Abstract
The expression and functions of microRNAs (miRNAs) in chronic temporal lobe epilepsy (TLE), the most common type of refractory epilepsy in adults, are poorly understood currently. In this study, status epilepticus evoked by amygdala stimulation was used to establish rat chronic TLE model. Two months later, high-throughput sequencing was employed to investigate miRNA expression profile in rat hippocampus and six miRNAs were confirmed to be differentially expressed. Kyoto Encyclopedia of Genes and Genomes pathway analysis indicated that most of the target genes for these six miRNAs were associated with neuronal apoptosis. Meanwhile, the levels of miR-423-3p and miR-296-5p were correlated with the activity of caspase-3, an apoptosis indicator. Additionally, the loading of miR-423-3p was increased in RNA-induced silencing complex whilst caspase-6, a target of miR-423-3p, was reduced in chronic TLE rats. Collectively, our findings suggest that miRNAs may exert anti-apoptotic effects in chronic TLE.
Similar content being viewed by others
Introduction
Chronic temporal lobe epilepsy (TLE) is the most common type of partial epilepsy in adults1. Symptoms in chronic TLE consist of partial or generalized seizures that originate from the amygdala many years after an initial brain insult such as status epilepticus (SE), encephalitis or febrile convulsions2,3. To date, the underlying molecular mechanisms of chronic TLE are still unclear. MicroRNAs (miRNA) are small non-coding RNAs that regulate the expression of target genes by binding to the 3′-untranslated regions of target mRNA4. In mammalians, more than 50% of all miRNAs are expressed in the brain5, suggesting a particularly significant role for miRNAs in brain physiology6,7. In recent years, accumulating evidence suggested that miRNA was implicated in the pathogenesis of epilepsy8,9. Several studies indicated a differential expression of several miRNAs in brain of SE patients and animal models10,11,12. Meanwhile, by employing rat model of SE, our group and Hu et al. have demonstrated that several differentially expressed miRNAs were involved in modulation of neuronal apoptosis, a main pathological characteristics of SE13,14.
Considering the above evidence, we hypothesized that refractory epilepsy such as chronic TLE also led to dramatic alterations in brain miRNA levels and dysregulation of miRNA might be associated with neuronal apoptosis and brain damages in chronic TLE. In order to test this hypothesis, we employed high-throughput sequencing to investigate the miRNA expression profiles in hippocampus of chronic TLE rats. Meanwhile, Nissl staining was used to detected neuronal losses whilst terminal deoxynucleotidyl transferase-mediated dUTP end-labeling (TUNEL) and the activity of caspase-3, an apoptosis indicator, were used to assess neuronal apoptosis in hippocampus. In addition, the association between differentially expressed miRNAs and caspase-3 activity was evaluated and the loading of miRNAs in RNA-induced silencing complex (RISC) as well as the protein levels of their predict targets were also determined here. It should be noted that we induced chronic TLE in rats by using amygdala stimulation in this study, as this model could mimic behavioral, electroencephalographic and morphological phenomena of human chronic TLE better than chemoconvulsants-induced TLE model15,16,17.
Results
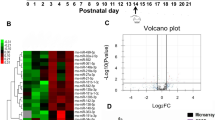
We obtained 8,839,947 and 9,080,314 raw reads from control group and chronic TLE group, respectively. As demonstrated by Supplementary Fig. S1, the most abundant size class was 22 nt, which accounted for 60.97% (control group) and 48% (chronic TLE group) of the total reads, respectively. After discarding the sequences shorter than 18 nt and low-quality sequences, 8,736,906 and 8,930,938 clean reads of 18–30 nt from the control group and chronic TLE group, respectively, were obtained for subsequent analysis.
The expression patterns of known miRNA in rat hippocampus at 2 months after amygdala stimulation were indicated by Supplementary Fig. S2. We identified novel miRNAs at 2 months after amygdala stimulation by using miRDeep software, which was designed to uncover novel miRNAs in deep sequencing data as previously described18,19. The algorithm of miRDeep took into account several factors to score the probability that a candidate small RNA represented a bona fide miRNA: (1) the presence in the deep sequencing data of reads corresponding to typical products of miRNA biogenesis; (2) the stability of the putative premiRNA hairpin; and (3) homology to previously identified miRNAs. After discarding reads corresponding to previously annotated regions, miRDeep analysis revealed the existence of a total of 63 novel miRNAs (Fig. S3).
After comparing the miRNA expression profile in control group and chronic TLE group, we detected 25 miRNAs that were differentially expressed in rat hippocampus. Among these miRNAs, fourteen miRNAs were found to be up-regulated whilst eleven miRNAs were down-regulated (Supplementary Table S1). Afterwards, six miRNAs (miR-455-3p, miR-345-3p, miR-423-3p, novel miR-54, miR-365-5p and miR-296-5p) were screened out based on the criteria mentioned in “Methods” section and qRT-PCR was performed to validate the alterations of these miRNA. As shown in Fig. 1, at 2 months after amygdala stimulation, the expression of miR-455-3p, miR-345-3p, miR-423-3p, novel miR-54 and miR-365-5p was significantly increased (P < 0.05) while the levels of miR-296-5p was markedly decreased in rat hippocampus (P < 0.05). These findings were consistent with results from high-throughput sequencing.
To predict the function of the six differentially expressed miRNAs that confirmed by qRT-PCR, the RNAhybrid and miRanda algorithms were used to obtain a list of potential target genes. Subsequent Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis indicated that most of the predicted target genes such as Fadd, Fas, Casp and Bax were closely relevant to neuronal apoptosis (Figure 2). Hence, these six miRNAs may play an important role in pathogenesis of chronic TLE by modulation of neuronal apoptosis in hippocampus.
We then employed Nissl staining to investigate the neuronal survival rate in rat hippocampus. As shown by Fig. 3A, in rats with chronic TLE, a dramatic reduction of the neuronal survival rate was observed in CA1 and CA3 regions of hippocampus at 2 months after amygdala stimulation. To further investigate whether apoptosis was involved in chronic TLE-induced neuronal losses, we performed TUNEL assay to evaluated neuronal apoptosis rate in rat hippocampus. As indicated by Fig. 3B, in hippocampus of chronic TLE rats, a significant increase in neuronal apoptosis rate was noted in CA1 and CA3 regions at 2 months after amygdala stimulation. In addition, we found that the activity of caspase-3, an indicator of apoptosis, was significantly higher in hippocampus of chronic TLE rats than that of controls (Fig. 3C). These results suggested that apoptosis represented part of the mechanisms underlying the chronic TLE-induced neuronal losses in hippocampus.
Neuronal apoptosis in rat hippocampus and its correlation with miRNA levels at 2 months after amygdala stimulation.
Neuronal survival was assessed by Nissl-staining. (A) Representative photo of Nissl-staining in CA1 region of rat hippocampus at 2 months after amygdala stimulation. Neurons with dark violet nucleus and intact morphology were identified as surviving neurons. Scale bars: 50 μm. The neuronal survival rate was defined as follows: neuronal surviving rate (%) = 100× (count of surviving neurons/total count of neurons). Neuronal apoptosis was determined by TUNEL assay. (B) Representative photo of TUNEL in rat hippocampus at 2 months after amygdala stimulation. Photos were converted to black and white to obtain a better contrast ratio. Neurons with deep black nuclei were identified as TUNEL-positive cells (indicated by red arrows). Scale bars: 50 μm. The neuronal apoptosis rate was defined as follows: neuronal apoptosis rate (%) = 100× (count of apoptotic neurons/total count of neurons). (C) The activity of caspase-3 in hippocampus was measured using a colorimetric assay kit (Abcam) according to the manufacturer's instructions. (D) Pearson's correlation analysis showed that the levels of miRNA-423-3p and miRNA-296-5p were significantly correlated with caspase-3 activity in hippocampus. All data are presented as the mean ± SD; n = 6 per group.
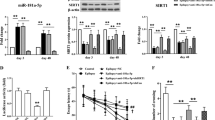
To evaluate whether the selected miRNAs were implicated in chronic TLE-induced neuronal apoptosis, we assessed the correlation between these six miRNAs and the caspase-3 activity in rat hippocampus at 2 months after amygdala stimulation. Pearson's correlation analysis showed a positive correlation between miR-423-3p and caspase-3 activity in hippocampus (Fig. 3D, r = 0.874, P = 0.023). In addition, a negative correlation was found between miR-296-5p and activity of caspase-3 (Fig. 3D, r = −0.881, P = 0.02). These data indicated that miR-423-3p and miR-296-5p might be implicated in the regulation of neuronal apoptosis in chronic TLE.
Subsequently, we investigated whether miR-423-3p exerted its function in brain of chronic TLE rats. We selected miR-423-3p rather than miR-296-5p mainly because its alteration is more apparent than that of miR-296-5p in chronic CLE (shown in Fig. 1). To determine whether up-regulation of miR-423-3p led to a corresponding increment in loading of the miRNA into the RISC, we immunoprecipitated Argonaute (Ago)-2, a crucial component of RISC, from hippocampus of controls and chronic TLE rats. It should be noted that no significant difference was observed in Ago-2 levels between controls and chronic TLE rats (Fig. 4A). As demonstrated by Fig. 4B, the levels of miR-423-3p bound to Ago-2 were dramatically increased in hippocampus of chronic TLE rats when compared with those of controls. Meanwhile, western blot analysis indicated a significant reduction in protein levels of caspase-6, a predicted target of miR-423-3p (shown in Fig. 2), in hippocampus of chronic TLE rats in comparison to controls (Fig. 4C).
Loading of miRNA-423-3p in RISC and protein levels of its predicted targets.
(A) Protein levels of Ago-2, a crucial component of RISC, in hippocampus of chronic TLE rats and controls were detected by western blot (cropped gels/blots are used here and the full-length gels and blots are shown in Supplementary Fig. S4A). (B) miRNA-423-3p levels eluted by immunoprecipitation of Ago-2 in hippocampus of chronic TLE rats and controls. (C) The protein levels of caspase-6, a predicted target of miRNA-423-3p, in hippocampus of chronic TLE rats and controls were assessed by western blot (cropped gels/blots are used here and the full-length gels and blots are shown in Supplementary Fig. S4B). (D) Illustration showing that miR-423-3p might be compensatorily up-regulated in response to cellular apoptosis and tried to inhibit apoptosis via down-regulation of proapoptotic proteins. All data are presented as the mean ± SD; n = 6 per group.
Discussion
Here, we provided the first evidence that chronic TLE induced by amygdala stimulation led to dramatic changes in the miRNA expression profile in rat hippocampus. Five miRNAs including miR-455-3p, miR-345-3p, miR-423-3p, novel miR-54 and miR-365-5p were significantly increased whist miR-296-5p was remarkably decreased in brain at 2 months after amygdala stimulation. These observations were in agreement with a previous study from Risbud and colleagues, as they found that miR-423-3p was up-regulated in a chronic TLE model induced by pilocarpine20. In addition, our findings were also supported by a recent study from Hu et al., as miR-296-5p was revealed to be down-regulated in rats with chronic TLE13. Interestingly, in contrast to our results, the expression of miR-455-3p, miR-345-3p, miR-423-3p, novel miR-54 and miR-365-5p remained unchanged in a recent study from Gorter and colleagues15. Meanwhile, Bot et al. found a moderate but significant reduction in miR-345-3p in rats with chronic TLE16. The lack of reproducibility in the miRNA expression profile can be explained by the diversity in TLE models, time-point and the criteria for miRNA screening. Of note, the miRNA expression was profiled using microarrays in studies from Gorter et al. and Bot et al., whereas we employed high-throughput sequencing to characterized miRNA expression here15,16. The differences in the sensibility and specificity between microarrays and high-throughput sequencing may also be responsible for these contradictory findings. It should be noted that another study from our group indicated that miR-345-3p and miR-365-5p were also dramatically increased in rats with status epilepticus, implying that miRNAs play a crucial role not only in chronic TLE but also in other types of epilepsy14. Nevertheless, the expression of miR-345-3p and miR-365-5p was increased at 24 hour and started to be recovered at 3 weeks after onset of SE whilst that was up-regulated at 2 month after induction of chronic TLE, possibly attributing to the differences in pathophysiology mechanisms underlying these two types of epilepsy.
As a form of programmed cell death, apoptosis was implicated in chronic TLE-induced neuron losses and brain damage12,21,22,23,24. In the current study, we found that chronic TLE dramatically increased the number of apoptotic neurons in rat hippocampus. These findings were supported by a recent study from Hu et al., which demonstrated that apoptosis represents part of the mechanisms underlying the chronic TLE-induced neuronal losses13. Chronic TLE-induced apoptosis can be triggered by activation of intrinsic pathway components including pro-apoptotic proteins belonging to Bcl-2 and caspase family. In addition, the extrinsic pathway mediated by TNFR1 and Fas is also found to be activated following chronic TLE and triggers neuronal apoptosis25,26. Interestingly, the subsequent KEGG pathway analysis indicated that these six miRNAs were targeted to genes involved in both intrinsic and extrinsic pathway of apoptosis, such as Fas, Casp6, Casp9 and Bax. Meanwhile, we found that the expression of miR-423-3p and miR-296-5p were significantly correlated with the activity of caspase-3, an indicator of apoptosis, further implying that these miRNAs participate in the pathogenesis of chronic TLE by modulating neuronal apoptosis in hippocampus. These findings were in agreement with a previous study from Hu and colleagues, which demonstrated expression of several miRNAs was dramatically changed and provided modulation on neuronal apoptosis in rats with chronic TLE13. More importantly, the loading of miR-423-3p was found to be increased in RISC where the miRNA-based RNA silencing occurred8. In addition, the protein levels of caspase-6, a predicted target of miR-423-3p, showed expected reduction in hippocampus of chronic TLE rats. These results further implied that miR-423-3p might be compensatorily up-regulated in response to cellular apoptosis and exerted anti-apoptotic effects in chronic TLE.
In conclusion, we employed high-throughput sequencing to characterize miRNA expression profile in hippocampus of rats with chronic TLE. Six miRNAs including miR-455-3p, miR-345-3p, miR-423-3p, novel miR-54 and miR-365-5p were confirmed to be differentially expressed at 2 months after amygdala stimulation. In addition, miR-423-3p and miR-296-5p were significantly correlated with neuronal apoptosis in hippocampus. Moreover, the loading of miR-423-3p was increased in RISC whilst the protein levels of caspase-6, a predicted target of miR-423-3p, showed expected reduction in hippocampus of chronic TLE rats. These findings indicate that miRNAs may provide protective effects against neuronal apoptosis in chronic TLE and therapies targeting miRNA may open up new avenues for the treatment of refractory epilepsy.
Methods
Adult male Sprague−Dawley rats weighing 230–270 g were obtained from Experimental Animal Center of Qingdao University. They were allowed free access to food and water and were maintained on a 12 h light/dark cycle with a controlled temperature and humidity and given free access to food and water. All experiments were performed in strict accordance with National Institute of Health Guide for the Care and Use of Laboratory Animals. Animal care and sacrifice were conducted according to methods approved by the Qingdao University Animal Experimentation Committee (permit No. QUEC-130629).
Rats were fixed in a stereotactic apparatus (Stoelting, USA) under deep anesthesia (10% chloral hydrate, 3.5 mL/kg, i.p.). An electrodes were implanted into the right basolateral amygdala as previously described (AP: −3.0 mm; L: −4.8 mm; V: −8.8 mm)27,28. The electrode was connected to a miniature receptacle, which was well embedded in the skull with screws and dental acrylic cement. After the surgery, the rats were treated with penicillin for 3 days to prevent infection and allowed to recover from surgery for 10 days.
Seizure was induced by 20 min amygdala stimulation (100 ms train of 1 ms, 60 Hz bipolar pulses, 400 μA, every 0.5 s) using a ML1101 electronic stimulator. Electroencephalograms of the right amygdala were recorded with a digital amplifier (AD Instrument, USA). Seizure severity was assessed according to a 5-stage scale29: (I) facial movement; (II) head nodding; (III) unilateral forelimb clonus; (IV) bilateral forelimb clonus and rearing; and (V) bilateral forelimb clonus and rearing and falling. Following electrical stimulation, rats were video-monitored for 2 months. The rats with chronic TLE were identified by occurrence of frequent seizures (at least 2 times IV/V spontaneous seizures per week) from 1 week after electrical stimulation13,15. The time between the last spontaneous seizure and the animals were killed was 2 h. Rats in control group were handled in the same manner while did not receive any electrical stimulation.
At 2 months after amygdala stimulation, rats with chronic TLE were anesthetized with 10% chloral hydrate (3.5 mL/kg, i.p.) and were transcardially perfused with ice-cold saline. Afterwards, brains were rapidly removed and frozen in liquid nitrogen for subsequent analysis.
Total RNA was extracted by the mirVana™ miRNA Isolation Kit (Ambion, USA) according to the manufacturer's instructions. RNA quality and quantity were evaluated using Agilent 2100 Bioanalyzer. Total hippocampal RNA from rats with chronic TLE as well as control rats was pooled separately and used for miRNA sequencing (n = 6 for each group). The small RNAs were ligated to a 5′ and a 3′ adaptor sequentially and then converted to cDNA by reverse transcription. The two generated small cDNA libraries were amplified by PCR with primers complementary to the adaptor sequences. Afterwards, the libraries were deep sequenced directly using the Illumina Cluster Station and Genome Analyze (Illumina Inc, USA) at Beijing Genomics Institute30. The deep sequencing data have been deposited in NCBI SRA database and are accessible through GEO series accession number GSE52443 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE52443). The differences in the quantities of miRNAs were determined by comparing the log2-ratio of the chronic TLE rats and control rats copies. A miRNA was considered “differentially expressed” only if it met the following criteria: (a) having at least 10 copies in either chronic TLE or control group. (b) fold-change (log2) > 1.5 or fold-change (log2) < −1.5 between these two groups (P < 0.05 and P ≠ 0). Of note, the Benjamini and Hochberg multiple testing correction was applied to adjust the P-values31.
To validate the initial results of high-throughput sequencing in this study, we selected miRNAs that met the following criteria for additional qRT-PCR analysis: (a) having at least 30 copies in either chronic TLE or control group. (b) Showing fold-change (log2) > 1.5 or fold-change (log2) < −1.5 between these two groups (P < 0.01 and P ≠ 0). As a result, six differentially expressed miRNAs were screened out for subsequent qRT-PCR confirmation. The detailed protocol of qRT-PCR was described as previously14. All experiments were performed in triplicate.
Potential target genes of the five known miRNAs were predicted using the database of RNAhybrid and miRanda as previously described32,33,34. Meanwhile, the potential target genes of novel miR-54 were predicted using a method described by Barozai et al35. and Wu et al36. First, we submitted the novel miRNA sequences as queries to the NCBI Basic local alignment search tool program with the following parameters: (1) database reference: RefSeq RNA; (2) organism: Rattus norvegicus; and (3) program selection: highly similar sequences (megablast). Afterwards, the mRNA sequences showing 75% query coverage were selected and submitted to RNAhybrid for the confirmation of the potential targets. Only targets having stringent seed site located at positions 2–7 from the 5′ end of the miRNA along or with the supplementary site were selected and further submitted to the miRanda to reconfirm the RNAhybrid results. Additionally, the KEGG pathway database was used to filter the enriched pathways of miRNA targets.
Nissl staining was used to detect surviving neurons in hippocampus. Briefly, the frozen slices were stained with 1% cresyl violet at 50°C for 5 min as previously described37 and the neurons with dark violet nucleus and intact morphology were identified as surviving neurons. Six fields of CA1 and CA3 regions in hippocampus of each section were randomly selected and the number of surviving neurons was counted. The neuronal survival rate was defined as follows: neuronal surviving rate (%) = 100× (count of surviving neurons/total count of neurons). Meanwhile, TUNEL was performed using a modified protocol described by Stähelin and colleagues to assess neuronal apoptosis38. TUNEL-positive neurons with condensate nuclear were identified as apoptotic neurons39,40. Six different areas were selected at random from the CA1 and CA3 regions of each section and the number of TUNEL-positive neurons in these areas was counted. The neuronal apoptosis rate was defined as follows: neuronal apoptosis rate (%) = 100× (count of apoptotic neurons/total count of neurons). In addition, the activity of caspase-3 in hippocampus was measured using a colorimetric assay kit (Abcam) according to the manufacturer's instructions.
The association between miRNAs and Ago-2, a crucial component in RISC, was investigated as described by Henshall's group10,41. Briefly, the hippocampus of six rats from each group were pooled and homogenized in 0.7 ml of ice-cold immunoprecipitation buffer. The homogenate was centrifuged at 16 000 × g for 15 min at 4°C and the supernatant was retained. Antibodies against Ago-2 (1:50; Cell Signaling Technology Inc., USA) was added to 400 mg of lysate in a final volume of 1 ml, mixed and incubated overnight. Next, 20 ml of 50% Protein-A/G-agarose beads was added for 1 h at 4°C and then centrifuged at 16 000 × g for 15 min. The pellet was washed in immunoprecipitation buffer and miRNA was extracted. Afterwards, qRT-PCR was performed to quantify the expression of miRNA.
Western blot analysis was performed as previously described42,43. Briefly, hippocampus of each rat was homogenized and the total proteins were extracted by RIPA lysis buffer. Different samples with an equal amount of protein were separated on 8–12% SDS polyacrylamide gels, transferred to nitrocellulose membranes and blocked in 5% BSA powder in 1 × trisbuffered saline with 0.1% Tween 20 (1 × TBST) at room temperature for 2 h. Membranes were incubated overnight at 4°C with a mouse monoclonal antibody against Ago-2 (1:1000; Cell Signaling Technology Inc., USA) and Caspase-6 (1:800; Cell Signaling Technology Inc., USA), then washed with 1 × TBST and incubated with HRP-coupled secondary antibody for 2 h at room temperature. After washing, protein bands were detected with chemiluminescent HRP substrate (Thermo Scientific Inc., USA) for 5 min at room temperature and exposed to an X-ray film. The band intensity was analysed using Quantity One software 4.6.2 (Bio-Rad Laboratories Inc., USA) and normalized to a loading control β-actin.
Statistical analysis was conducted using the SPSS 17.0 software. Statistical differences were determined by independent sample t-test. Pearson's correlation analysis was used to investigate to the correlation between the miRNA levels and caspase-3 activity. All data are expressed as mean ± SD. P < 0.05 was considered statistically significant.
References
Palmigiano, A., Pastor, J., Garcia de Sola, R. & Ortega, G. J. Stability of synchronization clusters and seizurability in temporal lobe epilepsy. PLoS One 7, e41799 (2012).
Geary, E. K., Seidenberg, M. & Hermann, B. Atrophy of basal ganglia nuclei and negative symptoms in temporal lobe epilepsy. J Neuropsychiatry Clin Neurosci 21, 152–9 (2009).
Meng, X. F., Yu, J. T., Song, J. H., Chi, S. & Tan, L. Role of the mTOR signaling pathway in epilepsy. J Neurol Sci 332, 4–15 (2013).
Bartel, D. P. MicroRNAs: target recognition and regulatory functions. Cell 136, 215–33 (2009).
Lagos-Quintana, M. et al. Identification of tissue-specific microRNAs from mouse. Curr Biol 12, 735–9 (2002).
Wang, W., Kwon, E. J. & Tsai, L. H. MicroRNAs in learning, memory and neurological diseases. Learn Mem 19, 359–68 (2012).
Krichevsky, A. M., King, K. S., Donahue, C. P., Khrapko, K. & Kosik, K. S. A microRNA array reveals extensive regulation of microRNAs during brain development. RNA 9, 1274–81 (2003).
Jimenez-Mateos, E. M. & Henshall, D. C. Epilepsy and microRNA. Neuroscience 238, 218–29 (2013).
Li, M. M., Li, X. M., Zheng, X. P., Yu, J. T. & Tan, L. MicroRNAs dysregulation in epilepsy. Brain Res 10.1016/j.brainres.2013.09.049 (2013).
Jimenez-Mateos, E. M. et al. miRNA Expression profile after status epilepticus and hippocampal neuroprotection by targeting miR-132. Am J Pathol 179, 2519–32 (2011).
McKiernan, R. C. et al. Expression profiling the microRNA response to epileptic preconditioning identifies miR-184 as a modulator of seizure-induced neuronal death. Exp Neurol 237, 346–54 (2012).
Jimenez-Mateos, E. M. et al. Hippocampal transcriptome after status epilepticus in mice rendered seizure damage-tolerant by epileptic preconditioning features suppressed calcium and neuronal excitability pathways. Neurobiol Dis 32, 442–53 (2008).
Hu, K. et al. MicroRNA expression profile of the hippocampus in a rat model of temporal lobe epilepsy and miR-34a-targeted neuroprotection against hippocampal neurone cell apoptosis post-status epilepticus. BMC Neurosci 13, 115 (2012).
Sun, Z. et al. Genome-Wide microRNA Profiling of Rat Hippocampus after Status Epilepticus Induced by Amygdala Stimulation Identifies Modulators of Neuronal Apoptosis. PLoS One 8, e78375 (2013).
Gorter, J. A. et al. Hippocampal subregion-specific microRNA expression during epileptogenesis in experimental temporal lobe epilepsy. Neurobiol Dis 62, 508–20 (2014).
Bot, A. M., Debski, K. J. & Lukasiuk, K. Alterations in miRNA Levels in the Dentate Gyrus in Epileptic Rats. PLoS One 8, e76051 (2013).
Nissinen, J., Halonen, T., Koivisto, E. & Pitkanen, A. A new model of chronic temporal lobe epilepsy induced by electrical stimulation of the amygdala in rat. Epilepsy Res 38, 177–205 (2000).
Friedlander, M. R. et al. Discovering microRNAs from deep sequencing data using miRDeep. Nat Biotechnol 26, 407–15 (2008).
Inukai, S., de Lencastre, A., Turner, M. & Slack, F. Novel microRNAs differentially expressed during aging in the mouse brain. PLoS One 7, e40028 (2012).
Risbud, R. M. & Porter, B. E. Changes in microRNA expression in the whole hippocampus and hippocampal synaptoneurosome fraction following pilocarpine induced status epilepticus. PLoS One 8, e53464 (2013).
Houseweart, M. K. et al. Apoptosis caused by cathepsins does not require Bid signaling in an in vivo model of progressive myoclonus epilepsy (EPM1). Cell Death Differ 10, 1329–35 (2003).
Wang, Y. et al. alpha-Amino-3-hydroxy-5-methylisoxazole-4-propionic acid subtype glutamate receptor (AMPAR) endocytosis is essential for N-methyl-D-aspartate-induced neuronal apoptosis. J Biol Chem 279, 41267–70 (2004).
Henshall, D. C. & Simon, R. P. Epilepsy and apoptosis pathways. J Cereb Blood Flow Metab 25, 1557–72 (2005).
Lewis, D. V. Losing neurons: selective vulnerability and mesial temporal sclerosis. Epilepsia 46 Suppl 7, 39–44 (2005).
Danial, N. N. & Korsmeyer, S. J. Cell death: critical control points. Cell 116, 205–19 (2004).
Henshall, D. C. Apoptosis signalling pathways in seizure-induced neuronal death and epilepsy. Biochem Soc Trans 35, 421–3 (2007).
Zhang, Q. et al. Anticonvulsant effect of unilateral anterior thalamic high frequency electrical stimulation on amygdala-kindled seizures in rat. Brain Res Bull 87, 221–6 (2012).
Sun, Z. et al. Activation of Adenosine Receptor Potentiates the Anticonvulsant Effect of Phenytoin against Amygdala Kindled Seizures. CNS Neurol Disord Drug Targets 10.2174/18715273113129990078 (2013).
Racine, R. J. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol 32, 281–94 (1972).
Yu, J. T. et al. Triggering receptor expressed on myeloid cells 2 variant is rare in late-onset Alzheimer's disease in Han Chinese individuals. Neurobiol Aging 35, 937.e1–3 (2014).
Benjamini, Y., Drai, D., Elmer, G., Kafkafi, N. & Golani, I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res 125, 279–84 (2001).
Rehmsmeier, M., Steffen, P., Hochsmann, M. & Giegerich, R. Fast and effective prediction of microRNA/target duplexes. RNA 10, 1507–17 (2004).
Enright, A. J. et al. MicroRNA targets in Drosophila. Genome Biol 5, R1 (2003).
Min, H. & Yoon, S. Got target? Computational methods for microRNA target prediction and their extension. Exp Mol Med 42, 233–44 (2010).
Barozai, M. Y. Identification and characterization of the microRNAs and their targets in Salmo salar. Gene 499, 163–8 (2012).
Wu, Q., Wang, C., Guo, L., Ge, Q. & Lu, Z. Identification and characterization of novel microRNA candidates from deep sequencing. Clin Chim Acta 415, 239–44 (2013).
Jiang, T. et al. Triggering receptor expressed on myeloid cells 2 knockdown exacerbates aging-related neuroinflammation and cognitive deficiency in senescence-accelerated mouse prone 8 mice. Neurobiol Aging 35, 1243–51 (2014).
Stahelin, B. J., Marti, U., Solioz, M., Zimmermann, H. & Reichen, J. False positive staining in the TUNEL assay to detect apoptosis in liver and intestine is caused by endogenous nucleases and inhibited by diethyl pyrocarbonate. Mol Pathol 51, 204–8 (1998).
Jiang, T. et al. Angiotensin-(1–7) modulates renin-angiotensin system associated with reducing oxidative stress and attenuating neuronal apoptosis in the brain of hypertensive rats. Pharmacol Res 67, 84–93 (2013).
Jiang, T. et al. Temsirolimus promotes autophagic clearance of amyloid-β and provides protective effects in cellular and animal models of Alzheimer's disease. Pharmacol Res 81, 54–63 (2014).
Sano, T. et al. MicroRNA-34a upregulation during seizure-induced neuronal death. Cell Death Dis 3, e287 (2012).
Jiang, T. et al. Suppressing inflammation by inhibiting the NF-kappaB pathway contributes to the neuroprotective effect of angiotensin-(1-7) in rats with permanent cerebral ischaemia. Br J Pharmacol 167, 1520–32 (2012).
Jiang, T. et al. Acute metformin preconditioning confers neuroprotection against focal cerebral ischemia by pre-activation of AMPK-dependent autophagy. Br J Pharmacol 10.1111/bph.12655 (2014).
Acknowledgements
This work was supported by the grants from the National Natural Science Foundation of China to Lan Tan (81171209, 81371406) and Jin-Tai Yu. (81000544), the grants from the Shandong Provincial Natural Science Foundation to Lan Tan (ZR2011HZ001) and Jin-Tai Yu. (ZR2010HQ004), the Medicine and Health Science Technology Development Project of Shandong Province to Lan Tan (2011WSA02018) and Jin-Tai Yu. (2011WSA02020) and the Innovation Project for Postgraduates of Jiangsu Province to Teng Jiang (CXLX13_561). The authors would like to thank Lin Tan, Qiu-Yan Liu, Zhong-Chen Wu and Xiao-Ling Zhong for technical assistance.
Author information
Authors and Affiliations
Contributions
J.T.Y. and L.T. design the whole study. M.M.L. and Z.S. performed the experiment. Q.Z. and C.C.T. analyzed the data. T.J. wrote the main manuscript text and prepared all figures. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. The images in this article are included in the article's Creative Commons license, unless indicated otherwise in the image credit; if the image is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the image. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Li, MM., Jiang, T., Sun, Z. et al. Genome-wide microRNA expression profiles in hippocampus of rats with chronic temporal lobe epilepsy. Sci Rep 4, 4734 (2014). https://doi.org/10.1038/srep04734
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep04734
This article is cited by
-
Applications of cell resealing to reconstitute microRNA loading to extracellular vesicles
Scientific Reports (2021)
-
A microRNA signature of toxic extrasynaptic N-methyl-D-aspartate (NMDA) receptor signaling
Molecular Brain (2020)
-
MicroRNA-15a-5p serves as a potential biomarker and regulates the viability and apoptosis of hippocampus neuron in children with temporal lobe epilepsy
Diagnostic Pathology (2020)
-
Whole transcriptome approach to evaluate the effect of aluminium hydroxide in ovine encephalon
Scientific Reports (2020)
-
Astrocyte-Derived Exosomal microRNA miR-200a-3p Prevents MPP+-Induced Apoptotic Cell Death Through Down-Regulation of MKK4
Neurochemical Research (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.