Abstract
Research based on event-related potential (ERP) reported mediofrontal negativities following unexpected negative feedback or performance error. Some authors proposed that these signals reflect reward prediction error for worse than expected outcomes, while others suggested that mediofrontal negativities express medial prefrontal cortex coding for unexpected non-occurrence of a predicted outcome, whether worse or better than expected. Many studies found mediofrontal negativities coding for unexpected negative outcomes; however, few studies found them after unexpected positive outcomes. The present study investigated ERP and skin conductance response (SCR) to the unexpected omission of electric shocks during Pavlovian aversive conditioning. To manipulate expectancies, participants were presented with visual stimuli paired with electric shock on either 80% (CS+1) or 20% (CS+2) of trials. SCR analysis confirmed higher shock-delivery expectancy for CS+1, relative to CS+2. ERP analysis evidenced a stronger negative frontocentral ERP component after unexpected, relative to expected, shock-omission. Methodological and theoretical implications are discussed.
Similar content being viewed by others
Introduction
The ability to use learned associations between events in order to guide ongoing behavior carries an important adaptive value that has been mainly attributed to the prefrontal cortex1. In particular, medial prefrontal cortex (mPFC) activation has been reported to code prediction error signals2,3, which represents the extent to which an event occurs surprisingly or unpredictably4. People form representations of events based on experience and, consequently, create predictions about what is going to happen in specific situations. The actual event that ultimately occurs is then compared with the expectancy and, if different, a prediction error signal is generated and used to update knowledge so that future predictions will be more accurate5.
Many event-related potential (ERP) studies reported the presence of mediofrontal negative components6,7,8,9,10,11,12, originated in mPFC13, which seem to code prediction error signals: the so-called Error Related Negativity (ERN)14,15, after performance error and Feedback Error Related Negativity (fERN or FRN)6,16, following unexpected negative feedback. Reinforcement Learning Theory of ERN (RL-ERN)12,17 proposed that these mediofrontal negativities reflect a reward prediction error signal to worse than expected events. This signal is thought to be triggered by either internal information (e.g., when a performance error occurs instead of the expected correct response) or external information (e.g., when a choice is followed by negative feedback). A more recent hypothesis comes from the Predicted Response-Outcome (PRO) model18, which states that mediofrontal cortex responds to violations of expectancies (thus, signaling prediction errors) for both positive and negative action outcomes. Specifically, the authors proposed that mPFC codes the unexpected non-occurrence of a predicted outcome, regardless of its affective valence. When an expected outcome is surprisingly omitted (i.e., an expected reward or punishment are not delivered) an unexpected non-occurrence signal is reflected by mPFC activation, regardless of whether outcome is worse or better than expected. This signal should be maximal when an expected outcome fails to occur, while it is inhibited when the predicted outcome actually occurs18. The PRO model18 holds that mediofrontal negativities, such as ERN and fERN, reflect mPFC activation for the unexpected non-occurrence of a predicted outcome. Consequently, both positive and negative unexpected non-occurrence events should be able to generate an ERN/fERN-like component. It has to be noticed that the omission of a positive outcome has a negative valence (e.g., frustration if you don't get a monetary win), while the omission of a negative outcome has a positive valence (e.g., relief if you don't get an electric shock)19. In line with both hypotheses, many findings reported frontal negative ERP signals for unexpected negative outcomes7,8,9,10,17,20,21; but few studies also reported similar mediofrontal negativities to unexpected positive outcomes22,23,24, a finding that is coherent with PRO model18 but not with RL-ERN theory17.
The aim of the present study was to investigate ERPs for the unexpected omission of a physical pain (an electric shock); thus, a prediction error signal for a positively valenced event (in this case, a relief condition). Previous experiments that found fERN after unexpected positive outcomes did not aim to directly test the unexpected non-occurrence hypothesis postulated by the PRO model18. Instead, fERN was reported after the presentation of a different stimulus than the one expected (i.e., a stimulus with the opposite valence or a neutral stimulus), rather than after the omission of the expected stimulus itself. For example, in a study from Talmi and colleagues23, participants explicitly knew the outcome probability and a “truth cue” informed about the subsequent presence or absence of the outcome. In this paradigm, the fERN was related to “truth cue” onset rather than to the omission of the outcome itself. In a different study, Oliveira and colleagues22, reported fERN after a better than expected performance-feedback during an anticipation-timing task. Again, rather than the unexpected omission of the predicted feedback, participants were presented with a positive feedback when a negative one was expected. In a further study by Ferdinand and colleagues24, fERN was found after a rare and thus unexpected, positive or negative feedback (relative to an intermediate feedback of high frequency) during a time-estimation task.
This study aims to directly test the prediction made by the PRO model18 that the unexpected non-occurrence of a salient negative event (i.e., a relief event) triggers mediofrontal negativity. To this end, the unexpected non-occurrence has been operationalized as the absence of a predicted electric shock, rather than as the presentation of a different stimulus than the one expected. A mediofrontal negativity triggered by the unexpected omission of a predicted negative outcome would lead to the conclusion that an event was expected and that its omission produced a prediction error signal.
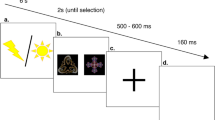
For this purpose, a Pavlovian aversive conditioning paradigm with a partial reinforcement schedule was used. On each trial, participants were presented with a visual stimulus (Japanese kana) displayed for 4 seconds, with a variable 7–9 seconds inter-trial interval (ITI). On some trials, a mild electric shock was delivered during the final 200 ms of stimulus presentation (Fig. 1). Participants performed a total of 640 trials divided in 16 blocks. During a single block, 2 different stimuli were presented 20 times each. Subjects were required to press a keyboard button correspondent to the left or right presentation of the stimulus and they were informed that this response had no effect on shock-delivery. In order to manipulate expectancies, one of the two stimuli presented in each block was paired with an electric shock on 80% of trials (CS+1), whereas the other was paired with an electric shock on 20% of trials (CS+2). Thus, by learning associations between stimuli and shock-delivery probability, participants should build up a high shock-delivery expectancy for CS+1 stimuli and a low shock-delivery expectancy for CS+2 stimuli. Two critical conditions were compared: unexpected shock-omission (CS+1 trials without shock-delivery: CS+1ws) and expected shock-omission (CS+2 trials without shock-delivery: CS+2ws). On CS+1ws trials, not receiving a shock represents an unexpected omission, while on CS+2ws trials, not receiving a shock represents a predicted event. Skin Conductance Response (SCR) and electroencephalogram (EEG) were recorded during the task: SCR analysis was used as a somatic indicator of expectancy, thus it was used to test the presence of a different shock-delivery expectancy between CS+1ws and CS+2ws25. The EEG was recorded to examine ERPs associated with expected and unexpected shock-omission. If unexpected omission of a negative event (i.e, a positively valenced relief event) triggers mediofrontal negativity as predicted by the PRO model18, then a stronger mediofrontal negativity for unexpected shock omission relative to expected shock omission should be observed. Moreover, in order to investigate possible relationships between personality traits related to behavioral responsiveness to reward26 and ERPs associated with positively valenced unexpected omission, Behavioral Activation System (BAS) inventory27 was filled by participants at the end of the task.
Results
SCR
SCR analysis was performed to check if there was a higher shock-delivery expectancy in the CS+1ws condition relative to the CS+2ws. SCR was calculated as the peak-to-peak amplitude difference of the largest deflection in the 0.5–4.5 seconds latency window after stimulus onset25. To analyze how rapidly expectancies were build up and to determine possible habituation effects on SCR level28, each block was divided in two hemiblocks, containing 20 stimuli each. Assumptions of normal distribution, independence of residuals and sphericity were verified. A 2x2 repeated measure ANOVA with Time (first/second hemiblock) and Condition (CS+1ws/CS+2ws) as within factors was performed. A significant main effect of Time (F(1, 14) = 26.27; two-tailed p < .0001; partial η2 = .68; N = 15) was found, with the first hemiblock (mean = .48 µS) presenting a significantly higher SCR level than the second hemiblock (mean = .37 µS). This indicates that the general electrodermal responsiveness to the task is reduced in the second hemiblock. Furthermore, a significant interaction Time X Condition (F(1, 14) = 11.06; two-tailed p = .006; partial η2 = .48; N = 15) was found. Bonferroni-corrected post-hoc analysis on the interaction effect revealed a significant difference (p = .04) between CS+1ws (mean = .52 µS; sd = .05 µS) and CS+2ws (mean = .44 µS; sd = .04 µS) in the first hemiblock; no significant effect emerged for the second hemiblock (p = .90) (Fig. 2). This indicates that subjects showed a higher arousal level when presented with the CS+1ws stimulus relative to CS+2ws in the first hemiblock consistent with a higher shock-delivery expectancy on these trials25. No further effects reached significance (ps > .27).
The analysis of SCR data confirmed that different expectancies were successfully generated in the first hemiblock as subjects presented a higher SCR in the CS+1ws condition, as compared with CS+2ws condition25. In sum, the SCR data suggest that the build up of expectancy was very rapid during the first hemiblock and then habituation hid the effect in the second hemiblock28.
Behavioral data
Percentage of errors and reaction times were also analyzed. Assumptions of normal distribution, independence of residuals and sphericity were verified. Two separate 2x2 repeated measure ANOVAs with Time (first/second hemiblock) and Condition (CS+1ws/CS+2ws) as within factors were performed.
Neither main effects, nor interaction effect emerged from the analysis on the percentage of errors (ps > .17). The average percentage of errors was 1.24 (first hemiblock: CS+1ws = 0.96 and CS+2ws = 1.20; second hemiblock: CS+1ws = 1.37 and CS+2ws = 1.42).
The analysis of reaction times showed a significant main effect of Condition (F(1, 14) = 8.63; two-tailed p = .01; partial η2 = .38; N = 15), with CS+1ws (mean = 520.86 ms; sd = 150.87 ms) presenting faster reaction times than CS+2ws (mean = 534.24 ms; sd = 159.43 ms). A marginal effect of Time (p = .06) and a significant Time X Condition interaction were found. Bonferroni-corrected post-hoc analysis on the interaction effect revealed a significant difference (p = .03) between CS+1ws (mean = 523.77 ms; sd = 149.42 ms) and CS+2ws (mean = 540.63 ms; sd = 161.03 ms) in the second hemiblock; a marginally significant effect emerged also for the first hemiblock (p = .058), with CS+1ws (mean = 517.36 ms; sd = 142.10 ms) presenting slightly faster reaction times than CS+2ws (mean = 527.55 ms; sd = 147.02 ms). These results suggests that, although shock-delivery was not relevant for the task, subjects were faster in indicating the side of presentation of the stimulus when presented with the cue which was highly associated with shock. This effect was stronger in the second hemiblock, relative to the first, when stimulus contingencies were better learned and expectations were stronger.
EEG
The aim of EEG analysis was to investigate the presence of an fERN-like component during unexpected, relative to expected, shock omission. fERN has been reported to present its maximum negative peak between 250 and 350 ms following the event indicating the prediction error12. In the present task, participants can be completely sure about shock omission and consequently, about whether their expectancy is confirmed or violated by the actual course of events only upon stimulus offset. Thus, this event represents the point in time where the prediction error occurs. Therefore, ERPs were calculated as the most negative peak in a 250–350 ms interval after stimulus offset, in unexpected shock-omission trials (CS+1ws) and expected shock-omission trials (CS+2ws). Grandaverage scalp topographies showed a frontal maximum activation for the difference between these two conditions (Fig. 3).
Statistical analysis were conducted on Fz electrode, where fERN has been previously reported12,23. Assumptions for a correct use of t-test (normal distribution, independence of residuals and sphericity) were verified. A paired t-test to compare CS+1ws (mean trial number = 58; sd = 4) and CS+2ws (mean trial number = 288; sd = 16) was performed. A significant difference between CS+1ws (mean = −0.31 μV; sd = 2.40 μV) and CS+2ws (mean = .95 μV; sd = 1.82 μV) was found (t(14) = −2.73; two-tailed p = .016; partial η2 = .34; N = 15). These results indicate that the unexpected omission of a shock (CS+1ws condition) generates a stronger frontal negative ERP component relative to the expected omission of a shock (CS+2ws condition) (Fig. 4).
Moreover, to further investigate the relationship between shock-delivery expectancy and neural activity consequent to its violation, a correlation between shock-delivery expectancy (as expressed by the difference in SCR signal during the two expectancy conditions CS+1ws and CS+2ws) and unexpected omission neural signal (as indexed by the difference in ERPs originated in Fz during CS+1ws and CS+2ws) was analyzed. A significant positive correlation (r = .61, one-tailed p = .01; N = 15) was found. Critically, this effect indicates that the stronger the shock-delivery expectancy (as measured by psychophysiological arousal) is, the stronger is the prediction error for unexpected shock-omission (as reflected by cortical ERPs) (Fig. 5).
BAS Inventory
To examine the relationship between reward responsiveness26 and neural activation consequent to the unexpected omission of shock-delivery, participants were required to fill the Behavioral Activation System (BAS) inventory27. Correlation between BAS (total score and subscales) and ERP peak amplitude during unexpected shock-omission condition was calculated. A marginally significant (for Bonferroni-corrected α = .0125) negative correlation emerged with BAS-Drive (BASD) subscale (r = −.54; one-tailed p = .019; N = 15). This negative correlation indicates that the higher BASD is, the more negative is the peak for unexpected omission. Thus, people with high levels of reward responsiveness tended to present a more negative ERP peak when a shock unexpectedly did not occur, a situation that is better than expected (i.e., a relief) (Fig. 6). No correlations with other BAS subscales or the total score reached significance (ps > .08).
Discussion
RL-ERN theory12,17 holds that mediofrontal negativities (such as, ERN and fERN) signal worse than expected outcomes, while the PRO model18 proposes that mediofrontal activity can be explained as unexpected non-occurrence signals, consequent to the unexpected omission of both positive and negative outcomes. Many studies reported the presence of mediofrontal negativities after unexpected negative outcomes7,8,9,10,17,29,30, but only few studies explored the presence of such ERPs after unexpected positive outcomes22,23,24. However, the unexpected non-occurrence hypothesis postulated by the PRO model18 has never been directly tested. The aim of this study was to investigate mediofrontal ERPs associated with the unexpected omission of an aversive outcome.
EEG and SCR were recorded during a Pavlovian aversive conditioning procedure, in which participants learned to associate visual stimuli to a high (CS+1) or a low (CS+2) shock-delivery probability. This made it possible to contrast and compare two critical conditions: unexpected shock-omission (CS+1ws) and expected shock-omission (CS+2ws). SCR analysis allowed ensuring that subjects actually differentiated between the two stimuli and that different shock-delivery expectancies were created25. This evidence was also supported by reaction time analysis. A larger SCR response indicating a higher arousal level at CS+1ws, relative to CS+2ws, was found. From this result, it can be inferred that there was a higher shock-delivery expectancy for the CS+1ws condition relative to the CS+2ws condition. ERPs in the 250–350 ms interval after stimulus offset, i.e., the point in time when subjective expectancy was confirmed or violated, were calculated for the unexpected (CS+1ws) and the expected (CS+2ws) shock-omission conditions. Analysis showed the presence of a stronger frontocentral negative ERP component in the CS+1ws condition, relative to CS+2ws. Thus, the unexpected omission of a predicted shock generated a stronger frontocentral negativity, signaling unexpected omission.
Time window, frontocentral localization and negative deflection detected in the unexpected omission condition are coherent with an fERN-like component12. Moreover, previous fMRI studies revealed mPFC activity after unexpected shock-omission during Pavlovian aversive conditioning paradigm31,32, which is in line with ERN and fERN source localization reported in the literature33.
Since an fERN-like component was found after a positively valenced unexpected omission event, the present results are not in line with RL-ERN theory account of mediofrontal negativities as selectively coding for worse than expected outcomes17. The present findings are, instead, coherent with the view that mediofrontal negativities can also be triggered by a positive event22,23,24, thus supporting PRO model conception that the mPFC detects both positive and negative unexpected omissions18. This view seems to be able to account for a wide range of findings, thus representing a promising unifying framework for mPFC functioning. It assumes that the fERN6,7,9,12 reflects general outcome monitoring on the basis of expectancy violations irrespective of outcome-valence aspects18,22, thus explaining that positive and negative unexpected feedback elicit similar mediofrontal negativities. Similarly, also the ERN8,10,11 following performance errors in rapid choice tasks can be explained by the fact that an error violates the expectancy of a correct response17,22. fMRI studies also reported enhanced mPFC activation to unexpected wins as compared with losses, when losses are more frequent than wins, thus suggesting that the error effect reflected in the fERN results from a comparison between actual and expected outcomes, rather than by the error feedback per se34.
According to the PRO model, the size of the fERN is proportional to the unexpectedness of the outcome18. Critically, as a demonstration of the link between expectancies and the observed negative ERP component, correlation analysis in the present study showed that higher shock-delivery expectancy, as expressed in SCR, is linked to a greater neural unexpected omission signal, as expressed in frontal negative ERPs at stimulus offset. Thus, in accordance with the predictions of the PRO model, the frontal unexpected omission signal is larger when a stronger expectancy is disconfirmed, i.e., the higher expectancy, the higher mPFC response to expectancy-violations.
Although the current study supports PRO model18, its results also challenge the idea that unexpected omission is specifically related to action-outcomes. Since a Pavlovian aversive conditioning paradigm was used, no action was required during the task. Thus, no action-outcome association was possible and the acquired expectancy about shock-delivery was only attributable to a stimulus-outcome association. The ERP results reported here clearly indicate that mediofrontal activity can be also triggered by stimulus-outcome expectancy violations. Hence, as also reported by other studies23,35,36, mPFC seems to code for unexpected outcomes even when there is no need for action.
An important contribution of this study to the literature is the direct demonstration that the observed neural signal to unexpected omission is conveyed by the absence of the expected event, rather than by the elaboration of a different stimulus. In the present study, shock absence itself acted as the feedback, while previous studies22,23,24 operationalized the unexpectedness by presenting a stimulus different than the one expected. The presence of a neural response to the absence of a stimulus leads unambiguously to the conclusion that an event was expected and that its absence produced a prediction error signal. Thus, the present results strongly support the PRO model18 conception of mPFC coding for the unexpected omission of a predicted event.
A further observation concerns the correlation between BAS measures26,27 and frontocentral negative component for positive unexpected omission. The observed trend indicates that this fERN-like component triggered by an unexpected positive event is stronger for people who are more responsive to rewards. Indeed, as compared with shock-delivery, shock-omission should be perceived as a positively valenced event37. The influence of reward responsiveness to ERP components (such as ERN) between subjects that are highly sensitive to punishment or to rewards has already been reported6,38,39,40,41, but these differences mainly concerned high-BAS as more responsive to ERPs associated with reward omission. To our knowledge, the present study for the first time reports the presence of a relation between BAS responsiveness and mediofrontal negativities coding for positive unexpected omission of a predicted punishment. However, this correlation could reflect a relationship between reward responsiveness and either positive events or omission events. Although this latter possibility seems extremely unlikely, future studies could specifically aim at disentangling these alternative explanations.
The present study tested a central claim of the PRO model18, namely that mPFC is specifically involved in coding unexpected omission events. Still, it remains to be clarified whether the role of mPFC is selectively related to the unexpected omission of predicted outcomes, as demonstrated here, or whether it also includes the unexpected occurrence of unpredicted outcomes, as some studies seem to indicate22,23,24,34. Although it would be functionally inefficient to have separate processors for detecting two kinds of unexpectedness, it could make sense considering the different strategic processing adjustments that these two events could drive42. For example, if the prediction error signal concerns an unexpectedly omitted punishment, the behaviorally relevant information to learn could be to stop avoiding the associated cue or choice; while, if it signals an unexpectedly delivered punishment, it would be relevant to learn to avoid the associated cue or choice. The inverse relation should be valid if the unexpected outcome is a reward omission or delivery. PRO model's assumptions about the role of mPFC in the detection of unexpected events could be strengthened by addressing this issue.
Methods
Participants
Twenty volunteers with no history of neurological diseases were randomly recruited from the student population of the University of Bologna. Two participants completed only one of the three testing sessions and three other participants were excluded for excessive eye artifacts. Thus, the final sample included fifteen (7 female, 1 left-handed) subjects between 19 and 29 years of age (mean = 23.93; sd = 2.37). All participants gave informed consent. The study was conducted in accordance with institutional guidelines and was approved by the Department of Psychology's ethical committee.
Stimuli and materials
The task consisted in a Pavlovian aversive conditioning paradigm with a partial reinforcement schedule. Visual stimuli were used as conditioned stimuli (CSs) and mild electrical shocks served as unconditioned stimuli (USs). A CS consisted in a 3 cm white square with a Japanese kana on it. All the stimuli were balanced for luminance, complexity and color saturation and were displayed on a 17-inch color monitor with a black background, at a viewing distance of 80 cm. Electrical shocks were pulses of 200 ms duration generated by a Digitimer Stimulator (Model DS7, Digitimer Ltd., Hertfordshire, United Kingdom). Mild shocks were administered to the inner wrist of the dominant hand, to which two SU15N1 electrodes (SEI EMG, Padova) were attached. The shock intensity was individually set before the task: stimulation was initially set at 0.5 and the intensity was gradually increased (1 mA increments) to a level the participant indicated as “uncomfortable, but not painful”25. The mean shock intensity was 4.6 mA (sd = 2.1 mA; min = 1.8 mA; max = 8.9 mA).
A single trial consisted in the presentation of one of two possible CSs, on the left or the right side of the screen: one was paired with an electric shock on 80% of trials (CS+1) and one was paired with shock on 20% of trials (CS+2). The CSs were presented for 4 seconds, with a 7–9 seconds intertrial interval (ITI) during which a fixation point was presented (Fig. 1). Stimuli were presented 8.5 cm to the left or to the right of fixation and participants were asked to press a left or a right button corresponding to the side of stimulus presentation. This procedure was introduced in order to keep subject's attention focused on the stimuli. Participants performed a total of 640 trials divided in 16 blocks. Each block consisted of 40 trials, equally divided between the two conditions (CS+1 and CS+2). In each block, 2 out of 32 different Japanese kana were used as CSs and their assignment to a specific category (CS+1 and CS+2) was counterbalanced across subjects. Each block was internally divided in two identical hemiblocks containing half of the stimuli. At the very beginning of each block, there was a brief learning phase during which 5 stimuli for each category (10 stimuli total) were visualized. This phase was not considered in the subsequent analysis. The 16 blocks were spread across three testing days: 5 on the first day, 6 on the second day and 5 on the last day. Between individual testing sessions there was a variable interval lasting from 1 to 4 days. A PC running Presentation software (Neurobehavioral Systems, Albany, CA) controlled stimulus presentation.
Procedure
On arrival, participants were comfortably seated in a silent room and their position was centered relative to the screen. Both SCR and EEG signals were collected continuously during the task and stored for off-line analysis. Participants were required to remain as quiet and still as possible, in order to avoid confounding effects on measurements. The experimental session began with a 10 minutes rest period during which the participant acclimated to the environment and a correct attachment and conductance of the electrodes - for both SCR and EEG - was ensured. Then, the participants' ability to generate SCRs to external stimuli was tested by generating loud sounds (i.e., clapping of the hands). Subsequently, the intensity of the electrical stimulation was individually set as previously described. Before the beginning of the task, participants were told that they would see visual images, on the right or left side of a computer screen, that might be followed by a mild electrical shock. They were required to pay attention to the screen and press a keyboard button correspondent to the left or right presentation of the stimulus. Participants were informed that their response about side of presentation had no effect on shock-delivery. Subjects were also told that they could take a short break between each block, if they wished. At the end of each day, all participants underwent an extinction session in order to eliminate the effects of the aversive conditioning. At the end of the last session, all subjects filled the Italian version43 of BAS inventory27.
SCR recording and analysis
The skin conductance response (SCR) was recorded using Ag/AgCl electrodes (TSD203 Model; Biopac Systems, Goleta, CA), filled with isotonic hyposaturated conductant. Electrodes were attached to the volar surface of the middle and index fingertip of the nondominant hand and held with Velcro straps. The signal was recorded using a DC amplifier (Biopac GSR100) with a gain factor of 5 μS/V and low-pass filter set at 10 Hz. The analog signal was digitized using the MP-150 digital converter (Biopac Systems) at a rate of 200 Hz and fed into AcqKnowledge 3.9 recording software (Biopac Systems).
SCR data acquired during the task were offline analyzed by using custom made scripts realized on MATLAB (2011a, The MathWorks, Inc., Natick, Massachusetts, United States). Statistical analyses were performed with SPSS 19.0 (IBM Corp. Released 2010. IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp.). The skin conductance response was transformed into microsiemens and calculated for each trial. SCR was calculated as the peak-to-peak amplitude difference of the largest deflection in the 0.5–4.5 sec latency window after stimulus onset25. The minimal response criterion was 0.02 µs and smaller responses were encoded as zero; raw skin conductance scores were square root transformed to normalize the distributions and scaled to each subject's maximal US response to account for interindividual variability25. Only trials without shock occurrence were analyzed.
EEG recording and analysis
Electroencephalographic (EEG) signal was recorded with Ag/AgCl electrodes (Fast'n Easy Electrodes, Brain Products, Gilching, Germany) from 26 electrode sites (Fp1, F3, F7, FC1, FC5, C3, T7, CP1, P3, P7, O1, Fz, FCz, Cz, Pz, Fp2, F4, F8, FC2, FC6, C4, T8, CP2, P4, P8, O2), as well as from the right mastoid. The left mastoid was used as reference electrode and the ground electrode was placed on the right cheek. All electrodes were offline rereferenced to the average of both mastoids. Vertical and horizontal electrooculogram (EOG) was recorded from above and below the left eye and from the outer canthi of both eyes. Both the EEG and EOG were recorded with 0.01–100 Hz band-pass and amplified by BrainAmp DC amplifier (Brain Products, Gilching, Germany). The amplified signals were digitized at a sampling rate of 500 Hz and were offline filtered with a 25 Hz low-pass filter.
EEG data acquired during the task were offline analyzed by using MATLAB (2011a, The MathWorks, Inc., Natick, Massachusetts, United States) and EEGLAB 11.0.5.4b free toolbox (Delorme & Makeig, 2004). Statistical analyses were performed with SPSS 19.0 (IBM Corp. Released 2010. IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp.). Epochs of 400 ms before and 800 ms after stimulus offset were extracted from the continuous EEG. The average voltage during the time interval from 400 ms to 200 ms before stimulus offset was used as baseline, as the shock was delivered 200 ms before stimulus offset on trials with shock-delivery. In this way, no influence of expectancy violation or confirmation on the ERP was possible during the baseline window. First, epochs presenting large artifacts were identified and excluded using a rejection method implemented in EEGLAB (pop_autorej, Onton & Delorme, SCCN/INC/UCSD, 2007 with default parameters). Second, EOG artifacts were corrected using a regression approach44. The mean percentage of excluded trials was 12% (sd = 0,4%). Epochs were then averaged separately for each participant and each condition. ERPs were calculated as the local peak amplitude in a 250–350 ms interval after stimulus offset.
BAS Inventory
The Behavioral Activation System (BAS) inventory is a personality self-reported questionnaire proposed by Carver and White27 based on Gray's personality theory26. It is composed of 13 items, each one is a statement that a person may either agree or disagree with. It is required to indicate how much you agree or disagree with that item on a 4 point likert scale (from “totally agree” to “totally disagree”). The inventory can be divided in three subscales: Drive (BASD, reflecting the strength with which reward outcome guides subsequent behavior), Reward Responsiveness (BASRR, indexing the degree to which a person derives pleasure from reward) and Fun Seeking (BASFS, reflecting a novelty-seeking trait). The BAS is generally believed to control appetitive motivation and is sensitive to signals of reward and escape from punishment. Activity in this system elicits movement toward goals and to engage in goal-directed efforts. Greater BAS sensitivity is reflected in greater experience for positive feelings and is supposed to be unrelated to negative affect27.
BAS scales were scored according to the procedures indicated from the authors and final scores (BASTOT mean = 40.47, sd = 3.51; BASD mean = 12.14, sd = 1.88; BASRR, mean = 18.4, sd = 1.68; BASFS mean = 9.94, sd = 2.40) were consistent with those reported in literature27.
References
Kehagia, A. A., Murray, G. K. & Robbins, T. W. Learning and cognitive flexibility: frontostriatal function and monoaminergic modulation. Curr. Opin. Neurobiol. 20, 199–204 (2010).
Schoenbaum, G., Roesch, M. R., Stalnaker, T. A. & Yuji, K. A new perspective on the role of the orbitofrontal cortex in adaptive behavior. Nat. Rev. Neurosci. 10, 885–892 (2009).
Holroyd, C. B., Larsen, J. T. & Cohen, J. D. Context dependence of the event-related brain potential associated with reward and punishment. Psychophysiology 41, 245–253 (2004).
Schultz, W. & Dickinson, A. Neuronal coding of prediction errors. Annu. Rev. Neuoscience 23, 473–500 (2000).
Gläscher, J., Daw, N., Dayan, P. & Doherty, J. P. O. States versus rewards: dissociable neural prediction error signals underlying model-based and model-free reinforcement learning. Neuron 66, 585–595 (2011).
Gehring, W. J. & Willoughby, A. R. The medial frontal cortex and the rapid processing of monetary gains and losses. Science 295, 2279–2282 (2002).
Crowley, M. J., Wu, J., Bailey, C. A. & Mayes, L. C. Bringing in the negative reinforcements: the avoidance feedback-related negativity. Neuroreport 20, 1513–1517 (2009).
Holroyd, C. B., Nieuwenhuis, S., Yeung, N. & Cohen, J. D. Errors in reward prediction are reflected in the event-related brain potential. Neuroreport 14, 2481–2484 (2003).
Chase, H. W., Swainson, R., Durham, L. & Benham, L. Feedback-related negativity codes prediction error but not behavioral adjustment during probabilistic reversal learning. J. Cogn. Neurosci. 23, 936–946 (2010).
Holroyd, C. B. & Krigolson, O. E. Reward prediction error signals associated with a modified time estimation task. Psychophysiology 44, 913–917 (2007).
Hajcak, G. What we've learned from mistakes: insights from error-related brain activity. Assoc. Psychol. Sci. 21, 101–106 (2012).
Hajcak, G., Moser, J. S., Holroyd, C. B. & Simons, R. F. The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biol. Psychol. 71, 148–154 (2006).
Nieuwenhuis, S., Holroyd, C. B., Mol, N. & Coles, M. G. H. Reinforcement-related brain potentials from medial frontal cortex: origins and functional significance. Neurosci. Biobehav. Rev. 28, 441–448 (2004).
Gehring, W. J., Goss, B., Coles, M. G., Meyer, D. E. & Donchin, E. A neural system for error detection and compensation. Psychol. Sci. 4, 385–390 (1993).
Falkenstein, M., Hohnsbein, J., Hoormann, J. & Blanke, L. Effects of errors in choice reaction tasks on the ERP under focused and divided attention. Psychophysiological Brain Res. 1, 192–195 (1990).
Kreussel, L. et al. The influence of the magnitude, probability and valence of potential wins and losses on the amplitude of the feedback negativity. Psychophysiology 49, 207–219 (2012).
Holroyd, C. B. & Coles, M. G. H. The neural basis of human error processing: Reinforcement learning, dopamine and the error-related negativity. Psychol. Rev. 109, 679–709 (2002).
Alexander, W. H. & Brown, J. W. Medial prefrontal cortex as an action-outcome predictor. Nat. Neurosci. 14, 1338–1344 (2011).
Rolls, E. T. Précis of the brain and emotion. Behav. Brain Sci. 23, 177–233 (2000).
Holroyd, C. B., Krigolson, O. E., Baker, R., Lee, S. & Gibson, J. When is an error not a prediction error? An electrophysiological investigation. Cogn. Affect. Behav. Neurosci. 9, 59–70 (2009).
San Martín, R., Manes, F., Hurtado, E., Isla, P. & Ibañez, A. Size and probability of rewards modulate the feedback error-related negativity associated with wins but not losses in a monetarily rewarded gambling task. Neuroimage 51, 1194–1204 (2010).
Oliveira, F. T. P., Mcdonald, J. J. & Goodman, D. Performance monitoring in the anterior cingulate is not all error related: expectancy deviation and the representation of action-outcome associations. J. Cogn. Neurosci. 19, 1994–2004 (2007).
Talmi, D., Atkinson, R. & El-Deredy, W. The feedback-related negativity signals salience prediction errors, not reward prediction errors. J. Neurosci. 33, 8264–8269 (2013).
Ferdinand, N. K., Mecklinger, A., Kray, J. & Gehring, W. J. The processing of unexpected positive response outcomes in the mediofrontal cortex. J. Neurosci. 32, 12087–12092 (2012).
Schiller, D., Levy, I., Niv, Y., Ledoux, J. E. & Phelps, E. A. From fear to safety and back: reversal of fear in the human brain. J. Neurosci. 28, 11517–11525 (2008).
Gray, J. A. Brain systems that mediate both emotion and cognition. Cogn. Emot. 4, 269–288 (1990).
Carver, C. S. & White, T. L. Behavioral inhibition, behavioral activation and affective responses to impending ieward and punishment: the BIS/BAS scales. J. Personal. Scocial Psychol. 67, 319–333 (1994).
Dawson, M. E., Schell, A. M. & Filion, D. L. [The electrodermal system]. Handbook of psychophysiology [Cacioppo, J. T., Tassinary, L. G., Berntson, G. G. (ed.)] [295–334] (Cambridge Univ. Press., Cambridge1990).
San Martín, R., Manes, F., Hurtado, E., Isla, P. & Ibañez, A. Size and probability of rewards modulate the feedback error-related negativity associated with wins but not losses in a monetarily rewarded gambling task. Neuroimage 51, 1194–204 (2010).
Holroyd, C. B., Krigolson, O. E., Baker, R., Lee, S. & Gibson, J. When is an error not a prediction error? An electrophysiological investigation. Cogn. Affect. Behav. Neurosci. 9, 59–70 (2009).
Dunsmoor, J. E. & Labar, K. S. Brain activity associated with omission of an aversive event reveals the effects of fear learning and generalization. Neurobiol. Learn. Mem. 97, 301–312 (2012).
Spoormaker, V. I. et al. Additional support for the existence of skin conductance responses at unconditioned stimulus omission. Neuroimage 63, 1404–1407 (2012).
Nieuwenhuis, S., Holroyd, C. B., Mol, N. & Coles, M. G. H. Reinforcement-related brain potentials from medial frontal cortex: origins and functional significance. Neurosci. Biobehav. Rev. 28, 441–8 (2004).
Jessup, R. K., Busemeyer, J. R. & Brown, J. W. Error Effects in anterior cingulate cortex reverse when error likelihood is high. J. Neurosci. 30, 3467–3472 (2010).
Yeung, N., Botvinick, M. M. & Cohen, J. D. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol. Rev. 111, 931–959 (2004).
Donkers, F. C. L., Nieuwenhuis, S. & van Boxtel, G. J. M. Mediofrontal negativities in the absence of responding. Brain Res. Cogn. Brain Res. 25, 777–87 (2005).
Konorski, J. Integrative activity of the brain: an interdisciplinary approach. (University of Chicago Press, Chicago, 1987).
Helden, J. & Boksem, M. A. S. The importance of failure: feedback-related negativity predicts motor learning efficiency. Cereb. Cortex 20, 1596–15603 (2010).
Hajcak, G., McDonald, N. & Simons, R. F. Error-related psychophysiology and negative affect. Brain Cogn. 56, 189–197 (2004).
Boksem, M. a. S., Tops, M., Wester, A. E., Meijman, T. F. & Lorist, M. M. Error-related ERP components and individual differences in punishment and reward sensitivity. Brain Res. 1101, 92–101 (2006).
Boksem, M. a. S., Tops, M., Kostermans, E. & De Cremer, D. Sensitivity to punishment and reward omission: evidence from error-related ERP components. Biol. Psychol. 79, 185–192 (2008).
Egner, T. Surprise! A unifying model of dorsal anterior cingulate function? Nat. Neurosci. 14, 1219–1220 (2011).
Leone, L., Pierro, A. & Lucia, M. Validità della versione italiana delle scale BIS/BAS di Carver e White (1994): generalizzabilità della struttura e relazioni con costrutti affini. G. Ital. di Psicol. 2, 413–434 (2002).
Gratton, G., Coles, M. G. & Donchin, E. A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol. 55, 468–484 (1983).
Acknowledgements
We thank Marco Steinhauser, for providing useful advices for experimental design and Irene Sciulli, Irene Silvestri and Daniele Migliorati for collaboration during data collection. This work was supported by grants from the University of Bologna (Ricerca Fondamentale Orientata) and from the Ministero Istruzione Università e Ricerca (PRIN 2010, protocol number: 2010XPMFW4_009) to GdP.
Author information
Authors and Affiliations
Contributions
All authors conceived and designed the experiment; S.G. performed the experiment; S.G. and M.E.M. analyzed data; S.G. and G.d.P. wrote the main manuscript text; S.G. prepared figures; all authors read, corrected and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. The images in this article are included in the article's Creative Commons license, unless indicated otherwise in the image credit; if the image is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the image. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Garofalo, S., Maier, M. & di Pellegrino, G. Mediofrontal negativity signals unexpected omission of aversive events. Sci Rep 4, 4816 (2014). https://doi.org/10.1038/srep04816
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep04816
This article is cited by
-
Changes in brain rhythms and connectivity tracking fear acquisition and reversal
Brain Structure and Function (2023)
-
Subliminal determinants of cue-guided choice
Scientific Reports (2020)
-
Neural Mechanisms of Attentional Switching Between Pain and a Visual Illusion Task: A Laser Evoked Potential Study
Brain Topography (2018)
-
Reduced anticipation of negative emotional events in alexithymia
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.