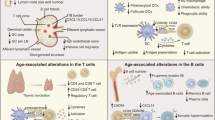
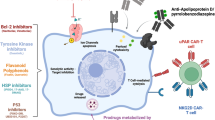
Abstract
Aging is a major risk factor for numerous chronic diseases. Vaccination offers a promising strategy to combat these age-related diseases by targeting specific antigens and inducing immune responses. Here, we provide a comprehensive overview of recent advances in vaccine-based interventions targeting these diseases, including Alzheimer’s disease, type II diabetes, hypertension, abdominal aortic aneurysm, atherosclerosis, osteoarthritis, fibrosis and cancer, summarizing current approaches for identifying disease-associated antigens and inducing immune responses against these targets. Further, we reflect on the recent development of vaccines targeting senescent cells, as a strategy for more broadly targeting underlying causes of aging and associated pathologies. In addition to highlighting recent progress in these areas, we discuss important next steps to advance the therapeutic potential of these vaccines, including improving and robustly demonstrating efficacy in human clinical trials, as well as rigorously evaluating the safety and long-term effects of these vaccine strategies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
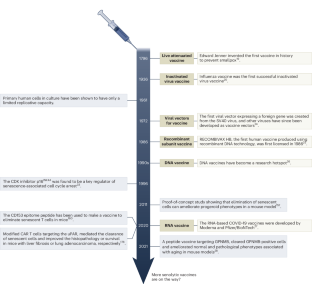





Similar content being viewed by others
References
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell 186, 243–278 (2023).
Benz, C. C. & Yau, C. Ageing, oxidative stress and cancer: paradigms in parallax. Nat. Rev. Cancer 8, 875–879 (2008).
Wu, Z., Qu, J., Zhang, W. & Liu, G. H. Stress, epigenetics, and aging: unraveling the intricate crosstalk. Mol. Cell 84, 34–54 (2024).
Cai, Y. et al. The landscape of aging. Sci. China Life Sci. 65, 2354–2454 (2022).
Dehghan, A. et al. Risk of type 2 diabetes attributable to C-reactive protein and other risk factors. Diabetes Care 30, 2695–2699 (2007).
Bussian, T. J. et al. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 562, 578–582 (2018).
Yan, H. et al. Degeneration Directory: a multi-omics web resource for degenerative diseases. Protein Cell https://doi.org/10.1093/procel/pwad066 (2023).
Katz, D. L. & Meller, S. Can we say what diet is best for health? Annu. Rev. Public Health 35, 83–103 (2014).
Newman, J. C. et al. Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metab. 26, 547–557 (2017).
Hojman, P., Gehl, J., Christensen, J. F. & Pedersen, B. K. Molecular mechanisms linking exercise to cancer prevention and treatment. Cell Metab. 27, 10–21 (2018).
Harrison, D. E. et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395 (2009).
Vaiserman, A., De Falco, E., Koliada, A., Maslova, O. & Balistreri, C. R. Anti-ageing gene therapy: not so far away? Ageing Res. Rev. 56, 100977 (2019).
Yan, P. et al. FOXO3-engineered human ESC-derived vascular cells promote vascular protection and regeneration. Cell Stem Cell 24, 447–461 (2019).
Liu, F. et al. Identification of FOXO1 as a geroprotector in human synovium through single-nucleus transcriptomic profiling. Protein Cell https://doi.org/10.1093/procel/pwad060 (2023).
Huang, D. et al. CRL2APPBP2-mediated TSPYL2 degradation counteracts human mesenchymal stem cell senescence. Sci. China Life Sci. https://doi.org/10.1007/s11427-023-2451-3 (2023).
Jing, Y. et al. Single-nucleus profiling unveils a geroprotective role of the FOXO3 in primate skeletal muscle aging. Protein Cell 14, 497–512 (2023).
Yusheng Cai, Z. J., Si Wang. et al. Genetic enhancement: an avenue to combat aging-related diseases. Life Med. https://doi.org/10.1093/lifemedi/lnac054 (2022).
Rudin, C. M. et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin. Cancer Res. 18, 3163–3169 (2012).
Riedel, S. Edward Jenner and the history of smallpox and vaccination. Proc. Bayl Univ. Med. Cent. 18, 21–25 (2005).
Thomas, F. Jr & Magill, T. Vaccination of human subjects with virus of human influenza. Proc. Soc. Exp. Biol. Med. 33, 604–606 (1936).
Liu, J. et al. Cancer vaccines as promising immuno-therapeutics: platforms and current progress. J. Hematol. Oncol. 15, 28 (2022).
Pulendran, B. & Ahmed, R. Immunological mechanisms of vaccination. Nat. Immunol. 12, 509–517 (2011).
Pollard, A. J. & Bijker, E. M. A guide to vaccinology: from basic principles to new developments. Nat. Rev. Immunol. 21, 83–100 (2021).
Jackson, D. A., Symons, R. H. & Berg, P. Biochemical method for inserting new genetic information into DNA of Simian Virus 40: circular SV40 DNA molecules containing lambda phage genes and the galactose operon of Escherichia coli. Proc. Natl Acad. Sci. USA 69, 2904–2909 (1972).
McAleer, W. J. et al. Human hepatitis B vaccine from recombinant yeast. Nature 307, 178–180 (1984).
Kutzler, M. A. & Weiner, D. B. DNA vaccines: ready for prime time? Nat. Rev. Genet. 9, 776–788 (2008).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Suda, M. et al. Senolytic vaccination improves normal and pathological age-related phenotypes and increases lifespan in progeroid mice. Nat. Aging 1, 1117–1126 (2021).
Aging Biomarker, C. et al. Biomarkers of aging. Sci. China Life Sci. 66, 893–1066 (2023).
Aging Biomarker Consortium; Suo, J. et al. A framework of biomarkers for skeletal aging: a consensus statement by the Aging Biomarker Consortium. Life Med. https://doi.org/10.1093/lifemedi/lnad045 (2023).
Aging Biomarker Consortium; Zhang, L. et al. A framework of biomarkers for vascular aging: a consensus statement by the Aging Biomarker Consortium. Life Med. https://doi.org/10.1093/lifemedi/lnad033 (2023).
Aging Biomarker Consortium et al. A biomarker framework for cardiac aging: the Aging Biomarker Consortium consensus statement. Life Med. https://doi.org/10.1093/lifemedi/lnad035 (2023).
Alcorta, D. A. et al. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc. Natl Acad. Sci. USA 93, 13742–13747 (1996).
Lu, H. et al. Aging hallmarks of the primate ovary revealed by spatiotemporal transcriptomics. Protein Cell https://doi.org/10.1093/procel/pwad063 (2023).
Boni-Schnetzler, M. et al. Increased interleukin (IL)-1beta messenger ribonucleic acid expression in beta -cells of individuals with type 2 diabetes and regulation of IL-1beta in human islets by glucose and autostimulation. J. Clin. Endocrinol. Metab. 93, 4065–4074 (2008).
Valiukas, Z. et al. Immunotherapies for Alzheimer’s disease—a review. Vaccines https://doi.org/10.3390/vaccines10091527 (2022).
Pecchi, E. et al. Induction of nerve growth factor expression and release by mechanical and inflammatory stimuli in chondrocytes: possible involvement in osteoarthritis pain. Arthritis Res. Ther. 16, R16 (2014).
Xia, X., Jiang, Q., McDermott, J. & Han, J. J. Aging and Alzheimer’s disease: Comparison and associations from molecular to system level. Aging Cell 17, e12802 (2018).
McDade, E., Llibre-Guerra, J. J., Holtzman, D. M., Morris, J. C. & Bateman, R. J. The informed road map to prevention of Alzheimer disease: a call to arms. Mol. Neurodegener. 16, 49 (2021).
Broussard, G. J., Mytar, J., Li, R. C. & Klapstein, G. J. The role of inflammatory processes in Alzheimer’s disease. Inflammopharmacology 20, 109–126 (2012).
Zhang, Y., Chen, H., Li, R., Sterling, K. & Song, W. Amyloid beta-based therapy for Alzheimer’s disease: challenges, successes and future. Signal Transduct. Target. Ther. 8, 248 (2023).
Ossenkoppele, R., van der Kant, R. & Hansson, O. Tau biomarkers in Alzheimer’s disease: towards implementation in clinical practice and trials. Lancet Neurol. 21, 726–734 (2022).
Yamazaki, Y., Zhao, N., Caulfield, T. R., Liu, C. C. & Bu, G. Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat. Rev. Neurol. 15, 501–518 (2019).
Depp, C. et al. Myelin dysfunction drives amyloid-beta deposition in models of Alzheimer’s disease. Nature 618, 349–357 (2023).
Gilman, S. et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 64, 1553–1562 (2005).
Reiss, A. B. et al. Alzheimer disease clinical trials targeting amyloid: lessons learned from success in mice and failure in humans. Neurologist 26, 52–61 (2021).
Ketter, N. et al. A randomized, double-blind, phase 2 study of the effects of the vaccine vanutide cridificar with QS-21 adjuvant on immunogenicity, safety and amyloid imaging in patients with mild to moderate alzheimer’s disease. J. Prev. Alzheimers Dis. 3, 192–201 (2016).
Plascencia-Villa, G. & Perry, G. Lessons from antiamyloid-beta immunotherapies in Alzheimer’s disease. Handb. Clin. Neurol. 193, 267–292 (2023).
Hardy, J. & Allsop, D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol. Sci. 12, 383–388 (1991).
Muhs, A. et al. Liposomal vaccines with conformation-specific amyloid peptide antigens define immune response and efficacy in APP transgenic mice. Proc. Natl Acad. Sci. USA 104, 9810–9815 (2007).
Hickman, D. T. et al. Sequence-independent control of peptide conformation in liposomal vaccines for targeting protein misfolding diseases. J. Biol. Chem. 286, 13966–13976 (2011).
Rafii, M. S. et al. Safety, tolerability, and immunogenicity of the ACI-24 vaccine in adults with Down syndrome: a phase 1b randomized clinical trial. JAMA Neurol. 79, 565–574, (2022).
Wang, C. Y. et al. UB-311, a novel UBITh amyloid beta peptide vaccine for mild Alzheimer’s disease. Alzheimers Dement. 3, 262–272 (2017).
Kwan, P., Konno, H., Chan, K. Y. & Baum, L. Rationale for the development of an Alzheimer’s disease vaccine. Hum. Vaccin. Immunother. 16, 645–653 (2020).
Yu, H. J. et al. Safety, tolerability, immunogenicity, and efficacy of UB-311 in participants with mild Alzheimer’s disease: a randomised, double-blind, placebo-controlled, phase 2a study. EBioMedicine 94, 104665 (2023).
Petrushina, I. et al. Characterization and preclinical evaluation of the cGMP grade DNA based vaccine, AV-1959D to enter the first-in-human clinical trials. Neurobiol. Dis. 139, 104823 (2020).
Davtyan, H. et al. The MultiTEP platform-based Alzheimer’s disease epitope vaccine activates a broad repertoire of T helper cells in nonhuman primates. Alzheimers Dement. 10, 271–283 (2014).
Lacosta, A. M. et al. Safety, tolerability and immunogenicity of an active anti-Abeta(40) vaccine (ABvac40) in patients with Alzheimer’s disease: a randomised, double-blind, placebo-controlled, phase I trial. Alzheimers Res. Ther. 10, 12 (2018).
Neff, R. A. et al. Molecular subtyping of Alzheimer’s disease using RNA sequencing data reveals novel mechanisms and targets. Sci. Adv. https://doi.org/10.1126/sciadv.abb5398 (2021).
Theunis, C. et al. Efficacy and safety of a liposome-based vaccine against protein Tau, assessed in tau.P301L mice that model tauopathy. PLoS ONE 8, e72301 (2013).
Song, C. et al. Immunotherapy for Alzheimer’s disease: targeting beta-amyloid and beyond. Transl. Neurodegener. 11, 18 (2022).
Novak, P. et al. FUNDAMANT: an interventional 72-week phase 1 follow-up study of AADvac1, an active immunotherapy against tau protein pathology in Alzheimer’s disease. Alzheimers Res. Ther. 10, 108 (2018).
Panza, F. & Logroscino, G. Anti-tau vaccine in Alzheimer’s disease: a tentative step. Lancet Neurol. 16, 99–100 (2017).
Novak, P. et al. ADAMANT: a placebo-controlled randomized phase 2 study of AADvac1, an active immunotherapy against pathological tau in Alzheimer’s disease. Nat. Aging 1, 521–534 (2021).
Sato, C. et al. Tau kinetics in neurons and the human central nervous system. Neuron 97, 1284–1298 (2018).
Park, H. H. et al. Novel vaccine peptide GV1001 effectively blocks beta-amyloid toxicity by mimicking the extra-telomeric functions of human telomerase reverse transcriptase. Neurobiol. Aging 35, 1255–1274 (2014).
Malonis, R. J., Lai, J. R. & Vergnolle, O. Peptide-based vaccines: current progress and future challenges. Chem. Rev. 120, 3210–3229 (2020).
Frenkel, D., Maron, R., Burt, D. S. & Weiner, H. L. Nasal vaccination with a proteosome-based adjuvant and glatiramer acetate clears beta-amyloid in a mouse model of Alzheimer disease. J. Clin. Invest. 115, 2423–2433 (2005).
Rafii, M. S. & Aisen, P. S. Detection and treatment of Alzheimer’s disease in its preclinical stage. Nat. Aging 3, 520–531 (2023).
Baruch, K. et al. PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer’s disease. Nat. Med. 22, 135–137 (2016).
Ogurtsova, K. et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 128, 40–50 (2017).
DeFronzo, R. A. et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Prim. 1, 15019 (2015).
Gallwitz, B. Clinical perspectives on the use of the GIP/GLP-1 receptor agonist tirzepatide for the treatment of type-2 diabetes and obesity. Front. Endocrinol. https://doi.org/10.3389/fendo.2022.1004044 (2022).
Vogt, A. S. et al. Anti-IAPP monoclonal antibody improves clinical symptoms in a mouse model of type 2 diabetes. Vaccines https://doi.org/10.3390/vaccines9111316 (2021).
Scarpa, E. S. et al. The combination of natural molecules naringenin, hesperetin, curcumin, polydatin and quercetin synergistically decreases sema3e expression levels and DPPIV activity in in vitro models of insulin resistance. Int. J. Mol. Sci. https://doi.org/10.3390/ijms24098071 (2023).
Voelker, J. et al. Anti-TGF-beta1 antibody therapy in patients with diabetic nephropathy. J. Am. Soc. Nephrol. 28, 953–962 (2017).
Galicia-Garcia, U. et al. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21176275 (2020).
Sandhu, H. et al. Glucagon-like peptide 1 increases insulin sensitivity in depancreatized dogs. Diabetes 48, 1045–1053 (1999).
Pang, Z. et al. Therapeutic vaccine against DPP4 improves glucose metabolism in mice. Proc. Natl Acad. Sci. USA 111, E1256–E1263 (2014).
Dinarello, C. A., Simon, A. & van der Meer, J. W. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 11, 633–652 (2012).
Malozowski, S. & Sahlroot, J. T. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 357, 302–303 (2007).
Cavelti-Weder, C. et al. Development of an interleukin-1beta vaccine in patients with type 2 diabetes. Mol. Ther. 24, 1003–1012 (2016).
Dhimolea, E. Canakinumab. MAbs 2, 3–13 (2010).
Zhang, Y. et al. Therapeutic vaccine against IL-1beta improved glucose control in a mouse model of type 2 diabetes. Life Sci. 192, 68–74 (2018).
Ekoru, K. et al. Type 2 diabetes complications and comorbidity in sub-Saharan Africans. EClinicalMedicine 16, 30–41 (2019).
Danser, A. H. & Deinum, J. Renin, prorenin and the putative (pro)renin receptor. Hypertension 46, 1069–1076 (2005).
Kanda, A. & Ishida, S. Prorenin receptor: involvement in diabetic retinopathy and development of molecular targeted therapy. J. Diabetes Investig. 10, 6–17 (2019).
Yokota, H. et al. Effect of prorenin peptide vaccine on the early phase of diabetic retinopathy in a murine model of type 2 diabetes. PLoS ONE 17, e0262568 (2022).
Abdul-Ghani, M. A. & DeFronzo, R. A. Pathogenesis of insulin resistance in skeletal muscle. J. Biomed. Biotechnol. 2010, 476279 (2010).
Shimizu, I. et al. Semaphorin3E-induced inflammation contributes to insulin resistance in dietary obesity. Cell Metab. 18, 491–504 (2013).
Garay-Gutierrez, N. F., Hernandez-Fuentes, C. P., Garcia-Rivas, G., Lavandero, S. & Guerrero-Beltran, C. E. Vaccines against components of the renin–angiotensin system. Heart Fail. Rev. 26, 711–726 (2021).
Downham, M. R. et al. Evaluation of two carrier protein-angiotensin I conjugate vaccines to assess their future potential to control high blood pressure (hypertension) in man. Br. J. Clin. Pharm. 56, 505–512 (2003).
Ambuhl, P. M. et al. A vaccine for hypertension based on virus-like particles: preclinical efficacy and phase I safety and immunogenicity. J. Hypertens. 25, 63–72 (2007).
Tissot, A. C. et al. Effect of immunisation against angiotensin II with CYT006-AngQb on ambulatory blood pressure: a double-blind, randomised, placebo-controlled phase IIa study. Lancet 371, 821–827 (2008).
Brown, M. J. Success and failure of vaccines against renin–angiotensin system components. Nat. Rev. Cardiol. 6, 639–647 (2009).
Chen, X. et al. Effectiveness and safety of a therapeutic vaccine against angiotensin II receptor type 1 in hypertensive animals. Hypertension 61, 408–416 (2013).
Zhou, Y. et al. ATRQbeta-001 vaccine prevents atherosclerosis in apolipoprotein E-null mice. J. Hypertens. 34, 474–485 (2016).
Pan, Y. et al. The ATRQbeta-001 vaccine improves cardiac function and prevents postinfarction cardiac remodeling in mice. Hypertens. Res. 42, 329–340 (2019).
Ferrario, C. M. Importance of the renin-angiotensin-aldosterone system (RAS) in the physiology and pathology of hypertension. An overview. Drugs 39, 1–8 (1990).
Li, C. et al. Vaccine targeted alpha 1D-adrenergic receptor for hypertension. Hypertension 74, 1551–1562 (2019).
Tanoue, A. et al. The alpha 1D-adrenergic receptor directly regulates arterial blood pressure via vasoconstriction. J. Clin. Invest. 109, 765–775 (2002).
Docherty, J. R. Subtypes of functional alpha1-adrenoceptor. Cell. Mol. Life Sci. 67, 405–417 (2010).
Nordon, I. M., Hinchliffe, R. J., Loftus, I. M. & Thompson, M. M. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat. Rev. Cardiol. 8, 92–102 (2011).
Greenhalgh, R. M. et al. Early elective open surgical repair of small abdominal aortic aneurysms is not recommended: results of the UK Small Aneurysm Trial. Steering Committee. Eur. J. Vasc. Endovasc. Surg. 16, 462–464 (1998).
Kurashiki, T., Miyake, T., Nakagami, H., Nishimura, M. & Morishita, R. Prevention of progression of aortic aneurysm by peptide vaccine against Ang II (angiotensin II) in a rat model. Hypertension 76, 1879–1888 (2020).
Xuan, H. et al. Inhibition or deletion of angiotensin II type 1 receptor suppresses elastase-induced experimental abdominal aortic aneurysms. J. Vasc. Surg. 67, 573–584 (2018).
Zhang, H. et al. ATRQbeta-001 vaccine prevents experimental abdominal aortic aneurysms. J. Am. Heart Assoc. 8, e012341 (2019).
Wang, J. C. & Bennett, M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ. Res. 111, 245–259 (2012).
Kobiyama, K. & Ley, K. Atherosclerosis. Circ. Res. 123, 1118–1120 (2018).
Hansson, G. K. & Nilsson, J. Developing a vaccine against atherosclerosis. Nat. Rev. Cardiol. 17, 451–452 (2020).
Ait-Oufella, H. et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat. Med. 12, 178–180 (2006).
Gistera, A. et al. Low-density lipoprotein-reactive T cells regulate plasma cholesterol levels and development of atherosclerosis in humanized hypercholesterolemic mice. Circulation 138, 2513–2526 (2018).
Lichtman, A. H., Binder, C. J., Tsimikas, S. & Witztum, J. L. Adaptive immunity in atherogenesis: new insights and therapeutic approaches. J. Clin. Invest. 123, 27–36 (2013).
Kimura, T. et al. Regulatory CD4+ T cells recognize major histocompatibility complex class II molecule-restricted peptide epitopes of apolipoprotein B. Circulation 138, 1130–1143 (2018).
Momtazi-Borojeni, A. A., Jaafari, M. R., Badiee, A. & Sahebkar, A. Long-term generation of antiPCSK9 antibody using a nanoliposome-based vaccine delivery system. Atherosclerosis 283, 69–78 (2019).
Ma, Z. et al. Peptide vaccine against ADAMTS-7 ameliorates atherosclerosis and postinjury neointima hyperplasia. Circulation 147, 728–742 (2023).
Bourinbaiar, A. S. & Jirathitikal, V. Effect of oral immunization with pooled antigens derived from adipose tissue on atherosclerosis and obesity indices. Vaccine 28, 2763–2768 (2010).
Bourinbaiar, A. S. & Jirathitikal, V. Safety and efficacy trial of adipose-tissue derived oral preparation V-6 Immunitor (V-6): results of open-label, two-month, follow-up study. Lipids Health Dis. 9, 14 (2010).
Nilsson, J. & Hansson, G. K. Vaccination strategies and immune modulation of atherosclerosis. Circ. Res. 126, 1281–1296 (2020).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Loeser, R. F. The role of aging in the development of osteoarthritis. Trans. Am. Clin. Climatol. Assoc. 128, 44–54 (2017).
Shane Anderson, A. & Loeser, R. F. Why is osteoarthritis an age-related disease? Best. Pract. Res Clin. Rheumatol. 24, 15–26 (2010).
von Loga, I. S. et al. Active immunisation targeting nerve growth factor attenuates chronic pain behaviour in murine osteoarthritis. Ann. Rheum. Dis. 78, 672–675 (2019).
Lane, N. E. & Corr, M. Osteoarthritis in 2016: anti-NGF treatments for pain—two steps forward, one step back? Nat. Rev. Rheumatol. 13, 76–78 (2017).
Chen, Y. et al. Aging reprograms the hematopoietic-vascular niche to impede regeneration and promote fibrosis. Cell Metab. 33, 395–410 (2021).
Brack, A. S. et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317, 807–810 (2007).
Sobecki, M. et al. Vaccination-based immunotherapy to target profibrotic cells in liver and lung. Cell Stem Cell 29, 1459–1474 (2022).
Kang, H. M. et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 21, 37–46 (2015).
Wu, D. et al. Vaccine against PCSK9 improved renal fibrosis by regulating fatty acid beta-oxidation. J. Am. Heart Assoc. 9, e014358 (2020).
Melero, I. et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat. Rev. Clin. Oncol. 11, 509–524 (2014).
Markowitz, L. E. et al. Human papillomavirus vaccine introduction—the first five years. Vaccine 30, 139–148, (2012).
Crews, D. W., Dombroski, J. A. & King, M. R. Prophylactic cancer vaccines engineered to elicit specific adaptive immune response. Front. Oncol. 11, 626463 (2021).
Karpanen, T. & Olweus, J. The potential of donor T-cell repertoires in neoantigen-targeted cancer immunotherapy. Front. Immunol. 8, 1718 (2017).
Paston, S. J., Brentville, V. A., Symonds, P. & Durrant, L. G. Cancer vaccines, adjuvants, and delivery systems. Front. Immunol. 12, 627932 (2021).
Giaccone, G. et al. A phase III study of belagenpumatucel-L, an allogeneic tumour cell vaccine, as maintenance therapy for non-small cell lung cancer. Eur. J. Cancer 51, 2321–2329 (2015).
Verma, V. et al. PD-1 blockade in subprimed CD8 cells induces dysfunctional PD-1+CD38hi cells and anti-PD-1 resistance. Nat. Immunol. 20, 1231–1243 (2019).
Saxena, M., van der Burg, S. H., Melief, C. J. M. & Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 21, 360–378 (2021).
Lazaro, A. et al. Human leukocyte antigen (HLA) typing by DNA sequencing. Methods Mol. Biol. 1034, 161–195 (2013).
Jennings, L. J. et al. Guidelines for validation of next-generation sequencing-based oncology panels: a joint consensus recommendation of the association for molecular pathology and college of american pathologists. J. Mol. Diagn. 19, 341–365 (2017).
Duperret, E. K. et al. A Synthetic DNA, multi-neoantigen vaccine drives predominately MHC class I CD8+ T-cell responses, impacting tumor challenge. Cancer Immunol. Res. 7, 174–182 (2019).
Liu, S. et al. A DNA nanodevice-based vaccine for cancer immunotherapy. Nat. Mater. 20, 421–430 (2021).
Hammerich, L., Bhardwaj, N., Kohrt, H. E. & Brody, J. D. In situ vaccination for the treatment of cancer. Immunotherapy 8, 315–330 (2016).
Chen, J. et al. In situ cancer vaccination using lipidoid nanoparticles. Sci. Adv. 7, eadv.abf1244 (2021).
Chen, L. et al. Bacterial cytoplasmic membranes synergistically enhance the antitumor activity of autologous cancer vaccines. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.abc2816 (2021).
Lopez-Otin, C., Pietrocola, F., Roiz-Valle, D., Galluzzi, L. & Kroemer, G. Meta-hallmarks of aging and cancer. Cell Metab. 35, 12–35 (2023).
Wells, D. K. et al. Key parameters of tumor epitope immunogenicity revealed through a consortium approach improve neoantigen prediction. Cell 183, 818–834 (2020).
Liu, X. et al. Resurrection of endogenous retroviruses during aging reinforces senescence. Cell 186, 287–304 (2023).
Xiaoqian Liu, H. J. et al. Migrasomes trigger innate immune activation and mediate transmission of senescence signals across human cells. Life Med. https://doi.org/10.1093/lifemedi/lnad050 (2023).
Marin, I. et al. Cellular senescence is immunogenic and promotes antitumor immunity. Cancer Discov. 13, 410–431 (2023).
Sciorati, C. et al. Pharmacological blockade of TNF prevents sarcopenia and prolongs survival in aging mice. Aging 12, 23497–23508 (2020).
Ridker, P. M. et al. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 390, 1833–1842 (2017).
Kang, T. W. et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 479, 547–551 (2011).
Chen, H. A. et al. Senescence rewires microenvironment sensing to facilitate antitumor immunity. Cancer Discov. 13, 432–453 (2023).
Childs, B. G. et al. Senescent cells: an emerging target for diseases of ageing. Nat. Rev. Drug Discov. 16, 718–735 (2017).
Childs, B. G. et al. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354, 472–477 (2016).
Jeon, O. H. et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 23, 775–781 (2017).
Baker, D. J. et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 (2011).
Baker, D. J. et al. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature 530, 184–189 (2016).
Xue, W. et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445, 656–660 (2007).
Yoshida, S. et al. The CD153 vaccine is a senotherapeutic option for preventing the accumulation of senescent T cells in mice. Nat. Commun. 11, 2482 (2020).
Sharpless, N. E., Ramsey, M. R., Balasubramanian, P., Castrillon, D. H. & DePinho, R. A. The differential impact of p16INK4a or p19ARF deficiency on cell growth and tumorigenesis. Oncogene 23, 379–385 (2004).
Xu, W. & Larbi, A. Markers of T cell senescence in humans. Int. J. Mol. Sci. https://doi.org/10.3390/ijms18081742 (2017).
Mittelbrunn, M. & Kroemer, G. Hallmarks of T cell aging. Nat. Immunol. 22, 687–698 (2021).
Shirakawa, K. et al. Obesity accelerates T cell senescence in murine visceral adipose tissue. J. Clin. Invest. 126, 4626–4639 (2016).
Smith, C. A. et al. CD30 antigen, a marker for Hodgkin’s lymphoma, is a receptor whose ligand defines an emerging family of cytokines with homology to TNF. Cell 73, 1349–1360 (1993).
Blazar, B. R. et al. CD30/CD30 ligand (CD153) interaction regulates CD4+ T cell-mediated graft-versus-host disease. J. Immunol. 173, 2933–2941 (2004).
Sato, Y. et al. CD153/CD30 signaling promotes age-dependent tertiary lymphoid tissue expansion and kidney injury. J. Clin. Invest. https://doi.org/10.1172/JCI146071 (2022).
Yazdanbakhsh, M., Kremsner, P. G. & van Ree, R. Allergy, parasites, and the hygiene hypothesis. Science 296, 490–494 (2002).
Hansen, G., Berry, G., DeKruyff, R. H. & Umetsu, D. T. Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation. J. Clin. Invest. 103, 175–183 (1999).
Sallin, M. A. et al. Host resistance to pulmonary Mycobacterium tuberculosis infection requires CD153 expression. Nat. Microbiol. 3, 1198–1205 (2018).
Vanhoutte, P. M. Endothelial dysfunction and atherosclerosis. Eur. Heart J. 18, E19–E29 (1997).
Suda, M. et al. Glycoprotein nonmetastatic melanoma protein B regulates lysosomal integrity and lifespan of senescent cells. Sci. Rep. 12, 6522 (2022).
Ogawa, T. et al. Osteoactivin upregulates expression of MMP-3 and MMP-9 in fibroblasts infiltrated into denervated skeletal muscle in mice. Am. J. Physiol. Cell Physiol. 289, 697–707 (2005).
Saade, M., Araujo de Souza, G., Scavone, C. & Kinoshita, P. F. The role of GPNMB in inflammation. Front. Immunol. 12, 674739 (2021).
Demaria, M. et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 31, 722–733 (2014).
Hernandez-Segura, A. et al. Unmasking transcriptional heterogeneity in senescent cells. Curr. Biol. 27, 2652–2660 (2017).
Van Damme, H. et al. Therapeutic depletion of CCR8+ tumor-infiltrating regulatory T cells elicits antitumor immunity and synergizes with anti-PD-1 therapy. J. Immunother. Cancer https://doi.org/10.1136/jitc-2020-001749 (2021).
Amor, C. et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 583, 127–132 (2020).
Amor, C. et al. Prophylactic and long-lasting efficacy of senolytic CAR T cells against age-related metabolic dysfunction. Nat. Aging https://doi.org/10.1038/s43587-023-00560-5 (2024).
Grove, L. M. et al. Urokinase-type plasminogen activator receptor (uPAR) ligation induces a raft-localized integrin signaling switch that mediates the hypermotile phenotype of fibrotic fibroblasts. J. Biol. Chem. 289, 12791–12804 (2014).
Elberling, C. et al. Urokinase receptor forms in serum from non-small cell lung cancer patients: relation to prognosis. Lung Cancer 74, 510–515 (2011).
Li, Y. & Cozzi, P. J. Targeting uPA/uPAR in prostate cancer. Cancer Treatment Rev. 33, 521–527, (2007).
Kiyan, J., Smith, G., Haller, H. & Dumler, I. Urokinase-receptor-mediated phenotypic changes in vascular smooth muscle cells require the involvement of membrane rafts. Biochem. J. 423, 343–351 (2009).
Bajou, K. et al. Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat. Med. 4, 923–928 (1998).
Sagiv, A. et al. NKG2D ligands mediate immunosurveillance of senescent cells. Aging 8, 328–344 (2016).
Yang, D. et al. NKG2D-CAR T cells eliminate senescent cells in aged mice and nonhuman primates. Sci. Transl. Med. 15, eadd1951 (2023).
Michieletto, D., Lusic, M., Marenduzzo, D. & Orlandini, E. Physical principles of retroviral integration in the human genome. Nat. Commun. 10, 575 (2019).
Melenhorst, J. J. et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature 602, 503–509 (2022).
Hasegawa, T. et al. Cytotoxic CD4+ T cells eliminate senescent cells by targeting cytomegalovirus antigen. Cell 186, 1417–1431 (2023).
Gasek, N. S., Kuchel, G. A., Kirkland, J. L. & Xu, M. Strategies for targeting senescent cells in human disease. Nat. Aging 1, 870–879 (2021).
Wang, T. W. et al. Blocking PD-L1–PD-1 improves senescence surveillance and ageing phenotypes. Nature 611, 358–364 (2022).
Lin, M. J. et al. Cancer vaccines: the next immunotherapy frontier. Nat. Cancer 3, 911–926 (2022).
Kim, K. M. et al. Identification of senescent cell surface targetable protein DPP4. Genes Dev. 31, 1529–1534 (2017).
Ambrosi, T. H. et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell 20, 771–784 (2017).
Valencia, I. et al. DPP4 promotes human endothelial cell senescence and dysfunction via the PAR2–COX-2–TP axis and NLRP3 inflammasome activation. Hypertension 79, 1361–1373 (2022).
Tian, X. L. & Li, Y. Endothelial cell senescence and age-related vascular diseases. J. Genet Genomics 41, 485–495 (2014).
Mund, A. et al. Deep Visual Proteomics defines single-cell identity and heterogeneity. Nat. Biotechnol. 40, 1231–1240 (2022).
Li, S. et al. Multiregional profiling of the brain transmembrane proteome uncovers novel regulators of depression. Sci. Adv. https://doi.org/10.1126/sciadv.abf0634 (2021).
Schmidt, J. et al. Prediction of neo-epitope immunogenicity reveals TCR recognition determinants and provides insight into immunoediting. Cell Rep. Med. 2, 100194 (2021).
Sohail, M. S., Ahmed, S. F., Quadeer, A. A. & McKay, M. R. In silico T cell epitope identification for SARS-CoV-2: progress and perspectives. Adv. Drug Deliv. Rev. 171, 29–47 (2021).
Chiang, E. Y. et al. Targeted depletion of lymphotoxin-alpha-expressing TH1 and TH17 cells inhibits autoimmune disease. Nat. Med. 15, 766–773 (2009).
O’Connor, R. A. et al. Cutting edge: Th1 cells facilitate the entry of Th17 cells to the central nervous system during experimental autoimmune encephalomyelitis. J. Immunol. 181, 3750–3754 (2008).
Liu, Z. et al. Immunosenescence: molecular mechanisms and diseases. Signal Transduct. Target. Ther. 8, 200 (2023).
Goronzy, J. J. & Weyand, C. M. Understanding immunosenescence to improve responses to vaccines. Nat. Immunol. 14, 428–436 (2013).
Pera, A. et al. Immunosenescence: implications for response to infection and vaccination in older people. Maturitas 82, 50–55 (2015).
Weinberger, B. Adjuvant strategies to improve vaccination of the elderly population. Curr. Opin. Pharmacol. 41, 34–41 (2018).
Mannick, J. B. et al. TORC1 inhibition enhances immune function and reduces infections in the elderly. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aaq1564 (2018).
Akinc, A. et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol. Ther. 18, 1357–1364 (2010).
Pardi, N., Hogan, M. J., Porter, F. W. & Weissman, D. mRNA vaccines - a new era in vaccinology. Nat. Rev. Drug Discov. 17, 261–279 (2018).
Krizhanovsky, V. et al. Senescence of activated stellate cells limits liver fibrosis. Cell 134, 657–667 (2008).
Meyer, K., Hodwin, B., Ramanujam, D., Engelhardt, S. & Sarikas, A. Essential role for premature senescence of myofibroblasts in myocardial fibrosis. J. Am. Coll. Cardiol. 67, 2018–2028 (2016).
Ritschka, B. et al. The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev. 31, 172–183 (2017).
Jun, J. I. & Lau, L. F. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat. Cell Biol. 12, 676–685 (2010).
Grosse, L. et al. Defined p16high senescent cell types are indispensable for mouse healthspan. Cell Metab. 32, 87–99 (2020).
Cai, Y. et al. Decoding aging-dependent regenerative decline across tissues at single-cell resolution. Cell Stem Cell 30, 1674–1691 (2023).
Cullen, N. C. et al. Efficacy assessment of an active tau immunotherapy in Alzheimer’s disease patients with amyloid and tau pathology: a post hoc analysis of the “ADAMANT” randomised placebo-controlled double-blind multi-centre phase 2 clinical trial. eBioMedicine 99, 104923 (2024).
Acknowledgements
We are grateful to Z. Wu for providing critical advice for this Review, and L. Bai for her administrative assistance. This work was supported by the National Natural Science Foundation of China (81921006 to G.-H.L., 92149301 to G.-H.L.), the National Key Research and Development Program of China (2020YFA0804000 to G.-H.L., 2020YFA0803401 to J.R., 2022YFA1103700 to W.Z. and 2019YFA0802202 to J.R.), the National Natural Science Foundation of China (92168201 to G.-H.L., 92049116 to W.Z., 32121001 to W.Z. and J.R., and 31970597 to J.R.), the Strategic Priority Research Program of the Chinese Academy of Science (XDB0570100 to J.R.), the CAS Project for Young Scientists in Basic Research (YSBR-076 to G.-H.L. and J.R., YSBR-012 to W.Z.) and the New Cornerstone Science Foundation through the XPLORER PRIZE (2021-1045 to G.-H.L.). All figures were created with or modified from BioRender.com.
Author information
Authors and Affiliations
Contributions
G.-H.L., J.R. and W.Z. designed and supervised the review and reviewed the manuscript; R.W. and F.S. wrote the manuscript and constructed the figures and tables. All the authors read and approved the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Aging thanks Tohru Minamino and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, R., Sun, F., Zhang, W. et al. Targeting aging and age-related diseases with vaccines. Nat Aging 4, 464–482 (2024). https://doi.org/10.1038/s43587-024-00597-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-024-00597-0