Abstract
We present a multi-functional electroporation method for delivery of biomolecule utilizing a high-density distributed electrode network (HDEN) under tri-phase electric stimulation. The HDEN device, with which drastic pH change during the electroporation was avoided,was demonstrated to be highly effective for transfection of not only DNA plasmids and small interfering RNAs (siRNA), but also a small molecular anti-cancer drug, into cells in adjustable volumes of cell suspension. The method constitutes a very flexible electroporation approach in a wide range of in vitro or ex vivo scenarios in various tubes, standard multi-well plates as well as flow chambers.
Similar content being viewed by others
Introduction
Electroporation is a powerful method for delivery of a large variety of molecules ranging from ions, drugs, dyes, tracers, antibodies and oligonucleotides to RNA and DNA for various biological research applications1,2,3. Comparing with chemical transfection methods, electroporation has the advantages of broad applicability and avoidance of the use of toxic chemicals4. Comparing with viral transfection methods, on the other hand, electroporation enjoys rapidity and technical simplicity. However, the use of electroporation is still limited mostly to transfection of nucleic acids5,6,7.
The most commonly used commercial electroporation devices are cuvette-type electroporators, which normally suffer from low survival rates of treated cells8,9,10. Besides cuvette-type devices, several other electroporation systems have been developed, for example Neon Transfection System (Life Technologies). The Neon system is based on a pipette tip chamber and can deal with varied volumes of cell suspension ranging from 10 μL to 100 μL. Several multi-well-plate compatible electroporation devices have been commercially available (such as Ambion siPORTer-96 electroporation chamber, Bio-Rad Gene Pulser MXcell and Amaxa 96-well Shuttle). These electroporation devices are all based on specially built multi-well plate and this limits the use of these devices and engenders relatively high cost for each experiment. Guignet and Meyer have proposed a novel suspended-drop electroporation (SDE) multi-well plate8, which can process small volumes of cells (10–20 μl per well) at high throughput, but it is difficult to have larger volumes in such a format in an individual well, as necessary for many biological experiments.
In this paper, we present a multi-functional and highly-efficient electroporation method, which is suitable for either single tube (or cuvette in demand) or multi-well plate applications and can execute electroporation in a wide range of sample volumes ranging from 10−20 μL to 300 μL per well. A flow-through type electroporation device has also been constructed for ultra-high speed processing of large volumes of cells for electroporation.
Results
Design of the HDEN device
As shown in Figure 1A, thirty-seven pillared electrodes were joined and welded on a printed circuit board (PCB). The electrodes were modified from bio-compatible stainless steel acupuncture needles. When electroporation was performed, pillared electrodes were inserted into the well of a 96-well plate or single tubes and immersed in cell suspension with various sample volumes. Pillared electrodes were arranged as a hexagonal cellular array (Figure 1B) and connected to three polarities by the patterned wires on the PCB (as shown in Supplementary Figure 1) to ensure the relatively uniform coverage of electric field. An improved tri-phase electric stimulation mode was proposed (Figure 1B). The electrodes were divided into three groups, as colored in blue, red and green and each group was connected to anode alternately during a three phasic stimulation controlled by a circuit consisted of three switches. The circuit detected the electric stimulation and then alternated the switches so that the polarity would have been shifted before that next pulse starts. The electric field generated by the electrode network is simulated as shown in Figure 1C. For a single pulse, electric traps emerged between homo-electric-polar electrodes where electric field intensity was approximately zero. However, positions of electric traps varied among three phases and electric field covered most areas of the entire well by tri-phase method. Compared with single-phase electroporation mode, tri-phase stimulation generated a rather uniform electric field and the electric field covered the entire area (Supplementary Figure 2).
The design of HDEN electroporation devices.
(A) Detailed illustration of a naked single HDEN device and the schematic view of electroporation procedure compatible with 96-well-plate. (B) The distribution of electrode network and schematic diagram of tri-phase electroporation method. Electrodes colored in blue, red and green are connected to pole I, II and III respectively and electric polarity is alternated during three phases. (C) Simulation of electric field distribution during three phases and the equivalent field intensity. The abbreviations t1, t2, t3 represent three phases and Max means the equivalent electric field if three phases are merged.
Performance of HDEN
All available commercial electroporation apparatuses utilize single phase electric pulse to increase the permeability of cells. It was demonstrated that the single phase electric pulsing leads to intensified electrolysis of water and accumulation of OH− ions locally thus causes a steep pH gradient between the electrodes11. The high pH around cathodes has been found to have great effects on cell death12. We have shown that the tri-phase electroporation is able to avoid polarized OH− accumulation and thus diminishing its lethal side effects. As shown in Figure 2A, under a single phase stimulation mode, the phenolphthalein solution near cathodes became fuchsia, indicating that the pH was quickly elevated to above 10.0. In contrast, the pH change in tri-phase electroporation was very well controlled, since the phenolphthalein solution remained colorless all the time during electropoartion, indicating a pH below 8.2.
(A) The pH changes during electroporation as indicated by phenolphthalein (below pH 8.2: colorless, above pH 10.0: fuchsia). Single-phase electroporation approaches engender considerable changes in pH, while three-phase method is able to maintain a pH value below 8.2. (B) Cell viability and transfection efficiency of HDEN and commercial cuvette (Bio-Rad or Eppendorf). The distance between the two electrodes of cuvette is 2 mm, whereas distance between any two electrodes of HDEN is 0.7 mm. HEK-293A cells are used in the experiments. The HDEN has a wider range of optimal electric field than cuvette. The data is averaged from at least three experiments and the error bars stand for standard error. (C) The optimization of pulse number for electroporation. The voltage and pulse duration is fixed as 110 V and 100 μs. The number of pulses is varied from 3 to 18, which means the number of three-phase circle is varied from 1 to 9. (D) The consistency of electroporation performance for varied volume of cell suspension in an individual well. The data are averaged from at least three experiments and the error bar stands for the standard error. No statistically significant differences are observed between the different groups in this case.
This unique tri-phase stimulation approach of HDEN dramatically reduced the damage of cell during electroporation and thus helped to maintain cell viability at a relatively high level under a wide range of electroporation conditions. In order to achieve both high transfection efficiency and high cell viability simultaneously, the condition of electroporation was optimized. Here we set the targeted goal that both the transfection efficiency and cell viability were higher than 70%. As presented in Figure 2B, cell viability during electroporation using the HDEN device maintained higher than 70% when the average electric field intensity was no higher than 240 V/mm, while transfection rate was higher than 70% when the electric field was ~150 V/mm or higher. Therefore, the optimal range was determined to be from 140 V/mm (variability of ~93% and transfection rate of ~70%) to 240 V/mm (variability of ~70% and transfection rate of ~95%) for the HDEN device in the dimension that was used in our current format, whereas the transfection efficiency and cell viability were both about 90% at around 180 V/mm (the corresponding voltage was 130 V). In contrast, such condition to keep both viability and transfection higher 70% for electroporation in the cuvette was found to be in a very narrow range between ~70 V/mm and ~80 V/mm. This made it very hard to optimize cuvette-type eletropoartion devices. Thus, HDEN was found to be much superior than cuvette-type eletropoartion devices in terms of flexibility in optimization.
The performance of the HDEN device under different number of pulses was further tested by transfecting DNA plasmids to HEK-293A cells. It was found that the transfection performance was influenced by the numbers of electric pulses. As shown in Figure 2C, transfection efficiency remained at a stable level (~90%) when pulses increased from 3 to 9. However, cell viability decreased rapidly when the number of pulses was 18. Thus, we used three electric pulses as a standard electroporation condition in subsequent experiments.
We further tested the electroporation with the HDEN device under different cell volumes and found it could accommodate electroporation from small volumes to large amount of sample and results in similar transfection efficiencies (Figure 2D).
Applications of HDEN on different cell types and molecules
We performed electroporation for different cell types using the HDEN device. DNA plasmids (pEGFP-C3) were successfully transfected into HEK-293A, Hela, Neuro-2A, MCF-7, C2C12, 3T3-L1, CHO, MDCK, HL-60 and HUVEC cells (as shown in Figure 3A) and A375, U251 cells (Supplementary Figure 3). The detailed electric parameters for these cell types are listed in Supplementary Table 1. The transfection efficiency was 50%–90% and the cell viability was 60%–90% depending on the cell types (listed in Supplementary Table 1).
Applications of HDEN electroporation devices in delivery of biomolecules.
(A) The performance of DNA plasmids electroporation for various types of cells. The duration and number of pulses are fixed as 100 μs and three. (B) Electroporation of siRNA. Firefly luciferase expression level is depressed to about 20% which is as low as transfection by Lipofectamine 2000. Cells without any treatment serve as negative control (NC), Mock: electric stimulation without siRNA, siLuc: siRNA targeting firefly luciferase. E-80V: electroporation at 80 V, E-100V: electroporation at 100 V, E-120V: electroporation at 120 V. The data are averaged from at least three experiments and the error bars stand for the standard error. Statistically significant differences in luciferase activity between Mock and electroporation groups are observed (***P < 0.001). (C) The tumor cell killing efficiency of bleomycin electrochemotherapy. The concentration of bleomycin is varied from 10−6 M to 10−4 M. The data are averaged from at least three experiments and the error bars stand for the standard error. Differences in cell viability are statistically compared between 0 M and other concentration of bleomycin. **P < 0.01 and ***P < 0.001 are plotted when observed.
We further transfected siRNA into Hela cells to inhibit the expression of firefly luciferase using HDEN device (Figure 3B). The enzyme activity was silenced to 20% when siRNA was electroporated by using HDEN, suggesting that HDEN could be as efficient as chemical transfection by using Lipofectamine 2000 (Invitrogen).
The HDEN device has been further used to explore the delivery of an antitumor drug into tumor cells by electroporation. Bleomycin was introduced into MCF-7 cells and cell viability was evaluated after 48 hours. Bleomycin alone only slightly affected the viability of MCF-7 cancer cells at concentrations from 10−6 M to 10−4 M. Similarly MCF-7 cell viability was only slightly reduced with only electric stimulation alone even when the voltage was as high as 170 V (Fig 3C). When the two approaches were combined, however, we found a dramatic synergistic effect and cancer cells were killed much more effectively. It was very encouraging to observe that fifty percent cell death was achieved using as low as 10−6 M bleomycin with the assistance of electropermeabilization at voltage between 130 V and 150 V. When the concentration of bleomycin was increased to 10−4 M, even an electroporation treatment as low as 70 V was found to result in 50% cell death, suggesting a synergistic effect between the compound and electric electroration.
HDEN based instruments
In order to explore the utility of HDEN device in a wide range of applications for excellent delivery of biomolecule, we developed several types of HDEN based electroporation instruments. A single hand-hold HDEN electroporation pen (Figure 4A) suitable for a small volume of tests in single tubes was first developed. We have also developed multi-functional electroporation apparatuses based on HDEN devices. The multi-HDEN was constructed by paralleling PCBs, thereby obtaining higher throughput up to 96-well formats (Figure 4B). Performance of siRNA transfection using a multi-HDEN device is shown in Figure 4C. Firefly luciferase suggested electroporation was efficient, reproducible and consistent. The electroporation device was also installed on a three-dimensional manipulator to enable automation and standardization (Figure 4D).
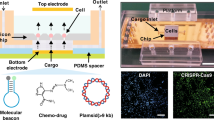
Diverse applications of HDEN based electroporation devices.
(A) A single HDEN is packaged in an electroporation pen to be used for electroporation in a single well or a tube. (B) Multi-HDEN devices to be used for high throughput electroporation in 96-well plate. (C) Delivery of siRNA using multi-HDEN. NC: negative control is cells with no treatment, EP: siRNA electroporation group. The voltage for electroporation was 100 V. The data are averaged from at least three experiments and the error bars stand for the standard error. Statistically significant differences in luciferase activity between NC and EP are observed (***P < 0.001). And no statistically significant differences are observed within EP groups. (D) A multi-HDEN device installed on a three-dimensional manipulator as an automatic instrument.
A flow-through type electroporation instrument was created by using a flow-through chamber with an HDEN device packaged inside and sealed by polydimethylsiloxane (PDMS), a material that was widely used in biology and medical applications with good bio-compatibility (as shown in Figure 5A). In our flow-through electroporation experiment, the HDEN device was shown to be able to electroporate up to about 5 ml cell suspension per minute, which was far more efficient than any micro fluidic electroporation devices created to date13, while excellent transfection performance was obtained (Figure 5B).
Discussion
Our study has explored a novel method of electroporation that enables the use of tri-phasic electric stimulation in order to maintain optimized cell viability while pushing for best electroporation efficiency. We further created the electroporation devices to execute these tasks. We have shown this novel method can be used to achieve high transfection efficiency and low toxic effects in a wide number of cell types by using this unique tri-phase stimulation approach to dramatically reduce the damage of cells during electroporation. Biomolecules such as DNA plasmids and siRNA were transfected into various types of cells using the apparatus. Delivery of antitumor drug was also found to be enhanced dramatically by electroporation using the HDEN device as demonstrated by sharply elevated cell death at a wide range of drug concentration. The HDEN device is compatible with multi-well plate and is amenable to automation of electroporation. We have also demonstrated that the HDEN device can be formatted to have very high processing capacity which might provide a possible approach for either transfection of a large amount of cells for transient protein production or for ex vivo gene therapy of blood cells.
Methods
Cell culture
HEK-29A, Hela, MCF-7, A-375, Neuro-2A, U251, C2C12, 3T3-L1 and MDCK cells were grown in DMEM culture medium (Hyclone) supplemented with 10% fetal bovine serum (Sigma), 100 units/ml penicillin and 100 mg/ml streptomycin (Sigma). CHO cells were grown in F-12 culture medium (Hyclone) supplemented with 10% fetal bovine serum (Sigma), 100 units/ml penicillin and 100 mg/ml streptomycin (Sigma). HL-60 cells were grown in RPMI-1640 culture medium (Hyclone) supplemented with 10% fetal bovine serum (Sigma), 100 units/ml penicillin and 100 mg/ml streptomycin (Sigma). HUVECs were maintained in ECM culture medium (ScienCell) supplemented with 20% FBS (ScienCell). Each cell type was cultured under recommended cell-specific culture conditions. All cells were seeded in a culture flask (Corning) 2–3 days prior to the experiments.
Plasmids, siRNA and drug
Transfection efficacy of plasmid DNA was determined by using pEGFP-C3 plasmid encoding an enhanced green fluorescent protein (Clontech). Purifications of plasmid DNA were performed using an EndoFree Plasmid Maxi Kit (Qiagen, German). DNA oligonucleotides used for vector construction were from Invitrogen (Beijing, China) and siRNA oligonucleotides were supplied by Suzhou Ribo Life Science Co., Ltd. (Jiangsu, China).The sequences were as follows: siLuc: sense:5′-CCCUAUUCUCCUUCUUCGCdTdT-3′, antisense: 5′-GCGAAGAAGGAGAAUAGGGdTdT-3′. The siLuc was delivery into Hela-Luc cells to silence the expression of luciferase. Hela-Luc cell was a cervical cancer line that stably expressed luciferase. As a commonly clinically used broad-spectrum antitumor drug, bleomycin was electroporated in terms of the enhancement of cell membrane permeability induced by external electric stimulation. In order to offer useful guidance, we purchased clinical used Bleomycin Hydrochloride for Injection (Takasaki Plant, NIPPON KAYAKU CO. LTD., Japan). The powder was dissolved in PBS buffer to a concentration of 15 mg/ml for use.
MTT assay and luciferase assay
Cell viability was evaluated by MTT assay. Briefly, cell culture medium was replaced by 100 ml fresh complete DMEM and 2 μl MTT (5 mg/ml) each well. After incubated for 4 h, all medium was removed again and 50 μl DMSO was added into each well and further incubated for 10 min at 37°C. Finally, the absorbance was read at 540 nm with a reference wavelength of 650 nm and the absolute absorbance (ODnet540) was OD540 minus OD650. Cell viability was calculated as:

In vitro gene silencing efficacy was verified by luciferase assay. Hela-Luc cells were grown in DMEM supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 units/mL penicillin and 100 μg/mL streptomycin (Life Technologies, Gibco). The cells were seeded into 24-well plates at a density of ~1 × 105 cells/well one day before transfection. The concentration of siRNA was 125 nM, transfected into the cells at ~60% confluence.
For the luciferase assay, the medium was removed and cells were washed with cold PBS, then the cells were lyzed in 100 μl 1× reporter lysis buffer (Promega Co., Madison, WI) followed by shaking for 20 min to ensure complete lysis. The lysate was transferred into a 1 ml centrifuge vial, centrifuged for 30 s at 12,000 rpm and the supernatant was collected for luminescence measurements. The relative light units (RLUs) were measured with a fluorometer (Synergy HT, BioTek, USA). The silencing efficiency was calculated by comparison with a sample without treatment.
Electroporation procedure
Briefly, before electroporation, cells were detached using trypsin–EDTA (Invitrogen Corporation, USA) and then were harvested and re-suspended to a density of 2.5–5 × 105 cells/100 μl in electroporation buffer (25 mM KCl, 0.3 mM KH2PO4, 0.85 mM K2HPO4, 36 mM myo-insitol, pH 7.2, conductivity 3.5 mS/cm at 25°C). Plasmid DNA or siRNA was then added to a final concentration of 20 ng/μl or 4–6 pM/μl respectively.
For each transfection, 20–300 μl of the mixture were loaded into one well of a standard 96-well plate (Corning), treated with electrical pulses delivered by an ECM-830 stimulator (BTX, USA). Immediately after electroporation, every 20 μl of the mixture was transferred into one well. Then 200 μl of culture medium was added into each well. Twenty-four hours later, the number of GFP expressing cells was counted in five randomly chosen areas, under a fluorescence microscopy (Olympus IX-71). Surviving cells were assessed by propidium iodide (Invitrogen) exclusion assay. For each transfection, efficiency was calculated by dividing the number of GFP expressing cells by the number of living cells. After observation, the cell viability ratio was obtained by MTT assay. Presented data were the average of at least three independent assays.
Cuvette-type electroporator were purchased from Bio-Rad (Bio-Rad Laboratories, Inc., USA) and Eppendorf (Eppendorf Corporation, Germany). The gap of cuvette we used was 2 mm.
Flow-through type Electroporation
The flow type HDEN device was made up of specialized glassware constructed by glass blowing which was able to be mass-produced. The HDEN device was packaged inside and then PDMS was used to fix and seal the HDEN. The packaged device was joined with rubber tube. A peristaltic pump (Terufusion Infusion Pump, Terumo Corporation, Japan) was used to control the flow rate of the cell suspension. Cells were harvested and re-suspended to a density of 3 × 105 cells/100 μl in electroporation buffer with plasmid DNA added to a final concentration of 20 ng/μl. Electroporated cell suspension was collected and then separated with buffer by centrifugation. Cells were re-suspended in culture medium and then transferred in 96-well plate. The supernate was recycled, from which DNA plasmids were extracted and reused.
References
Gehl, J. Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiologica Scandinavica. 177, 437–447 (2003).
Hockemeyer et al. Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nature biotechnology. 27, 851–857 (2009).
Pekarik et al. Screening for gene function in chicken embryo using RNAi and electroporation. Nature biotechnology. 21, 93–96 (2002).
Tryfona & Mark, T. Enhancement of biomolecule transport by electroporation: a review of theory and practical application to transformation of Corynebacterium glutamicum. Biotechnology and bioengineering. 93, 413–423 (2006).
Wolf et al. Control by pulse parameters of electric field-mediated gene transfer in mammalian cells. Biophysical journal. 66, 524–531 (1994).
Kotnik & Damijan Analytical description of transmembrane voltage induced by electric fields on spheroidal cells. Biophysical Journal. 79, 670–679 (2000).
Deng et al. The effects of intense submicrosecond electrical pulses on cells. Biophysical journal. 84, 2709–2714 (2003).
Guignet, G. & Tobias Suspended-drop electroporation for high-throughput delivery of biomolecules into cells. Nature methods. 5, 393–395 (2008).
Zwaka, T. P. & Thomson, J. A. Homologous recombination in human embryonic stem cells. Nature biotechnology. 21, 319–321 (2003).
Kim, J. A., Cho, K., Shin, M. S. et al. A novel electroporation method using a capillary and wire-type electrode. Biosensors and Bioelectronics. 23, 1353–1360 (2008).
Friedrich, U. et al. High efficiency electrotransfection with aluminum electrodes using microsecond controlled pulses. Bioelectrochemistry and bioenergetics. 47, 103–111 (1998).
Kim, Ah. et al. A novel electroporation method using a capillary and wire-type electrode. Biosensors and Bioelectronics. 23, 1353–1360 (2008).
Wang & James Lee, L. Micro-/nanofluidics based cell electroporation. Biomicrofluidics. 7, 011301 (2013).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 61176111) and the National High Technology Research and Development Program of China (2012AA022501). We thank Dr. Jincai Luo for providing HUVECs. We are grateful to Dr. Yuan Xiao, Qiang Cheng, Xing Meng, Xiao Zhou and all the members of Prof. Zhihong Li's group for their helpful advices.
Author information
Authors and Affiliations
Contributions
M.W. and W.Z. designed and fabricated the devices. D.Z. designed and performed the experiments. H.Y. and X.W. performed the composition of corresponding patents. M.W., Z.L1. and Z.L2. analyzed the data and wrote the manuscript. M.W. and D.Z. contributed equally to the work. All the authors discussed the results and commented on the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
supplementary figure and table
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareALike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Wu, M., Zhao, D., Zhong, W. et al. High-density distributed electrode network, a multi-functional electroporation method for delivery of molecules of different sizes. Sci Rep 3, 3370 (2013). https://doi.org/10.1038/srep03370
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep03370
This article is cited by
-
A Flow-Through Cell Electroporation Device for Rapidly and Efficiently Transfecting Massive Amounts of Cells in vitro and ex vivo
Scientific Reports (2016)
-
Electroporation on microchips: the harmful effects of pH changes and scaling down
Scientific Reports (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.