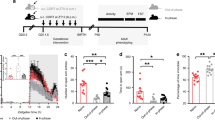
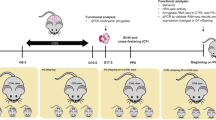
Abstract
Childhood and adolescent stress increase the risk of postpartum depression (PPD), often providing an increased probability of treatment refractoriness. Nevertheless, the mechanisms linking childhood and adolescent stress to PPD remain unclear. Here we investigated the longitudinal effects of adolescent stress on the hypothalamic–pituitary–adrenal axis and postpartum behaviors in mice and humans. Adolescent social isolation prolonged glucocorticoid elevation, leading to long-lasting postpartum behavioral changes in female mice. These changes were unresponsive to current PPD treatments but improved with post-delivery glucocorticoid receptor antagonist treatment. Childhood and adolescent stress significantly impacted hypothalamic–pituitary–adrenal axis dysregulation and PPD in human females. Repurposing glucocorticoid receptor antagonists for some cases of treatment-resistant PPD may be considered.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$59.00 per year
only $4.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Source data are provided with this paper.
References
Brunton, P. J. & Russell, J. A. The expectant brain: adapting for motherhood. Nat. Rev. Neurosci. 9, 11–25 (2008).
O’Hara, M. W. & McCabe, J. E. Postpartum depression: current status and future directions. Annu. Rev. Clin. Psychol. 9, 379–407 (2013).
Workman, J. L., Barha, C. K. & Galea, L. A. Endocrine substrates of cognitive and affective changes during pregnancy and postpartum. Behav. Neurosci. 126, 54–72 (2012).
Payne, J. L. & Maguire, J. Pathophysiological mechanisms implicated in postpartum depression. Front. Neuroendocrinol. 52, 165–180 (2019).
Viguera, A. C. et al. Episodes of mood disorders in 2,252 pregnancies and postpartum periods. Am. J. Psychiatry 168, 1179–1185 (2011).
Guintivano, J. et al. Adverse life events, psychiatric history, and biological predictors of postpartum depression in an ethnically diverse sample of postpartum women. Psychol. Med. 48, 1190–1200 (2018).
Williams, L. M., Debattista, C., Duchemin, A. M., Schatzberg, A. F. & Nemeroff, C. B. Childhood trauma predicts antidepressant response in adults with major depression: data from the randomized international study to predict optimized treatment for depression. Transl. Psychiatry 6, e799 (2016).
Nemeroff, C. B. et al. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc. Natl Acad. Sci. USA 100, 14293–14296 (2003).
Schiller, C. E., Meltzer-Brody, S. & Rubinow, D. R. The role of reproductive hormones in postpartum depression. CNS Spectr. 20, 48–59 (2015).
Axelrod, J. & Reisine, T. D. Stress hormones: their interaction and regulation. Science 224, 452–459 (1984).
Joels, M. & Baram, T. Z. The neuro-symphony of stress. Nat. Rev. Neurosci. 10, 459–466 (2009).
Sorrells, S. F., Caso, J. R., Munhoz, C. D. & Sapolsky, R. M. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron 64, 33–39 (2009).
Brummelte, S. & Galea, L. A. Postpartum depression: etiology, treatment and consequences for maternal care. Horm. Behav. 77, 153–166 (2016).
Guintivano, J., Arad, M., Gould, T. D., Payne, J. L. & Kaminsky, Z. A. Antenatal prediction of postpartum depression with blood DNA methylation biomarkers. Mol. Psychiatry 19, 560–567 (2014).
Osborne, L. et al. Replication of epigenetic postpartum depression biomarkers and variation with hormone levels. Neuropsychopharmacology 41, 1648–1658 (2016).
Meltzer-Brody, S. et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet 392, 1058–1070 (2018).
Bloch, M., Daly, R. C. & Rubinow, D. R. Endocrine factors in the etiology of postpartum depression. Compr. Psychiatry 44, 234–246 (2003).
Ahokas, A., Kaukoranta, J., Wahlbeck, K. & Aito, M. Estrogen deficiency in severe postpartum depression: successful treatment with sublingual physiologic 17β-estradiol: a preliminary study. J. Clin. Psychiatry 62, 332–336 (2001).
Bloch, M. et al. Effects of gonadal steroids in women with a history of postpartum depression. Am. J. Psychiatry 157, 924–930 (2000).
Gregoire, A. J., Kumar, R., Everitt, B., Henderson, A. F. & Studd, J. W. Transdermal oestrogen for treatment of severe postnatal depression. Lancet 347, 930–933 (1996).
Sichel, D. A., Cohen, L. S., Robertson, L. M., Ruttenberg, A. & Rosenbaum, J. F. Prophylactic estrogen in recurrent postpartum affective disorder. Biol. Psychiatry 38, 814–818 (1995).
Galea, L. A., Wide, J. K. & Barr, A. M. Estradiol alleviates depressive-like symptoms in a novel animal model of post-partum depression. Behav. Brain Res. 122, 1–9 (2001).
Green, A. D., Barr, A. M. & Galea, L. A. Role of estradiol withdrawal in ‘anhedonic’ sucrose consumption: a model of postpartum depression. Physiol. Behav. 97, 259–265 (2009).
Zhang, Z. et al. Postpartum estrogen withdrawal impairs hippocampal neurogenesis and causes depression- and anxiety-like behaviors in mice. Psychoneuroendocrinology 66, 138–149 (2016).
Stewart, D. E. & Vigod, S. N. Postpartum depression: pathophysiology, treatment, and emerging therapeutics. Annu. Rev. Med. 70, 183–196 (2019).
Molyneaux, E., Trevillion, K. & Howard, L. M. Antidepressant treatment for postnatal depression. JAMA 313, 1965–1966 (2015).
Langan, R. & Goodbred, A. J. Identification and management of peripartum depression. Am. Fam. Physician 93, 852–858 (2016).
Meltzer-Brody, S. & Kanes, S. J. Allopregnanolone in postpartum depression: role in pathophysiology and treatment. Neurobiol. Stress 12, 100212 (2020).
Kanes, S. et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet 390, 480–489 (2017).
Leader, L. D., O’Connell, M. & VandenBerg, A. Brexanolone for postpartum depression: clinical evidence and practical considerations. Pharmacotherapy 39, 1105–1112 (2019).
Blakemore, S. J. The social brain in adolescence. Nat. Rev. Neurosci. 9, 267–277 (2008).
Ibi, D. et al. Social isolation rearing-induced impairment of the hippocampal neurogenesis is associated with deficits in spatial memory and emotion-related behaviors in juvenile mice. J. Neurochem. 105, 921–932 (2008).
Kin, K., Gaini, R. & Niwa, M. in Encyclopedia of Behavioral Neuroscience 2nd edn, Vol. 1 (ed. Della Sala, S.) 360–371 (Elsevier, 2021).
Peters, Y. M. & O’Donnell, P. Social isolation rearing affects prefrontal cortical response to ventral tegmental area stimulation. Biol. Psychiatry 57, 1205–1208 (2005).
Lukkes, J. L., Watt, M. J., Lowry, C. A. & Forster, G. L. Consequences of post-weaning social isolation on anxiety behavior and related neural circuits in rodents. Front. Behav. Neurosci. 3, 18 (2009).
Walker, D. M., Cunningham, A. M., Gregory, J. K. & Nestler, E. J. Long-term behavioral effects of post-weaning social isolation in males and females. Front. Behav. Neurosci. 13, 66 (2019).
Niwa, M. et al. Adolescent stress-induced epigenetic control of dopaminergic neurons via glucocorticoids. Science 339, 335–339 (2013).
Niwa, M. et al. A critical period of vulnerability to adolescent stress: epigenetic mediators in mesocortical dopaminergic neurons. Hum. Mol. Genet. 25, 1370–1381 (2016).
Hikida, T. et al. Adolescent psychosocial stress enhances sensitization to cocaine exposure in genetically vulnerable mice. Neurosci. Res. 151, 38–45 (2020).
Matsumoto, Y. et al. Adolescent stress leads to glutamatergic disturbance through dopaminergic abnormalities in the prefrontal cortex of genetically vulnerable mice. Psychopharmacology 234, 3055–3074 (2017).
Kin, K., Francis-Oliveira, J., Kano, S. I. & Niwa, M. Adolescent stress impairs postpartum social behavior via anterior insula-prelimbic pathway in mice. Nat. Commun. 14, 2975 (2023).
Molendijk, M. L. & de Kloet, E. R. Immobility in the forced swim test is adaptive and does not reflect depression. Psychoneuroendocrinology 62, 389–391 (2015).
Melon, L., Hammond, R., Lewis, M. & Maguire, J. A novel, synthetic, neuroactive steroid is effective at decreasing depression-like behaviors and improving maternal care in preclinical models of postpartum depression. Front. Endocrinol. 9, 703 (2018).
Liu, M. Y. et al. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat. Protoc. 13, 1686–1698 (2018).
Millan, M. J. & Bales, K. L. Towards improved animal models for evaluating social cognition and its disruption in schizophrenia: the CNTRICS initiative. Neurosci. Biobehav. Rev. 37, 2166–2180 (2013).
Yang, M., Silverman, J. L. & Crawley, J. N. Automated three-chambered social approach task for mice. Curr. Protoc. Neurosci. Chapter 8, 8.26.21–28.26.16 (2011).
Hendrick, V., Altshuler, L. L. & Suri, R. Hormonal changes in the postpartum and implications for postpartum depression. Psychosomatics 39, 93–101 (1998).
Stoffel, E. C. & Craft, R. M. Ovarian hormone withdrawal-induced ‘depression’ in female rats. Physiol. Behav. 83, 505–513 (2004).
Suda, S., Segi-Nishida, E., Newton, S. S. & Duman, R. S. A postpartum model in rat: behavioral and gene expression changes induced by ovarian steroid deprivation. Biol. Psychiatry 64, 311–319 (2008).
Lupien, S. J., McEwen, B. S., Gunnar, M. R. & Heim, C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 10, 434–445 (2009).
Herman, J. P., Nawreen, N., Smail, M. A. & Cotella, E. M. Brain mechanisms of HPA axis regulation: neurocircuitry and feedback in context Richard Kvetnansky lecture. Stress 23, 617–632 (2020).
De Kloet, E. R. Why dexamethasone poorly penetrates in brain. Stress 2, 13–20 (1997).
Meijer, O. C. et al. Penetration of dexamethasone into brain glucocorticoid targets is enhanced in mdr1A P-glycoprotein knockout mice. Endocrinology 139, 1789–1793 (1998).
Liston, C. & Gan, W. B. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc. Natl Acad. Sci. USA 108, 16074–16079 (2011).
De Kloet, E. R., Vreugdenhil, E., Oitzl, M. S. & Joels, M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 19, 269–301 (1998).
McEwen, B. S. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 87, 873–904 (2007).
Ogino, S., Fuchs, C. S. & Giovannucci, E. How many molecular subtypes? Implications of the unique tumor principle in personalized medicine. Expert Rev. Mol. Diagn. 12, 621–628 (2012).
Waks, A. G. & Winer, E. P. Breast cancer treatment: a review. JAMA 321, 288–300 (2019).
Collisson, E. A., Bailey, P., Chang, D. K. & Biankin, A. V. Molecular subtypes of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 16, 207–220 (2019).
Walton, N. & Maguire, J. Allopregnanolone-based treatments for postpartum depression: why/how do they work? Neurobiol. Stress 11, 100198 (2019).
Mody, I. GABAAR modulator for postpartum depression. Cell 176, 1 (2019).
Belelli, D., Hogenkamp, D., Gee, K. W. & Lambert, J. J. Realising the therapeutic potential of neuroactive steroid modulators of the GABAA receptor. Neurobiol. Stress 12, 100207 (2020).
Althaus, A. L. et al. Preclinical characterization of zuranolone (SAGE-217), a selective neuroactive steroid GABAA receptor positive allosteric modulator. Neuropharmacology 181, 108333 (2020).
Beaudry, J. L. et al. Effects of selective and non-selective glucocorticoid receptor II antagonists on rapid-onset diabetes in young rats. PLoS One 9, e91248 (2014).
Asagami, T. et al. Selective glucocorticoid receptor (GR-II) antagonist reduces body weight gain in mice. J. Nutr. Metab. 2011, 235389 (2011).
Samuels, B. A. et al. 5-HT1A receptors on mature dentate gyrus granule cells are critical for the antidepressant response. Nat. Neurosci. 18, 1606–1616 (2015).
Locci, A., Geoffroy, P., Miesch, M., Mensah-Nyagan, A. G. & Pinna, G. Social isolation in early versus late adolescent mice is associated with persistent behavioral deficits that can be improved by neurosteroid-based treatment. Front. Cell. Neurosci. 11, 208 (2017).
Gehrand, A. L. et al. Glucocorticoid receptor antagonist alters corticosterone and receptor-sensitive mRNAs in the hypoxic neonatal rat. Endocrinology 163, 1–21 (2022).
Kroon, J. et al. Selective glucocorticoid receptor antagonist CORT125281 activates brown adipose tissue and alters lipid distribution in male mice. Endocrinology 159, 535–546 (2018).
McEwen, B. S. & Morrison, J. H. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 79, 16–29 (2013).
McEwen, B. S. & Gianaros, P. J. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann. N. Y. Acad. Sci. 1186, 190–222 (2010).
Arnsten, A. F. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 10, 410–422 (2009).
Craig, A. D. How do you feel–now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10, 59–70 (2009).
Critchley, H. D. & Harrison, N. A. Visceral influences on brain and behavior. Neuron 77, 624–638 (2013).
Nestler, E. J. & Hyman, S. E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 13, 1161–1169 (2010).
Perlman, R. L. Mouse models of human disease: an evolutionary perspective. Evol. Med. Public Health 2016, 170–176 (2016).
Castinetti, F., Brue, T. & Conte-Devolx, B. The use of the glucocorticoid receptor antagonist mifepristone in Cushing’s syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 19, 295–299 (2012).
Hunt, H. et al. Assessment of safety, tolerability, pharmacokinetics, and pharmacological effect of orally administered CORT125134: an adaptive, double-blind, randomized, placebo-controlled phase 1 clinical study. Clin. Pharmacol. Drug Dev. 7, 408–421 (2018).
Hunt, H. J. et al. Identification of the clinical candidate (R)-(1-(4-fluorophenyl)-6-((1-methyl-1H-pyrazol-4-yl)sulfonyl)-4,4a,5,6,7,8-hexahydro-1H-pyrazolo[3,4-g]isoquinolin-4a-yl)(4-(trifluoromethyl)pyridin-2-yl)methano ne (CORT125134): a selective glucocorticoid receptor (GR) antagonist. J. Med. Chem. 60, 3405–3421 (2017).
Vendruscolo, L. F. et al. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J. Clin. Invest. 125, 3193–3197 (2015).
Pineau, F. et al. New selective glucocorticoid receptor modulators reverse amyloid-beta peptide-induced hippocampus toxicity. Neurobiol. Aging 45, 109–122 (2016).
Hunt, H. J. et al. 1H-Pyrazolo[3,4-g]hexahydro-isoquinolines as potent GR antagonists with reduced hERG inhibition and an improved pharmacokinetic profile. Bioorg. Med. Chem. Lett. 25, 5720–5725 (2015).
Dulawa, S. C., Holick, K. A., Gundersen, B. & Hen, R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology 29, 1321–1330 (2004).
McMurray, K. M. J. et al. Identification of a novel, fast-acting GABAergic antidepressant. Mol. Psychiatry 23, 384–391 (2018).
Siopi, E. et al. Anxiety- and depression-like states lead to pronounced olfactory deficits and impaired adult neurogenesis in mice. J. Neurosci. 36, 518–531 (2016).
Amellem, I., Suresh, S., Chang, C. C., Tok, S. S. L. & Tashiro, A. A critical period for antidepressant-induced acceleration of neuronal maturation in adult dentate gyrus. Transl. Psychiatry 7, e1235 (2017).
Turcotte-Cardin, V. et al. Loss of adult 5-HT1A autoreceptors results in a paradoxical anxiogenic response to antidepressant treatment. J. Neurosci. 39, 1334–1346 (2019).
Vahid-Ansari, F. et al. Abrogated Freud-1/Cc2d1a repression of 5-HT1A autoreceptors induces fluoxetine-resistant anxiety/depression-like behavior. J. Neurosci. 37, 11967–11978 (2017).
Kazdoba, T. M., Hagerman, R. J., Zolkowska, D., Rogawski, M. A. & Crawley, J. N. Evaluation of the neuroactive steroid ganaxolone on social and repetitive behaviors in the BTBR mouse model of autism. Psychopharmacology 233, 309–323 (2016).
Savarese, A. M. et al. Targeting the glucocorticoid receptor reduces binge-like drinking in high drinking in the dark (HDID-1) mice. Alcohol. Clin. Exp. Res. 44, 1025–1036 (2020).
Pinna, G. & Rasmusson, A. M. Ganaxolone improves behavioral deficits in a mouse model of post-traumatic stress disorder. Front. Cell. Neurosci. 8, 256 (2014).
Cao, X. et al. Astrocyte-derived ATP modulates depressive-like behaviors. Nat. Med. 19, 773–777 (2013).
Rein, B., Ma, K. & Yan, Z. A standardized social preference protocol for measuring social deficits in mouse models of autism. Nat. Protoc. 15, 3464–3477 (2020).
Niwa, M. et al. Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron 65, 480–489 (2010).
Wada, R., Tifft, C. J. & Proia, R. L. Microglial activation precedes acute neurodegeneration in Sandhoff disease and is suppressed by bone marrow transplantation. Proc. Natl Acad. Sci. USA 97, 10954–10959 (2000).
Boyle, M. P. et al. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc. Natl Acad. Sci. USA 102, 473–478 (2005).
Pang, T. Y. et al. Positive environmental modification of depressive phenotype and abnormal hypothalamic-pituitary-adrenal axis activity in female C57BL/6J mice during abstinence from chronic ethanol consumption. Front. Pharmacol. 4, 93 (2013).
Andrikopoulos, S., Blair, A. R., Deluca, N., Fam, B. C. & Proietto, J. Evaluating the glucose tolerance test in mice. Am. J. Physiol. Endocrinol. Metab. 295, E1323–E1332 (2008).
Bowe, J. E. et al. Metabolic phenotyping guidelines: assessing glucose homeostasis in rodent models. J. Endocrinol. 222, G13–G25 (2014).
Kimmel, M. et al. Family history, not lack of medication use, is associated with the development of postpartum depression in a high-risk sample. Arch. Womens Ment. Health 18, 113–121 (2015).
Acknowledgements
We thank Corcept Therapeutics, especially H. Hunt and J. K. Belanoff for providing the GR antagonist CORT113176 and technical support for its use in an academic study. We also thank clinical study participants and staff members. We appreciate C. Erdly and D. Mallah for their technical help, and M. A. Landek-Salgado for scientific reading. This work was supported by the National Institutes of Health (MH-092443 (A.S.), MH-094268 Silvio O. Conte Center (A.S.), K99MH-094408 (M.N.), MH-105660 (A.S.), MH-107730 (A.S.), DA-040127 (A.S. and M.N.), and MH-116869 (M.N.)), NARSAD (A.S. and M.N.), Stanley (A.S.), S-R/RUSK (A.S.), JST PRESTO JPMJPR14M6 (M.N.), UAB Psychiatry startup funds (M.N.) and UAB Comprehensive Neuroscience Center Pilot Award (M.N. and S.K.).
Author information
Authors and Affiliations
Contributions
M.N. conceived and designed the project, and supervised S.L., D.J.W., J.F.-O., K.K. and A.A. for practical experiments with the help of S.K. and the guidance of A.S. Experiments were performed by M.N., S.L., D.J.W., J.F.-O., K.K. and A.A. Data were analyzed by M.N., K.Y., J.F.-O. and K.K. The first manuscript draft was written by M.N. and A.S. with input from all the authors. M.N. and A.S. revised and edited the manuscript for the final version. G.S.W. provided expertise of endocrinology in a translationally relevant manner. J.L.P. provided human plasma samples with participants’ information as well as expertise of postpartum mood disorders.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks Sylvie Lesuis, Fernanda B. Lima, Kiyofumi Yamada and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Experimental schedule of the preclinical study.
A, Virgin female mice were group-housed. B, Virgin female mice were isolated from 5 to 8 weeks of age. C, Virgin female mice were group-housed, mated with a male mouse, and gave birth to pups. D, Virgin female mice were isolated from 5 to 8 weeks of age, mated with a male mouse, and gave birth to pups. TST, tail suspension test; FST, forced swim test; SIT, three-chamber social interaction test. Different cohorts of mice subjected to behavioral tests at 0 week, 1 week, and 3 weeks postpartum were studied to avoid the repeated exposure to stressful behavioral procedures.
Extended Data Fig. 2 Long-lasting behavioral changes in the social interaction test in dams exposed to adolescent social isolation.
Sniffing time and indexes of sociability and social novelty recognition during the three-chamber social interaction test were measured at postpartum days 0, 7, and 21. No changes in sociability or social novelty recognition among the four groups were observed at postpartum day 0. Behavioral changes in social novelty recognition, but not sociability, were observed in stressed dams at postpartum days 7 and 21. Postpartum day 0/Unstressed virgins, N = 10; Postpartum day 0/Stressed virgins, N = 9; Postpartum day 0/Unstressed dams, N = 10; Postpartum day 0/Stressed dams, N = 9; Postpartum day 7/Unstressed virgins, N = 10; Postpartum day 7/Stressed virgins, N = 10; Postpartum day 7/Unstressed dams, N = 10; Postpartum day 7/Stressed dams, N = 11; Postpartum day 21/Unstressed virgins, N = 10; Postpartum day 21/Stressed virgins, N = 10; Postpartum day 21/Unstressed dams, N = 12; Postpartum day 21/Stressed dams, N = 11. Values are represented as mean ± SEM; **P < 0.01 and *P < 0.05. See Supplementary Table 1 for details on the statistical analyses.
Extended Data Fig. 3 No change in the levels of plasma estradiol, progesterone, oxytocin, and prolactin in mice exposed to adolescent social isolation.
Levels of estradiol, progesterone, oxytocin, and prolactin in plasma were measured at four-time points (virgin, late pregnancy, 0 week postpartum, and 1 week postpartum). No differences in the levels of estradiol, progesterone, oxytocin, and prolactin were observed between unstressed and stressed mice at any time point. N = 9–20 (precise values detailed in Supplementary Table 1). Values are represented as mean ± SEM. See Supplementary Table 1 for details on the statistical analyses.
Extended Data Fig. 4 Positive correlation between plasma corticosterone levels and immobility time in the tail suspension and forced swim tests at 1 week and 3 weeks after delivery.
The levels of plasma corticosterone (CORT) were positively correlated with immobility time in the tail suspension test (TST) and forced swim test (FST) at 1 week and 3 weeks after delivery. 1 week postpartum/Unstressed dams, N = 9; 1 week postpartum/Stressed dams, N = 10; 3 weeks postpartum/Unstressed dams, N = 12; 3 weeks postpartum/Stressed dams, N = 11. Spearman and Pearson rank correlation coefficients were examined for the data at 1 week and 3 weeks postpartum, respectively. See Supplementary Table 1 for details on the statistical analyses.
Extended Data Fig. 5 Dose-response effects of a SSRI, a GABAA receptor modulator, or a GR antagonist on behavioral changes in dams exposed to adolescent social isolation.
A–C, Stressed dams were treated with a SSRI fluoxetine (18, 36, 52 mg/kg, p.o.) (A), a GABAA receptor modulator ganaxolone (10, 20, 30 mg/kg, i.p.) (B), or a GR antagonist CORT113176 (40, 80, 160 mg/kg, p.o.) (C) once daily from postpartum day 0 to 24 h prior to sampling at postpartum day 9. Post-delivery treatment with only the GR antagonist at 40 and 80 mg/kg ameliorated the behavioral changes in the tail suspension test on postpartum day 7 in stressed dams. Post-delivery treatment with only the GR antagonist at 80 mg/kg ameliorated the behavioral changes in forced swim test on postpartum day 8 in stressed dams N = 12. Values are represented as mean ± SEM; **P < 0.01 and *P < 0.05. See Supplementary Table 1 for details on the statistical analyses.
Extended Data Fig. 6 No effect of a GR antagonist, a SSRI, and an allopregnanolone analog on body weight.
A, CORT113176 (80 mg/kg, p.o., once daily from gestation day 14 to 24 h prior to behavioral testing), a selective GR antagonist, did not affect body weight after the forced swim test at postpartum day 8 in either group. B, Post-delivery treatment with a SSRI fluoxetine (18 mg/kg, p.o.), a GABAA receptor modulator ganaxolone (10 mg/kg, i.p.), or CORT113176 (80 mg/kg, p.o.) once daily from postpartum day 0 to 24 h prior to behavioral testing did not affect body weight after the forced swim test at postpartum day 8 in any of the groups. N = 12. Values are represented as mean ± SEM. See Supplementary Table 1 for details on the statistical analyses.
Extended Data Fig. 7 No effect of a GR antagonist on blood glucose levels and weight of the thymus, spleen, and visceral fat.
A, After fasting for 6 hours, glucose (2 g/kg) was administered intraperitoneally and blood glucose levels at 0, 30, 60, 90, and 120 minutes were examined on postnatal day 8. CORT113176 (80 mg/kg, p.o., once daily from postpartum day 0 to 24 h prior to the blood glucose testing at postpartum day 8), a selective GR antagonist, did not affect blood glucose levels in either group. B-D, Thymus, spleen, and visceral fat weights were measured after blood glucose testing at postpartum day 8. CORT113176 (80 mg/kg, p.o., once daily from postpartum day 0 to 24 h prior to the blood glucose testing at postpartum day 8) had no effect on weight of the thymus (B), spleen (C), and visceral fat (D) in any groups. Unstressed/Vehicle, N = 6; Unstressed/GR antagonist, N = 6; Stressed/Vehicle, N = 6; Stressed/GR antagonist, N = 7. Values are represented as mean ± SEM. See Supplementary Table 1 for details on the statistical analyses.
Supplementary information
Supplementary Information
Supplementary Table 1: Statistical results.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Niwa, M., Lockhart, S., Wood, D.J. et al. Prolonged HPA axis dysregulation in postpartum depression associated with adverse early life experiences: a cross-species translational study. Nat. Mental Health (2024). https://doi.org/10.1038/s44220-024-00217-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44220-024-00217-1