabbit polyclonal antibodies for TLR8 (1:1000; Abcam, Cambridge, UK) and mouse monoclonal antibodies for synaptopodin (1:50; Fitzgerald, Acton, MA, USA) overnight at 4°C. After washes in PBS, the sections were incubated with Alexa Fluor 546-labelled donkey anti-rabbit IgG antibodies (1:500; Life Technologies) and Alexa Fluor 488-labelled donkey anti-mouse IgG antibodies (1:500; Life Technologies) for 30 min at room temperature and then washed again. For nuclear staining, the sections were incubated with Hoechst 33342 (1:2000; Dojindo, Kumamoto, Japan) for 5 min and examined using a fluorescence microscope (BZ-9000; Keyence, Osaka, Japan).
Reverse transcription and real-time PCR
mRNA expression was analysed as previously described6. Briefly, total RNA was isolated from the glomeruli, spleen and urine using an RNeasy kit (Qiagen, Hilden, Germany). cDNA was synthesized from total RNA by reverse transcription (RT) using the ReverTra Ace reverse transcriptase enzyme (Toyobo, Osaka, Japan) and random dT primers (Promega). cDNA was used in real-time PCR with Brilliant III SYBR Green QPCR master mix and Mx3000P (Agilent Technologies, La Jolla, CA, USA). Gene expression in the glomeruli and spleen was normalized to the expression of actin, beta (Actb). The primer pairs are shown in Table 3.
RT- and TaqMan-based real-time PCR
MicroRNA (miRNA) expression was analysed as described previously19. Briefly, total RNA including miRNA in the glomeruli and serum was isolated using an miRNeasy kit (Qiagen). Total RNA was reverse-transcribed using miRNA-specific stem-loop RT primers, reverse transcriptase, RT buffer, dNTPs and RNase inhibitor according to the manufacturer's instructions (Applied Biosystems, Foster City, CA, USA). Real-time PCR was performed with the resulting cDNA using miR-21-specific TaqMan primers with specific probes (Applied Biosystems), a TaqMan Universal PCR Master Mix (Applied Biosystems) and Mx3000P (Agilent Technologies).
Statistical analysis
The results were expressed as the mean ± standard error (s.e.) and were statistically analysed using a nonparametric Mann-Whitney U-test or Welch's t-test (P < 0.05). The correlation between two parameters was analysed using Spearman's rank correlation test (P < 0.05).
References
Ahearn, J. M., Liu, C. C., Kao, A. H. & Manzi, S. Biomarkers for systemic lupus erythematosus. Transl Res 159, 326–342 (2012).
Lech, M. & Anders, H. J. The pathogenesis of lupus nephritis. J Am Soc Nephrol 24, 1357–1366 (2013).
Santiago-Raber, M. L., Laporte, C., Reininger, L. & Izui, S. Genetic basis of murine lupus. Autoimmun Rev 3, 33–39 (2004).
Kimura, J. et al. BXSB-type genome causes murine autoimmune glomerulonephritis; pathological correlation between telomeric region of chromosome 1 and Yaa. Genes Immun 15, 182–189 (2014).
Kimura, J. et al. Quantitative and qualitative urinary cellular patterns correlate with progression of murine glomerulonephritis. PLoS One 31, e16472 (2011).
Kimura, J. et al. Close relations between podocyte injuries and membranous proliferative glomerulonephritis in autoimmune murine models. Am J Nephrol 38, 27–38 (2013).
Subramanian, S. et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci U S A 103, 9970–9975 (2006).
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11, 373–384 (2010).
Baccala, R. et al. Sensors of the innate immune system: their mode of action. Nat Rev Rheumatol 5, 448–456 (2009).
Garraud, O. & Cognasse, F. Platelet Toll-like receptor expression: the link between “danger” ligands and inflammation. Inflamm Allergy Drug Targets 9, 322–333 (2010).
Mersmann, J. et al. Attenuation of myocardial injury by HMGB1 blockade during ischemia/reperfusion is Toll-like receptor 2-dependent. Mediators Inflamm 2013, 174168 (2013).
Shichita, T. et al. Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat Med 18, 911–917 (2012).
Eleftheriadis, T., Pissas, G., Liakopoulos, V., Stefanidis, I. & Lawson, B. R. Toll-like receptors and their role in renal pathologies. Inflamm Allergy Drug Targets 11, 464–477 (2012).
Chassin, C. et al. TLR4 facilitates translocation of bacteria across renal collecting duct cells. J Am Soc Nephrol 19, 2364–2374 (2008).
Andersen-Nissen, E. et al. Cutting edge: Tlr5-/- mice are more susceptible to Escherichia coli urinary tract infection. J Immunol 178, 4717–4720 (2007).
Zhang, D. et al. A toll-like receptor that prevents infection by uropathogenic bacteria. Science 303, 1522–1526 (2004).
Wolfs, T. G. et al. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-alpha mediated up-regulation during inflammation. J Immunol 168, 1286–1293 (2002).
Shigeoka, A. A. et al. TLR2 is constitutively expressed within the kidney and participates in ischemic renal injury through both MyD88-dependent and -independent pathways. J Immunol 178, 6252–6258 (2007).
Ichii, O. et al. Altered expression of microRNA miR-146a correlates with the development of chronic renal inflammation. Kidney Int 81, 280–292 (2012).
Fabbri, M. et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A 109, E2110–2116 (2012).
Gurkan, S. et al. Inhibition of type I interferon signalling prevents TLR ligand-mediated proteinuria. J Pathol 231, 248–256 (2013).
Pawar, R. D. et al. Ligands to nucleic acid-specific toll-like receptors and the onset of lupus nephritis. J Am Soc Nephrol 17, 3365–3373 (2006).
Fu, Y. et al. Innate stimuli accentuate end-organ damage by nephrotoxic antibodies via Fc receptor and TLR stimulation and IL-1/TNF-alpha production. J Immunol 176, 632–639 (2006).
Muzio, M. et al. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol 164, 5998–6004 (2000).
Choubey, D. Interferon-inducible Ifi200-family genes as modifiers of lupus susceptibility. Immunol Lett 147, 10–17 (2012).
Osnes, L. T., Nakken, B., Bodolay, E. & Szodoray, P. Assessment of intracellular cytokines and regulatory cells in patients with autoimmune diseases and primary immunodeficiencies - novel tool for diagnostics and patient follow-up. Autoimmun Rev 12, 967–971 (2013).
Peng, G. et al. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science 309, 1380–1384 (2005).
Banas, M. C. et al. TLR4 links podocytes with the innate immune system to mediate glomerular injury. J Am Soc Nephrol 19, 704–713 (2008).
Machida, H. et al. Expression of Toll-like receptor 9 in renal podocytes in childhood-onset active and inactive lupus nephritis. Nephrol Dial Transplant 25, 2530–2537 (2010).
Bartel, D. P. MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233 (2009).
Ceribelli, A. et al. MicroRNAs in systemic rheumatic diseases. Arthritis Res Ther 13, 229 (2011).
Pan, W. et al. MicroRNA-21 and microRNA-148a contribute to DNA hypomethylation in lupus CD4+ T cells by directly and indirectly targeting DNA methyltransferase 1. J Immunol 184, 6773–6781 (2010).
Iyer, S. S. et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A 106, 20388–20393 (2009).
Niemir, Z. I. et al. Podocytes are the major source of IL-1 alpha and IL-1 beta in human glomerulonephritides. Kidney Int 52, 393–403 (1997).
Takano, Y. et al. Transcriptional suppression of nephrin in podocytes by macrophages: roles of inflammatory cytokines and involvement of the PI3K/Akt pathway. FEBS Lett 581, 421–426 (2007).
Timoshanko, J. R., Kitching, A. R., Iwakura, Y., Holdsworth, S. R. & Tipping, P. G. Leukocyte-derived interleukin-1beta interacts with renal interleukin-1 receptor I to promote renal tumor necrosis factor and glomerular injury in murine crescentic glomerulonephritis. Am J Pathol 164, 1967–1977 (2004).
Wickman, L. et al. Urine podocyte mRNAs, proteinuria and progression in human glomerular diseases. J Am Soc Nephrol 24, 2081–2095 (2013).
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research (for a graduate student) from the Graduate School of Veterinary Medicine, Hokkaido University; a Grant-in-Aid for JSPS Fellows (No. 25000961); a Grant-in-Aid for Young Scientists (No. 24688033); and a Grant-in-Aid for Scientific Research B (No. 24380156) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Contributions
J.K. designed and performed experiments and analysed data. O.I., T.H., S.O.-K. and Y.K. designed experiments and analysed data. K.M. and T.N. designed experiments and performed experiments. All authors were involved in writing the paper and had final approval of the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Kimura, J., Ichii, O., Miyazono, K. et al. Overexpression of Toll-like receptor 8 correlates with the progression of podocyte injury in murine autoimmune glomerulonephritis. Sci Rep 4, 7290 (2014). https://doi.org/10.1038/srep07290
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07290
This article is cited by
-
Does the renal expression of Toll-like receptors play a role in patients with IgA nephropathy?
Journal of Nephrology (2020)
-
Toll-like receptors in lupus nephritis
Journal of Biomedical Science (2018)
-
Modified scanning electron microscopy reveals pathological crosstalk between endothelial cells and podocytes in a murine model of membranoproliferative glomerulonephritis
Scientific Reports (2018)
-
UCH-L1 Expressed by Podocytes: a Potentially Therapeutic Target for Lupus Nephritis?
Inflammation (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.