Key Points
-
Highlights the challenges involved in treating peri-implant disease.
-
Provides a pilot clinical trial demonstrating porous titanium granules as a treatment option for peri-implantitis lesions.
-
Suggests that since peri-implantits is going to be one of our major concerns in the forthcoming decade, the development of predictable, evidence-based treatment protocol is urgently needed.
Abstract
Objectives The aim of the present study is to report on preliminary clinical and radiographic results of using porous titanium granules for treatment of peri-implantitis lesions.
Methods A retrospective cohort of 18 implants presenting with peri-implantitis in 16 consecutive patients from two private practices had been evaluated. Treatment included open flap debridement of the lesion, implant surface decontamination using tetracycline, filling of the defect with porous titanium granules and apically positioning of the flaps. Patients' demographics, site and implant characteristics, as well as clinical and radiographic evaluation as baseline and time of follow-up were recorded.
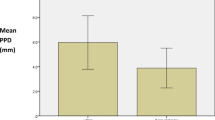
Results Patients' age ranged from 44 to 79 years with a mean of 61.3 ± 9.5 years. Follow-up time after treating peri-impantitis lesions ranged from 6 to 15 months (mean 7.5 ± 3.9). Two out of the 18 implants still presented with bleeding and suppuration at follow-up and thus the treatment was considered as a failure. This resulted in an overall success rate of 88% for the treatment. Mean bone loss prior to treatment was 4.4 ± 2.1 mm and was reduced following treatment to 2.3 ± 2.1 mm.
Conclusion The use of porous titanium granules might be a viable treatment option in cases of peri-implantitis lesions. Further large-scale long-term studies are warranted in order to assess the additional benefit from this treatment option compared to other available alternatives. assiduous
Similar content being viewed by others
Introduction
The inflammatory lesions that develop in the tissues around implants are collectively recognised as peri-implant diseases. In accordance with the classification of periodontal disease at teeth, peri-implant disease includes two entities: peri-implant mucositis that corresponds to gingivitis and peri-implantitis that corresponds to periodontitis.1,2 While peri-implant mucositis was defined as a reversible inflammatory reaction in the soft tissues surrounding a functioning implant, peri-implantitis was described as inflammatory reactions associated with loss of supporting bone around an implant in function.1,2
Peri-implant disease following successful integration of an endosseous implant is the result of an imbalance between bacterial load and host defence. If undiagnosed, peri-implant disease may lead to complete loss of osseointegration and implant loss.3
Despite evidence of excellent results of implant therapy peri-implant diseases do occur. Attempts have been made to determine the optimal treatment protocol for achievement of complete resolution of peri-implantitis.4 In addition to this resolution the treatment may also include regeneration of lost tissue and re-establishment of osseointegration along previously contaminated implant surfaces. Conservative, resective and regenerative treatments have been investigated in conjunction with various methods of additional surface decontamination.4
Access surgery combined with implant surface decontamination for treatment of peri-implantitis has scarcely been investigated. Moreover, no single method of surface decontamination (chemical agents, air abrasives and lasers) was found to be superior.4
A prerequisite for peri-implant bone healing seems to be the recruitment of osteogenic cells and their migration toward the implant surface.5
Publications have reported on blood cell reactions to titanium, demonstrating that the surface properties of an implant, such as oxide thickness, chemical composition and roughness, have an impact on bone response to an implant surface.5,6,7 Adapting these issues to the field of regenerative surgery, one may hypothesise that using a titanium graft material with a surface to volume ratio as large as possible may enhance osseous growth. Porous titanium granules represent one such strategy. A typical porous titanium granule is 500–1,000 mm in diameter but the total titanium surface of the ultra-porous granules is close to 2 cm2 according to analyses performed by the manufacturer.5 Porous titanium granules have also been introduced by Bystedt and Rasmusson as a bone graft substitute in the field of oral and maxillofacial surgery for use in sinus-lifts.8 They reported on the outcome after having treated 16 patients with a total of 23 implants. The implant survival rate, evaluated between 12 and 36 months after functional loading of the implants, was 87%.
The aim of the present study is to report on preliminary clinical and radiographic results of using porous titanium granules for treatment of peri-implantitis lesions.
Methods
A retrospective cohort of 18 implants presenting with peri-implantitis in 16 consecutive patients from two private practices had been evaluated. Since this is a retrospective chart study it was exempt for review board approval. Treatment included open flap debridement of the lesion, implant surface decontamination using tetracycline, filling of the defect with porous titanium granules (PTG, Natixs, Tigran Technologies AB, Malmo, Sweden) and apical positioning of the flaps (Figs 1–6). Patients' demographics, site and implant characteristics as well as clinical and radiographic evaluation as baseline and time of follow-up were recorded. Clinical evaluation included probing depth measurements, bleeding on probing and suppuration evaluation, while radiographic evaluation was performed to assess bone loss around the implant. Implants presenting no bleeding and suppuration with shallow probing pocket depth at follow-up were considered as success of the treatment.
Results
Overall, 16 patients received treatment for 18 implants with peri-impantitis lesions during the follow-up time. Patients' age ranged from 44 to 79 years with a mean of 61.3 ± 9.5 years. Follow-up time after treating peri-impantitis lesions ranged from 6 to 15 months (mean 7.5 ± 3.9). Table 1 presents the patients' demographics as well as clinical and radiographic results of the study's cases. The treatment was considered a failure for 2 out of the 18 implants, which still presented with bleeding and suppuration at follow-up. Therefore, the overall success rate for the treatment was 88%. Mean bone loss prior to treatment was 4.4 ± 2.1 mm and was reduced following treatment to 2.3 ± 2.1 mm.
Discussion
Peri-implantitis is mainly a feature around implants that have been functioning in the oral cavity for a long period of time. In a literature review regarding prevalence of peri-implantitis, Zitzmann and Berglundh reported that peri-implantitis was found in 28%-56% of subjects and in 12%-43% of implant sites.1 The therapies that have been proposed over the years for the treatment of peri-implantitis have been based on the evidence available for the treatment of periodontitis.9 There are, however, obvious differences between screw root-formed, rough surfaced implants and natural teeth. It is of great interest for the dental community to find ways to treat peri-implantitis and to regenerate the bone that was lost due to the infection. The ultimate goal is re-osseointegration on the exposed implant surface and several attempts have been made to determine a treatment protocol that could successfully achieve it.9 These attempts included conservative, resective and regenerative treatment in conjunction with various methods of additional surface decontamination.
In a review regarding surgical treatment of peri-implantitis, Claffey et al. stated that regenerative procedures such as bone graft techniques with or without the use of barrier membranes resulted in various degrees of success.4 However, it must be stressed that such techniques do not address disease resolution but rather merely attempt to fill the osseous defect.4 In order to improve the results of such treatments, new regenerative procedures are needed. A recent study in rabbits demonstrated that porous titanium granules are osteoconductive graft materials suitable for regenerative treatment of osseous defects and that the granules provide osteoconductive scaffolding that can also be used safely adjacent to titanium implants. However, the authors concluded that to address the efficacy of porous titanium granules when utilised as graft material in osseous regenerative surgery adjacent to functionally loaded titanium implants, clinical trials will be needed.5
This preliminary report provides a pilot clinical trial demonstrating the ability of porous titanium granules to serve as a treatment option in cases of peri-implantitis lesions. This is a preliminary report with no comparison group and thus future research is warranted, especially to determine the long term integration and bio-compatibility of the titanium granules.
Recently, Wohlfahrt et al. published a case report of a patient with a peri-implant osseous defect treated with porous titanium granules, which demonstrated newly formed bone in close connection with porous titanium granules and with re-osseointegration of the implant.10
The cumulative data from our study, as well as previous reports, are rather encouraging; however, long-term, large-scale studies are still warranted. Those studies should compare clinical and radiographic outcomes of various available treatment options for treating peri-implantitis. Since peri-implantits is going to be one of our major concerns in the upcoming decade, the development of predictable, evidence-based treatment protocol is urgently needed.
Conclusions
The use of porous titanium granules might be a viable treatment option in cases of peri-implantitis lesions. Further large-scale long-term studies are warranted in order to assess the additional benefit from this treatment option compared to other available alternatives.
References
Zitzmann N U, Berglundh T . Definition and prevalence of peri-implant diseases. J Clin Periodontol 2008; 35(Suppl. 8): 286–291.
Albrektsson T, Isidor F . Consensus report: implant therapy. In Lang N P, Karring T. (eds) Proceedings of the 1st European Workshop on Periodontology. pp 365–369. Berlin: Quintessence, 1994.
Heitz-Mayfield L J . Peri-implant diseases: diagnosis and risk indicators. J Clin Periodontol 2008; 35(Suppl. 8): 292–304.
Claffey N, Clarke E, Polyzois I, Renvert S : Surgical treatment of peri-implantitis. J Clin Periodontol 2008; 35(Suppl. 8): 316–332.
Wohlfahrt J C, Monjo M, Rønold H J, Aass A M, Ellingsen J E, Lyngstadaas S P . Porous titanium granules promote bone healing and growth in rabbit tibia periimplant osseous defects. Clin Oral Implants Res 2010; 21: 165–173.
Lamolle S F, Monjo, M, Lyngstadaas S P, Ellingsen, J E, Haugen H J . Titanium implant surface modification by cathodic reduction in hydrofluoric acid: surface characterization and in vivo performance. J Biomed Mater Res 2009; 88: 581–588.
Monjo, M, Lamolle S F, Lyngstadaas S P, Rønold H J, Ellingsen J E . In vivo expression of osteogenic markers and bone mineral density at the surface of fluoride-modified titanium implants. Biomaterials 2008; 29: 3771–3780.
Bystedt, H, Rasmusson L . Porous titanium granules used as osteoconductive material for sinus floor augmentation: a clinical pilot study. Clin Implant Dent Relat Res 2009; 11: 101–105.
Renvert S, Polyzois I, Maguire R . Re-osseointegration on previously contaminated surfaces: a systematic review. Clin Oral Implants Res 2009; 20(Suppl. 4): 216–227.
Wohlfahrt J C, Aass A M, Rønold H J, Lyngstadaas S P . Micro CT and human histological analysis of a peri-implant osseous defect grafted with porous titanium granules: a case report. Int J Oral Maxillofac Implants 2011; 26: e9–e14.
Author information
Authors and Affiliations
Corresponding author
Additional information
Refereed Paper
Rights and permissions
About this article
Cite this article
Mijiritsky, E., Yatzkaier, G., Mazor, Z. et al. The use of porous titanium granules for treatment of peri-implantitis lesions: preliminary clinical and radiographic results in humans. Br Dent J 214, E13 (2013). https://doi.org/10.1038/sj.bdj.2013.222
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bdj.2013.222