Abstract
Volcanic eruptions generate initially sterile materials where biological processes are absent, allowing for the fresh colonization by new organisms. This review summarizes the characteristics of volcanic habitats that are available for pioneer microbial colonization, including hot springs, fumaroles, lava tubes, and recently cooled rock surfaces and interiors. Eruptions provide unique insight into microbial community development in extreme environments. The trajectories that these ecosystems follow are largely dictated by the initial environmental conditions and identities of the colonizers, rather than the age of the system. The review also discusses how studies of microbial communities in young lava flow fields can provide insights into the possibility of life on Mars, which was volcanically and hydrologically active in the past. Understanding biosignature preservation as well as the metabolisms and survival mechanisms of microorganisms in volcanic systems has implications for how an ecosystem might have developed on early Earth and possibly Mars.
Similar content being viewed by others
Introduction
Pioneer species are organisms that first colonize a bare substrate created by a disturbance1, such as soil exposure after glacial retreat (primary succession), freshly emplaced lava flow fields (primary succession)2,3,4, or habitat destruction due to a wildfire (secondary succession)5. Investigating pioneer microorganisms following an eruption is essential because the initial microbial community composition and metabolisms will dictate the development of more complex ecosystems and biogeochemical processes. Most of the Earth’s surface (including the seafloor) is of volcanic origin6, which makes constraining succession in these systems fundamentally important for informing ecological processes.
Deepening the understanding of the taxonomic and metabolic identity, origin, and distribution of pioneer species is relevant in astrobiology because volcanic terrains were likely the first habitats to support life on Earth and could also have hosted, or could be hosting, life elsewhere. It is particularly timely because the search for life on Mars and other volcanically active planets is a top science priority in the Planetary Science and Astrobiology Decadal Survey 2023–20327. Previous reviews recognized lava rocks can provide habitable environments for life8,9,10, but there is a need for a holistic perspective regarding the abiotic and biotic factors that influence succession in the range of habitats within volcanic environments (Fig. 1).
We highlight the process of ecological succession within young terrestrial volcanic environments from the viewpoint of microbial communities and discuss key processes related to the habitability of basaltic rocks from an astrobiology perspective. We use the phrase “young volcanic terrains” to describe environments that have been affected by a geologically recent eruption (<2000 years), the phrase “volcanic rocks” to describe igneous rocks solidified on or near the surface6, and the term “terrestrial” to describe processes occurring on land, as opposed to oceanic systems.
The importance of volcanic terrains in understanding primary microbial succession
Volcanic rock is sterile upon emplacement due to its high initial temperature (e.g., basaltic lavas are typically erupted at ~1150 °C)11. This generates new surfaces where biological processes are initially absent. Colonizers of volcanic terrains must overcome low nutrient availability (e.g., <0.1 wt% carbon at some sites)12,13, highly variable temperatures, oxidative stress, and high ultraviolet radiation (UV) at young, unvegetated sites9. These freshly emplaced materials are consequently low biomass (often ~106 cells/g)14,15 and methodological limitations make it difficult to analyze biomarkers and to adequately document the early stages of succession. For example, the binding of cells to the hard mineral matrix in basalt and the enhanced potential for introducing exogenous contaminants further complicates the extraction of biomarkers16. These factors have limited the use of modern omics techniques, leaving little still known about the identity and metabolisms of initial colonizers.
Eukaryotic photoautotrophs such as algae (including lichen), mosses, and plants were previously thought to be the first colonizers of lava flows17,18,19,20,21. Yet, recent studies have shown that prokaryotes (bacteria and archaea; Supplementary Table 1) are able to establish limited communities within the lava as soon as three months after an eruption14, some with chemoautotrophic metabolisms (Supplementary Table 1) such as the oxidation of volcanically derived carbon monoxide (CO)22. Other studies have shown the initial microbial community is dominated by heterotrophs, suggesting organic carbon (C) must come from an external source such as the atmosphere, water percolation, or necromass23,24,25. However, uncertainties remain about the specific metabolisms because many of these studies relied on 16S rRNA gene sequencing data (Supplementary Table 1) for functional inferences.
Two types of processes—deterministic and stochastic—govern microbial community assembly during succession26. Deterministic processes involve abiotic and biotic factors that influence the type and relative abundance of certain species. For example, abiotic factors such as water availability, weathering, mineralogy, porosity, rock texture, and local weather control the conditions resulting in habitat formation, which directly affect the microbial community composition that can inhabit the cooled lava27,28. Stochastic processes are governed by random dispersal and changes in species abundance that are not due to environmental fitness29. An open question in the study of succession in volcanic environments is the relative importance of deterministic and stochastic processes over multiple timescales in generating variation in species composition across biogeographic regions30.
Volcanism is inextricably tied to the history of life on Earth since the crust and atmosphere result from volcanic processes. One of the major theories regarding the origin of life is that the last universal common ancestor (LUCA) lived at deep-sea hydrothermal vents31,32, which are driven by the rapid cooling of magma at oceanic plate boundaries33. Other ideas include the hypotheses that volcanic terrains were the first habitats to harbor life on land34,35, or that LUCA first formed in terrestrial hot spring pools and later moved to the ocean to develop complex life36. Floating islands of pumice (glassy, frothy, pyroclastic volcanic rock) have also been proposed as the setting for the emergence of life on Earth37. Volcanism provides a source of life-sustaining nutrients and contributes to the cycling of the volatiles (Supplementary Table 1) that play a role in stabilizing planetary climate38.
Understanding how microorganisms utilize and are preserved within habitats in volcanic systems has implications for our ability to detect possible life on other volcanically active planets. Mars has abundant evidence of both hydrological39 and volcanic activity in the past40,41,42,43,44, perhaps as recently as the past few million years45,46. For instance, Noachian-aged (Supplementary Table 1) craters on Mars, like Gusev47 and Jezero48, commonly exhibit evidence of paleolakes and lava that has undergone aqueous alteration49,50,51. The combination of liquid water and volcanic activity on ancient Mars has raised the question of whether it was habitable and once hosted life. Therefore, volcanic terrains on Earth have been used as analogs for similar Martian environments25,52,53. On icy moons, cryovolcanism is thought to be a plausible mechanism for potentially carrying biological material onto the surface ice54.
In volcanic systems, factors such as pH, elemental composition55,56, mineralogy57, temperature58, water content59, and pressure60,61 contribute to changes in microbial diversity and metabolic potential62, and these variations can be observed at fine spatial resolutions, specifically scales smaller than one meter. Substantial work remains to understand how physicochemical and geologic conditions impact different types of habitats (Fig. 1), leading to microbial colonization on Earth, and possibly Mars or other planets.
Hydrothermal systems
Long-lived hot springs
Long-lived hot springs (>decades old) are known to harbor diverse and abundant microbial communities due to the presence of various temperature regimes, reduced chemical species, and profuse mineral depositional processes63,64. These systems form when a persistent magmatic heat source interacts with groundwater (Fig. 2a, b). The resulting fluids have a diverse range of chemical compositions, but can be grouped into three end-member categories: (1) bicarbonate-rich springs that can create travertine (CaCO3; Fig. 2a); (2) alkali-chloride springs of nearly neutral pH that can form siliceous sinter deposits; and (3) acid-sulfate springs that develop when ascending hydrogen sulfide (H2S) is oxidized to sulfuric acid (H2SO4) and reacts with surrounding rocks to create clays and oxides (Fig. 2b)65. In neutral to alkaline water, Cyanobacteriota (i.e., photoautotrophs) dominate, whereas chemoautotrophs and heterotrophs are more abundant in acidic and higher temperature regimes64.
a Long-lived bicarbonate rich springs in the Geysir geothermal area, Iceland (photo credit: N. Hadland)236. b Long-lived acid-sulfate springs in the Krýsuvík geothermal area, Iceland (Photo credit: N. Hadland)176. c Ephemeral hot springs that formed following the 2014–2015 Holuhraun eruption in Iceland after the lava was emplaced into the Jökulsá á Fjöllum river (Photo credit: C. W. Hamilton). d Meteoric water percolating through permeable basalt to a hot center in a lava flow resulted in ephemeral fumaroles that lasted for several years after the 2018 Kīlauea eruption in Hawaiʻi (Photo credit: N. Hadland). e Evidence of past hydrothermal activity on Mars in the form of white nodular opaline silica deposits (amorphous SiO2 · nH2O) (Mars Exploration Rover Spirit Pancam false-color image P2388; Photo credit: NASA/JPL-Caltech)93. f Chains of ring-mound landforms in Athabasca Valles on Mars, which are interpreted to be volcanic rootless cones formed by explosive lava–water interaction. The overlapping cones indicate the features in the upflow direction (black arrow) are younger237 (High Resolution Imaging Science Experiment (HiRISE) image PSP_002938_1890; Photo credit: NASA/JPL-Caltech/UArizona).
Microbial mats are ubiquitous geobiological features, and gradients in physicochemical parameters throughout the mat can result in layering that leaves morphological biosignatures66,67. Useful insights into successional processes in hot springs can be drawn from studies of newly formed sinter deposits67,68,69 or by incubating submerged sterile microscope slides in mature systems to observe mat formation63,70,71, though caution should be taken when extrapolating these conclusions to newly formed hot springs. Colonization onto new surfaces by chemoautotrophs (e.g., arsenite oxidation by Hydrogenbaculum spp., Fe(II) oxidation by Metallosphaera yellowstonensis, etc.) or photoautotrophs occurs rapidly (~hours to days)70. Later, the development of mature mat structures can result from extracellular polymeric substances and filamentous cellular morphologies capturing free-floating cells or precipitates68, progressively building up layers. Oxygenic photosynthesis by Cyanobacteriota in the mat can locally increase the pH, leading to carbonate saturation and precipitation72, further building up layers. Extracellular polymeric substances—mainly composed of polysaccharides, lipids and proteins—also facilitate carbonate precipitation and crystal nucleation73. These biomineralization byproducts allow for the later colonization of heterotrophs or organisms with anaerobic metabolisms due to the lack of oxygen (O2) at the bottom of the mat, which is relevant to understanding early Earth ecosystems since they were also anaerobic69,70.
Temperature and pH also play roles in the development of a microbial mat on a newly exposed surface. For example, beyond the upper limits for phototrophy (~72 °C)74, species richness drops dramatically and morphologies in the biofilm become less diverse. Sinter deposition is even limited above 77 °C68,71. Consequently, temperature gradients can result in different biomineralization products and biosignatures on sub-meter scales.
Ephemeral hydrothermal systems
Lava–water interactions generate ephemeral hot springs and fumaroles (Fig. 2c, d). Lava from effusive eruptions tend to occupy topographic lows and commonly encounter near-surface water75. Water can flow beside, under, over, and through lava to generate hot springs76. These systems differ from long-lived hydrothermal systems because once the eruption stops, the supply of heat will diminish, returning the water to ambient temperature53. Ephemeral hydrothermal systems also form via meteorite impacts, such as within the Haughton Crater in Canada77 or the Chicxulub Crater in Mexico78. These systems are similar to lava flows in terms of primary succession since the impact effectively resets biological processes in the region79.
On Earth, the best recent example of a lava-induced hydrothermal system occurred during the 2014–2015 eruption of Holuhraun in the central highlands of Iceland, where the lava was emplaced into part of the Dyngjujökull glacial floodplain and tributaries to the Jökulsá á Fjöllum river. Water ponded against the lava and flowed through the porous basalt to the distal end of the lava flow, where heated water emerged to generate a hydrothermal system (Fig. 2c). The warmest recorded temperatures in the pools and outflow springs were measured at 48.4 °C six months after the eruption ended, and the water declined to ambient temperature two years later75,76. However, hotter temperatures are implied by the development of fumarolic steam plumes in the interior portions of the lava flow (Fig. 2c). The hot springs were quickly colonized by microorganisms, which showed little similarity to the taxonomic diversity of the glacial river. Culture-based analysis revealed the presence of endospore-forming thermophiles (Supplementary Table 1) such as Geobacillus stearothermophilus. Interestingly, incubating glacial water at the warm lava-heated sites resulted in increased cell growth and taxonomic diversity. Sequences that dominated glacial water such as the sulfur (S)-oxidizing Sulfuricurvum sp., reduced in the warm treatment while photosynthesizers (e.g., Cyanobacteriota and microalgae) increased in number53.
Another example of an ephemeral hydrothermal habitat is Surtsey, a volcanic island off the southern coast of Iceland created by a series of submarine, explosive, and effusive eruptions of basaltic lava in 1963–196780. The associated Surtseyan eruption style is characterized by ejecta rapidly expanding upward through a shallow water column and resulting in glass-rich cocktail sprays of ash, rock, and lava (collectively called tephra) and the formation of new land. Other Surtseyan explosions were observed in association with Capelinhos (Azores) in 1957–195881 and the Hunga Tonga–Hunga Haʻapai (HTHH) eruption in the South Pacific in 2014–201582. Human visitation to Surtsey has been relatively limited, creating an excellent natural laboratory for studying succession83. Microbial communities within Surtsey have been investigated using a borehole drilled in 1970 to access the subsurface hydrothermal environment that formed following the eruption. Temperature increased with depth up to biologically prohibitive temperatures but started to decrease ~100 m below the surface. A diverse unculturable microbial community was discovered at 145–172 m including methanogens (e.g., Methanobacterium), sulfate reducers (e.g., Desulfosporosinus, Desulfatiglans, and Desulfotomaculia), and sulfur oxidizers (e.g., Thiomicrospira and Sulfurospirillum), suggesting an active S cycle. Taxa typically found in seawater such as Halomonas and Pseudoalteromonas were also found, demonstrating that seawater infiltration into the porous lapilli layer is an important vector for colonization. However, many groups had little similarity in the 16S rRNA gene to any known lineages. Combined with a high-temperature ceiling at ~100 m (124 °C in 2017), this indicates this community is likely indigenous to the subsurface and suggests the eruption generated a massive underground biome for the thermophiles to occupy. Remnant heat has allowed the unique community to persist for decades after the eruption84,85,86.
Eruptions in 1967 and 1969 on Deception Island, Antarctica, produced isolated basaltic cinder cone islands87,88. Similar to Surtsey, seawater interacted with magmatic heat, and ephemeral hot spring crater ponds formed, resulting in a semi-isolated saltwater system with algae and high levels of phylogenetic diversity compared to surrounding Antarctic waters89,90. Remarkably, some of the most abundant phyla found are rarely seen in other Antarctic ecosystems and are typically identified in marine hydrothermal vents (e.g., the thermophilic Calditrichota). Samples also contained ammonia oxidizers, contributing to primary productivity and the nitrogen (N) cycle and thus playing an important role during the dark polar winter months when photoautotrophy is severely limited91.
From an astrobiology perspective, lava-induced hydrothermal systems are intriguing because lava flows on Mars may have interacted with near-surface water or ice-bearing permafrost40,41. These events may have created environmental conditions suitable for thermophiles53,92. Future missions could search for biosignatures in sites with evidence of lava–water interactions, such as in Gusev Crater (Fig. 2e)93, Athabasca Valles (Fig. 2f)41, or Jezero Crater51.
Volcano–ice interactions
Volcano–ice interactions can generate a range of habitats for microbial colonization94. This process has been reviewed in the context of astrobiology95. Subglacial eruptions can produce glacier caves96 or melt the overlying ice layer to form glacial springs, meltwater lakes, or ice-melt-fed surficial hot springs55. Magma intrusions into a glacier could produce massive subsurface hydrothermal environments97. These environments represent transient volcanogenic habitats hypothesized during Snowball Earth scenarios (Supplementary Table 1)98 or on Mars in the past97. On Mars, there could have been sufficient geothermal heat to cause hydrothermal circulation after melting the permafrost ice layer, potentially with enough exploitable redox gradients to support chemoautotrophic life99, including S, hydrogen gas (H2), and iron (Fe) driven metabolisms55.
Microorganisms can also occupy the basal layer of glaciers and subglacial lava edifices, but these environments are difficult to access and study. Basalt from subglacial eruptions in Iceland have been exposed following glacial retreat and glass products have been shown to harbor Actinomycetota, Pseudomonadota, and Bacteroidota23,24. Microbial communities are also found on the surface of ice near active volcanoes, such as on Deception Island, Antarctica, or Kamchatka Peninsula, Russia. Reduced forms of Fe and manganese (Mn) present in basaltic ash embedded within the ice could serve as an energy source for chemoautotrophs, such as the Fe-oxidizing Ferrimicrobium acidiphilum (Actinomycetota) or the Fe-reducing Rhodoferax ferrireducens (Pseudomonadota). Volcanic gas containing S could also explain why S-metabolizing bacteria, such as Thiomonas thermosulfata (Pseudomonadota) have been found within ice91,100.
Fumaroles
Fumaroles form when meteoric or subsurface (i.e., phreatic) water is heated to form steam and escapes through lava flow surface cracks (Fig. 2c, d). Fumaroles generate stark physicochemical gradients that contrast sharply with the surrounding environment. Acidic pH, variable temperatures of steam discharge (~45–180 °C), and heavy metals require a great degree of specialization for microbial life and thus result in generally low biomass101,102. For example, on the Big Island of Hawaiʻi, fumaroles are abundant and most lava flows are less than 1500 years old (Fig. 2d)103, allowing for the study of relatively young systems. Active fumaroles exhibit lower phylogenetic diversity compared to relict sites and unaltered basalt and microbial biomass is heterogeneous in distribution25,52, likely due to prohibitively high temperatures for many organisms104.
Fumaroles in more extreme environments, such as Mount Erebus in Antarctica or Socompa in the hyperarid Atacama Desert have a generally different trend compared to Hawaiʻi because the areas around the fumarole vents are the only abode of warmth or moisture, potentially creating islands of habitability. In these systems, there may be a higher spatial variability in phylogenetic diversity as a function of distance from the fumarole vent due to very large variations in temperature and moisture content58,59. In cold environments, steep thermal gradients can cause soil environments to transition quickly from thermophile to psychrophile-dominated communities58. Communities can also quickly transition in metabolic function—from photoautotrophs (e.g., Cyanobacteriota and algae) in the steam-heated soils near the fumarole to xerotolerant (Supplementary Table 1) endospore-forming heterotrophs on the outskirts (e.g., Actinomycetota)59. Communities with the highest phylogenetic diversity on Socompa were located in intermediary locations a few meters away from the fumarole and could still benefit from the moisture and nutrients from the steam vent without the extreme temperatures59.
On Mount Erebus, soil pH acidifies further away from the fumarole as a result of S-oxidizing bacteria creating H2SO4 combined with the buffering effects of high temperature and carbon dioxide (CO2) near the fumarole58. There may also be variations in the amount of organic C and other nutrients as a function of distance from the fumarole, which could influence the community105. At Marum Crater, Vanuatu, cell counts decreased with depth into the sediment surrounding a fumarole, but metabolic activity increased, suggesting that subsurface niches provide a more stable environment106. Together, these studies demonstrate that varying physicochemical factors likely result in gradients of microbial function and diversity as a function of distance away from the fumarole and depth into the sediment, because the environmental contrast between the near-fumarole ecosystem and the ambient surroundings is very large.
Discoveries of archaea and bacteria contained within condensed steam vent waters have opened insight into the primary succession of fumaroles and the dynamic coupling between subsurface and surface-based ecosystems107,108. Steam from Surtsey fumaroles harbored a distinct microbial community compared to subsurface hydrothermal fluids and seawater near the island. Cyanobacteriota, Deinococcota, and Acidobacteriota phyla were discovered in high proportions with some overlap with subsurface basaltic tuff deposits, suggesting that subsurface organisms could be transported to the surface by fumaroles84. In this way, fumaroles may be an important functional element in microbial community development by facilitating microbial transport from the subsurface to the surface.
Lava tubes and caves
Lava tubes are common features in volcanic terrains (Fig. 3). They commonly form when the outer surface of a channelized lava flow109 or a pāhoehoe (Supplementary Table 1) lobe network110 solidifies, thereby thermally insulating the core of the flow and enabling preferred pathways to develop within the interior of a lava unit. When the eruption stops or the lava is diverted, part of the lava within the preferred pathway can drain, leaving a partially evacuated tube111. Inflationary processes can also result in lava-rise pits and caves112,113. Lava tubes and caves have less variation in environmental parameters than the surface. After the tube drains, gravitational and/or erosional effects can create skylights111 that allow light to enter the otherwise thermally stable subsurface environment (Fig. 3b, d).
a Top-down view of point cloud LiDAR data of Four Windows Cave in El Malpais National Monument, New Mexico. Elevation values were converted to gray scale with dark colors corresponding to lower elevations. Shape edges were shaded with an eye-dome lighting model. The surface is removed in the center part of the figure to expose the subsurface lava tube (data from Bardabelias et al.238). The white boxes correspond to photographs b–c. b Top view of a skylight into Four Windows Cave (Photo credit: N. Hadland). c Dark interior of Four Windows Cave. Mineral precipitates and textured surfaces on the walls are visible (Photo credit: N. Hadland). d The edge of the twilight zone in Four Windows Cave where light progressively drops out. Directly beneath the skylight, moss and Cyanobacteriota films visibly dominate (Photo credit: N. Hadland).
Lava tube environments have distinct selection pressures compared to the overlying lava flow. Subsurface communities often include diverse microbial biofilms and mats114,115,116. For example, little overlap was found between surface soil/lava and cave microbial communities in Lava Beds National Monument in California. There was a much higher abundance of Actinomycetota in cave samples than in the overlying soil, some members of which were capable of nitrate-dependent Fe-oxidation117. Other work has shown that species within lava tube habitats may have special adaptations compared to overlying soil—such as resistance to metals118—or even lose certain functional genes, such as UV radiation repair mechanisms119.
The higher biomass environments in lava tubes generally occur in the twilight zone, or the areas around where light is reduced and photosynthetic organisms progressively drop out, but organisms can still benefit from the thermal stability (Fig. 3d)115,120. Deeper than this point in caves, entire communities can be supported by chemoautotrophic metabolisms (Fig. 3c)116,121. Pseudomonas sp. HerB isolated from lava tubes in the Oregon Cascades grew using olivine as an energy source via oxidation of structural Fe(II) during incubation experiments122. Heterotrophic microorganisms also can be supported by organic matter detritus that enters the cave through water percolation123. However, despite organic matter inputs, biofilms within caves at Lava Beds National Monument were supported primarily by active C fixation. Chemoautotrophs could have been the initial colonizing group in the newly formed lava tube and established a unique niche by providing fixed C to heterotrophs124. As overlying vegetation became established and nutrient input via water percolation increased and supported additional heterotrophs125, the original chemoautotroph colonizers may have maintained their independent niche124. Metagenomic analysis in lava caves in Craters of the Moon National Monument, Idaho, found similar C fixation pathways and anaerobic sulfate reduction, nitrate reduction, and methanogenesis126.
Phylogenetic diversity is lower in new geothermal caves compared to older lava tubes102. Similar to fumaroles, this suggests increased metabolic interactions among taxa are required to survive. Extreme environmental conditions in young geothermal caves with less organic C filter for certain species. In these environments, synergistic metabolic interactions may be important in the development of a microbial community. Primary producers in young geothermal cave environments have been described as hub organisms in phylogenetic network analyses—species linked with many other species—potentially depicting a symbiotic relationship with heterotrophs or a generally ecologically important role102,127,128.
Lava tubes are of interest to astrobiology because of their stable environmental conditions (e.g., temperature, humidity), lack of harmful UV, entrapment of water, and secondary mineral precipitation, leading to diverse microbial communities and biosignature preservation114,129. Several lava tubes have been identified in lunar maria and on Mars111,130,131. Tubes on Earth are generally restricted to lava flows that are younger than a few million years132 due to collapse via weathering processes or sediment/lava infilling. Tubes on Mars might be stable for much longer periods due to the lower weathering rates and lack of planet-wide tectonics activity. This stability may lead to a higher biosignature preservation potential for past life and possibly environmental conditions suitable for extant life126,133.
Lava texture and porosity
Lava can provide different types of habitats for endoliths (organisms that occupy the inner part of a rock) through variations in texture and porosity (Fig. 4). The correlation between porosity and microbial diversity is well known in soil at ~cm scales and drives the heterogeneous distribution of microbial biomass134. Surface area measurements and porosity are used in calculations of bulk microbial biomass in oceanic crustal basalts135 because these parameters correlate with the space available for microbial colonization, the rate of fluid flow, macronutrient deposition, and chemical leaching136. Vesicular basalts in oceanic environments host euendoliths (Fig. 4h) that can bore into the rock and leave morphological biosignatures, including filamentous fossils and microtubules137,138,139,140,141. Similarly, in terrestrial volcanic rocks, the initial texture and porosity potentially play important roles in habitat development since pore spaces and variability in the surface texture can provide protected locations from exterior stressors. Nonetheless, these trends remain understudied for terrestrial systems. From an astrobiology perspective, this is a critical task since these trends could influence sample site selection on Mars by identifying which lava type has the highest potential for containing remnant microbial biosignatures.
Example of a pāhoehoe lava flow and associated habitats. a Pāhoehoe ropes (formed by a cooling surface that deforms above a flowing interior) can create variations in surface texture to create sheltered habitats for epiliths (organisms that can colonize rock surfaces). Image from the 2018 Kīlauea lava flow, Hawaiʻi (Photo credit: N. Hadland). b Skylights formed by collapses often create rubble piles within a lava tube or cave, which are colonized by photosynthesizers. Image from Four Windows Cave in El Malpais National Monument, New Mexico (Photo credit: N. Hadland). c Vent proximal lava is commonly extremely vesicular due to high gas content and has a somewhat glassy surface that provide habitats for cryptoendoliths (organisms that occupy vesicles and primary pores). Image from the 2018 Kīlauea lava flow (Photo credit: N. Hadland). d Twilight zone in Four Windows Cave at the edge of where photosynthetic groups can occupy (green film at the top of the image). Variations in textures on lava tube walls are caused by lava drips, extrusions, or fluctuating levels of lava during tube formation (Photo credit: N. Hadland). e Crystalline core of a pāhoehoe flow with few vesicles (Photo credit: Quinn Dombrowski; https://commons.wikimedia.org/wiki/File:Pahoehoe_Lava_Flow_at_Kilauea_in_Hawaii_20071209_B.jpg). f Transition between pāhoehoe and ʻaʻā, which have dramatically different surface textures and vesicularity. Image from 2021 Fagradasfjall eruption, Iceland (Photo credit: N. Hadland). g Inflation processes can create large cracks and void spaces for chasmoendolith colonization (organisms that populate fractures and cracks). Perspective rendering from McCartys lava flow field in El Malpais National Monument (Photo credit: C. W. Hamilton)113. h Older lava is more weathered due to alteration processes as well as euendolith (actively penetrating/burrowing into a rocky surface) and autoendolith (metabolically induced mineral precipitation in pore spaces)239 activity. Image from Reykjanes Peninsula, Iceland (Photo credit: N. Hadland).
Fissure-fed lava systems exhibit a diverse range of lava types leading to variability in texture that result from magma composition, temperature and eruption conditions, and mode of emplacement. Pāhoehoe generally form from high-temperature lava with lower viscosity, which is commonly emplaced at lower local effusion rates and lower strain rates than ʻaʻā (Supplementary Table 1). If temperature decreases, underlying topographic slope increases, or effusions rates increase, then pāhoehoe can transition to ʻaʻā due to increasing crystallinity and viscosity as well as increased shear strain rates6,142. Subsequent episodic disruption events and passive cooling at different stages results in these various transitional lava types (Fig. 4f)143. The resultant surficial texture may play an important role in the initial colonization of organisms. For example, rough ʻaʻā flows will dampen surface windspeeds, thereby leading to increase boundary-level humidity, while individual spinose clinker clasts will have small surfaces cavities that favor the accumulation of aeolian sediments, which contain nutrients and help to hold water. Narrow, sharp, and protruding points of a rock face capture biomass at much higher rates than smoother surfaces, which are comparatively barren144. Moreover, the temperature and water retention rate are strongly dependent on the aspect of the surface exposure (e.g., north/south facing), with daily temperature fluctuations on rock surfaces often twice that of air temperature145. These temperature fluctuations could provide niches for mesophiles (Supplementary Table 1) or thermophiles in cold environments146. On Deception Island, Antarctica, coarser particle fractions and higher slopes in the topography correlate with richer communities on the volcanoes, likely due to increased water percolation90.
Vesicles in igneous rocks form as a result of the exsolution of supersaturated volatiles during magma ascent and cooling147. Vertical variations in vesicularity within a lava unit may affect the microbial biomass distributions. These spaces contribute to increased microhabitats and consequently higher levels of biomass relative to other lava types52,148,149. For example, the solid crystalline core shown Fig. 4e has few spaces for microbial colonization compared to the vesicular glassy surfaces (Fig. 4c). Vesicular rock can also shape microbial diversity by increasing the relative surface area available for colonization, improve macronutrient retention, and potentially provide electron donors and acceptors from the crystalline rock itself106,136,150. Basalt has a high compressive strength, which allow vesicles to remain open in buried lava flows at greater depths and could encourage the preservation of past biosignatures151. Exhumed vesicular igneous rocks should be considered as potential targets in the search for past life on Mars.
The amount of water retained in volcanic rocks affects the growth of microorganisms living inside them. Basaltic rocks lose near-surface water rapidly (~tens of hours after a wetting event), but retain water in deeper pores (~10 cm depth) for extended periods152. The frequency of precipitation and humidity in the environment also plays a role in the diffusion of water to deeper pores. Since water retention in basaltic environments is low due to low organic C153 and water distribution can be heterogeneous, endolithic organisms may be adapted to survive dry and wet cycles similar to hypolithic Cyanobacteriota in the hyperarid core of the Atacama Desert, which can be rapidly reactivated following salt-driven deliquescence154 or rare precipitation events155.
Diffusion of O2 to deeper pore spaces is much slower when the rock is saturated with water, creating transitory anoxic environments152. The heterogeneous distribution of nutrients deposited by the diffusion of water through the pores may also create pockets of higher biomass24. These regions of higher heterotrophic activity may exacerbate the development of localized anoxic environments in the pores through aerobic metabolic pathways152. These factors may restrict the initial endolith colonization of the interior of lava to facultatively or obligately anaerobic taxa, which have been frequently found within young basaltic rocks14,24.
Surprisingly, photosynthetic groups including Cyanobacteriota have been observed in deep pore spaces within volcanic rocks, including generally opaque obsidian156. The largest reasonable depth for photosynthetically active radiation to penetrate in solid volcanic glass is ~50 µm, but cracks and vesicular networks may increase this depth (Fig. 4c, g)148,156. This depth provides advantages since harmful UV radiation cannot penetrate the basalt157. Unconsolidated basaltic regolith (Supplementary Table 1) increases the depth for photosynthetically active radiation further, while still providing protection from UV, which some studies have hypothesized could allow for near-surface photosynthetic life on Mars158.
Mineralogy, major element composition, and alteration products
Initial composition and emplacement environment
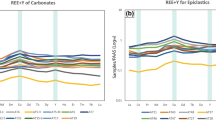
The composition of magma feeding a volcano is one of the major controlling parameters in the evolution of an eruption, largely influencing its eruption style, eruption products, and the mineralogy of the resultant igneous rocks. From a geomicrobiology perspective, mineralogy may control nutrient sequestration, inorganic nutrient availability for chemoautotrophs, and the ability for euendoliths to burrow into the rock8. Some microbes can off-load electrons onto Fe or Mn within the mineral matrix159,160 while others can access electrons from reduced structural Fe(II) in basalt161. Composition also influences the texture and porosity of the lava and the mineralogy, which can strongly affect microbial colonization57,162. Differences in community structure have been observed between different lava compositional endmembers (Box 1). For example, a rhyolitic lava flow in Iceland was shown to have a higher Shannon diversity index compared to a basaltic flow, though the difference could be due to porosity and water retention163. Additionally, cinder cone sites have a higher phylogenetic diversity than spatter cones, likely due to these compositional and morphological differences (Box 1)164.
Another major factor that has been shown to influence primary succession is the relative glassiness/crystallinity of the material due to the weatherability and homogeneity of basaltic glass compared to crystalline rocks9. Basaltic glass forms when lava is rapidly quenched upon contact with ice or water. The amorphous homogeneity of glasses results in greater nutrient availability while in crystalline materials bioessential nutrients may be less evenly distributed and concentrated in specific minerals (e.g., plagioclase or olivine) and therefore less accessible8,9,23,149,156. Microbial colonization of glasses also results in alteration textures such as tubular and granular microborings and secondary biogenic minerals useful for biosignatures in astrobiology8. However, substantial tunneling does not occur for at least 1000 years139.
In oceanic environments, colonization of freshly formed basaltic glass occurs rapidly ( ~ days) by species involved in C, N, S, and Fe cycles165. Some microorganisms, such as metal-oxidizing bacteria (e.g., Pseudomonas stutzeri or Mariprofundus ferrooxydans) are able to scavenge elements from glass within the oceanic crust161,166,167. There is a strong influence of nutrient availability on biofilm formation on oceanic basaltic glass161. Nutrient-deprived environments resulted in greater attachment because basalt may be the only source of phosphorus (P), Fe, or other biologically essential nutrients. However, these trends are likely to be different in terrestrial systems than in oceanic environments because the glass is exposed to variable precipitation/desiccation and nutrient deposition.
Floating rhyolitic pumice in a freshwater lake from a 2011 eruption in Chile was a major source of P for the initial microbial community before becoming a nutrient sink as the community grew168. Conversely, incubation experiments have found that although structural phosphorus pentoxide (P2O5) within the basalt—a bioavailable form of P169—can be leached into solution, it is not sufficient to sustain microbial growth13,161. Additional work is still needed to understand the effect of various P2O5 concentrations in basalt on microbial growth, particularly in terrestrial systems. Lava at Craters of the Moon, Idaho, has elevated concentrations of P2O5 and is comparable to Mars (up to 10× higher than Earth)170. P2O5 is also more abundant in basalt (up to 1 wt%) compared to rhyolite (as little as 0.02 wt%)171. Basalts are N-poor, generally less than 0.01%172. The addition of N to seafloor basalts in incubation experiments stimulated the growth of Fe-oxidizing taxa, which suggests that these groups are generally N-limited173,174. Additionally, multiple studies have shown that basalt can enhance the growth of organisms in Fe-limited media by providing structural Fe(II) for Fe-oxidation pathways13,161,167. These trends might be more important in lava tubes where chemoautotrophy is a major form of primary productivity and specific minerals drive microbial distribution and niche partitioning. For example, certain chemoautotrophic strains preferentially colonized basalt under simulated subsurface oligotrophic conditions175. Although basalt can be beneficial to microbial growth, the presence of transition metals bound in the glass matrix could be inhibitory to certain taxa. Some isolates in Icelandic basaltic glass have been found to have some degree of heavy metal resistance23.
Alteration products and weathering
Hydrothermal activity impacts the mineralogy and bulk chemistry of a substrate and therefore results in a range of microbial activity. In many cases, alteration mineral assemblages are controlled by acid supply, temperature, and meteoric water input, with local rock lithology playing a minor role56,176. Local geochemistry dictates the pH and bioavailability of electron acceptors and donors, such as various forms of S and Fe. When SO2 in shallow magma is emitted, it can interact with groundwater to create high-temperature H2S-enriched fluids. Pyrite, native S deposits, and other secondary phases subsequently form, resulting in a higher fraction of organisms with S-redox metabolisms56,177. Fumarolic and syn-emplacement alteration (interaction with volatiles during emplacement) create unique suites of minerals such as sulfate-bearing phases like natroalunite and thenardite178. In Hawaiʻi, the differences in phylogenetic diversity between unaltered and altered sites within lava flows (excluding trends with active fumaroles) were variable and lacked a consistent trend. Conversely, at Craters of the Moon in Idaho, the arid climate may require specialized adaptations and resulted in a concentration of microbial biomass in specific niches, specifically altered rock25,52.
Post-emplacement alteration (e.g., non-geothermal) also has been hypothesized to increase the relative habitability of terrestrial basalts. For example, basaltic glasses can weather via dissolution processes to palagonite under high water/rock ratios, which forms rinds on the interior surfaces of vesicles. These rinds have been shown to host higher concentrations of cells156 and potentially increase nutrient accessibility23. Basaltic glass in oceanic environments have an enrichment of amorphous Fe and titanium (Ti) on the rim of the palagonite, potentially encouraging the use of the palagonite sheet on fracture walls as an anchoring site for Fe-oxidizing organisms139,179. Additionally, P is often the limiting nutrient in basaltic glass environments and P2O5 and organic C have been suggested to be enriched in palagonite sheets139,179. Consequently, the need for P and organics may accelerate microbial alteration processes and increase the rate of microbial colonization of palagonite sheets in the pore spaces.
Basaltic rocks are major reservoirs of C as CO2 reacts with calcium (Ca) and magnesium (Mg) to form carbonates, often through biogenic processes in hot spring environments180. It has been estimated that microbially induced weathering (e.g., alteration textures, local dissolution, carbonate formation, etc.) is the dominant process of glass alteration in the upper 250 m of the oceanic crust139,181,182,183. Under high water/rock ratios, vesicles and fractures can be filled with authigenic mineral precipitates over time, potentially resulting in the entombment of the microbial biofilm and the preservation of biosignatures139. However, some of these weathering processes occur on longer timescales ( ~ centuries or longer) and therefore is unlikely to have an impact on the primary succession of volcanic materials in its early stages184,185, particularly in terrestrial systems.
Water plays an important role in cooling terrestrial lava flows and in the formation of unique secondary substrates that could serve as additional habitats for microorganisms. For example, explosions can occur when lava advances over a water-bearing substrate. Tephra from these interactions forms radially symmetric deposits that build into cratered cones (i.e., volcanic rootless cones) through successive explosion cycles (Fig. 2f)186,187. These features are important for identifying sites of past lava-induced hydrothermal activity40,41 and potentially fossil habitable environments on Earth and Mars53,188. Explosive lava–water interactions resulting in volcanic rootless cones are important targets for astrobiology because the transport of altered substrate material onto the surface could have carried remnant microbial biomass from subsurface hydrothermal environments187. Future Mars missions could investigate these deposits in search of biosignatures and microfossils.
Mechanisms of microbial deposition
The mechanisms of microbial deposition to young lava flows or hydrothermal systems are poorly understood. Rigorous statistical approaches such as Bayesian source tracking, which uses a probabilistic model to infer the origin of microbial communities to an environment189, have yet to be used to estimate the proportion of microorganisms in volcanic terrains coming from different sources. These could include soils, hydrothermal systems, bioaerosols, precipitation (e.g., rain and snow), or fluvial systems (Fig. 5). Still, broad ecological trends can provide insights into mechanisms of microbial deposition and colonization in volcanic terrains. For example, microorganisms can be transported globally as bioaerosols (Supplementary Table 1)190,191, or locally from nearby soil particles (Fig. 5a, b, d)192,193. Survival in the atmosphere requires tolerance to various selection pressures, which are also generally faced by microorganisms in volcanic terrains. Adaptations such as cell pigmentation, aggregation, endospore formation, and modifications to the cell membrane have been shown to be important protection mechanisms against desiccation and solar radiation in the atmosphere194,195 and should enhance the ability of a particular species to survive in young volcanic systems.
a Syn-emplacement mechanism for soil deposition on the surface of lava. In the foreground, lava scraped or bulldozed underlying soil followed by an inflationary process that lofted particles onto the surface of the flow. Saturated soils also flashed to steam as lava flowed over the substrate, carrying soil particles as the steam escaped through cracks. Dusty steam plumes are visible at the back of the image. Image from the 2021 Fagradasfjall eruption, Iceland (Photo credit: C. W. Hamilton). b Finer-grained soil particles have been blown onto the surface of lava at Fagradasfjall post lava emplacement (Photo credit: N. Hadland). c Wet sediment deposited onto the surface and interior of the lava from fluvial systems. Image of the 2014–2015 Holuhraun lava flow field, Iceland (Photo credit: C. W. Hamilton). d At Holuhraun, coarser-grained sand has been blown up against the side of the lava flow, progressively building up sand ramps to deposit material on the surface of the flow and even cover portions (Photo credit: N. Hadland). e Snow and rain fell on Fagradasfjall lava during emplacement, potentially carrying microorganisms (Photo credit: C. W. Hamilton). f Human and animal visitors to lava flows, such as shown here at Fagradasfjall, can also bring microbial colonizers (Photo credit: C. W. Hamilton).
After surviving airborne transport, microorganisms must also survive the depositional process. Bioaerosol particles can be removed from the air by wet (e.g., precipitation) or dry (e.g., collisions, gravitational settling) deposition196. For wet deposition, clouds may buffer the extreme conditions in the atmosphere, provide an oasis for life197, and support metabolically active microorganisms198. Microorganisms also serve as cloud condensation nuclei199. Precipitation may be an important driver of microbial colonization in volcanic terrains as microorganisms are deposited from the falling raindrops or snow (Fig. 5e)200. For dry deposition, major aeolian dust events in the Sahara201, Gobi202, and potentially polar deserts203 may allow certain organisms to spread via attachment to dust particles which mitigate stressors by reducing desiccation and shielding the organism from radiation. While it is unknown what the balance between local sources of soil and globally transported dust is during primary succession, dust-mediated transport increases bacterial viability compared to free-floating cells204,205. Therefore, both local and atmospheric dust are likely important inputs to volcanic systems.
Environmental filters, particularly in extreme climates, may impose additional selection pressures on colonizing organisms. Although certain taxa, such as endospore formers, are particularly adapted to global distribution in the troposphere206, it has been hypothesized that hyperthermophilic microorganisms may exhibit a higher degree of dispersal limitations due to the lack of cellular mechanisms for survival over the large distances between geothermal sites207. Nonetheless, spore-forming thermophilic groups such as the genus Geobacillus have been cultivated from Saharan dust deposited in Europe208 and from newly formed hydrothermal systems in Iceland53. Phylogenetic relationships were used to infer that the thermophiles colonizing fumaroles in Hawaiʻi likely came from other geothermal sites101. Thermophilic groups on Tramway Ridge, Antarctica, fumaroles were indistinguishable from groups found in geothermal sites in Yellowstone, likely because of long-range dispersa209. The subsurface communities, however, were largely endemic, suggesting these communities could outcompete exogenous sources of microorganisms before permanent establishment. The colonization of newly formed fumaroles and hydrothermal sites may in some cases be restricted to regional populations that are highly adapted to that particular combination of environmental filters210, and endemic microorganisms will only be found locally near the emission point196.
In addition to bioaerosols and dust, another mechanism for microbial deposition may be surface and subsurface water (Fig. 5c). For example, there were different fluvial inputs into the ephemeral hydrothermal system created by the 2014–2015 Holuhraun eruption in Iceland. Cold spring water, glacial meltwater, and water emerging from beneath the lava flow all could have contributed microorganisms to the hydrothermal system, though a wide range of temperature adaptations would be required. Several taxonomic groups showed the highest sequence similarity to basaltic aquifer ecosystems, suggesting that hydrological connections could potentially transport microorganisms from the subsurface to the hot springs53. For Surtseyan eruptions, seawater infiltration into subsurface habitats may be an important vector for colonization, with fumarolic activity helping to transport microorganisms to colonize the surface. Similarly, microorganisms colonizing the newly formed HTHH islands in the South Pacific were likely transported from the seafloor from nearby submarine hydrothermal systems during the eruption event or from nearby fumaroles on other volcanic islands as bioaerosols211.
In some systems, animal life may introduce certain taxa to the volcanic environment. Cell counts within different soil types on the island of Surtsey, Iceland, dramatically increased in those contaminated with bird feces and contained unique taxa, such as Enterobacteriacae86. On HTHH, however, bacterial taxa associated with the bird microbiome was rare, though they did not sample near bird nesting sites211. In addition to animal life, human visitation likely plays a role (Fig. 5f). Studies on Deception Island, Antarctica, after the formation of new cinder cones in 1967 noted that human and animal contamination was immense in the area, with boat wreckages and animal feces contributing to the microbial colonization of the new island89,100.
Finally, though it is difficult to imagine how organisms would survive such a blast, the dispersal of organisms via explosive volcanic eruptions has been observed in eukaryotes212 and hypothesized for prokaryotes209,213. The exact mechanism is unknown, but an explosion with enough force to eject lithic material (Supplementary Table 1) prior to superheating could potentially launch microorganisms into the atmosphere, enabling long-distance dispersal. Organisms could presumably remain viable by staying encased in the lithic material isolated from magmatic heat during the explosion212.
Age dependence
Trends in biomass and diversity
Overall trends in microbial biomass, phylogenetic diversity, and the complexity of metabolic interactions within a community may have some correlation to the age of the system. While it is impossible to select samples of different ages that have identical environmental parameters without conducting multi-year fixed-site samplings214, several studies have attempted to isolate the effect of age on ecological succession in lava flows15,28,102,215,216,217,218,219. In deglaciated soils3,220 and in seafloor lavas221, phylogenetic diversity generally increases with age. However, similar trends in lava flows have conflicting results15,102,216 and there may be variability in deterministic and stochastic processes at different successional stages, so age may change how the relative roles of different assembly processes impact the microbial community (Box 2)30,222. The rate and complexity of assembly is also likely highly affected by the geographical location and the general type of system. For example, a microbial mat within a mature hot spring system can form in a matter of days70,71, while an established community within a lava flow in a colder climate could take months to years14,15.
The earliest reported sampling of a freshly emplaced lava flow was conducted by Kelly et al.14 three months after the Eyjafjallajökull volcano in Iceland erupted in 2010. Their samples surprisingly already had ~106 cells/g, whereas ~decades-old lava flows in Iceland have ~107–108 cells/g15. Centuries-old lava flows in Idaho and Hawaiʻi have comparable numbers25,52,215, potentially suggesting an upper limit on cell abundance in basaltic rocks. However, widefield fluorescence microscopy and flow cytometry remain underused techniques for cell counts because of the low biomass and high background fluorescence25, which can be solved with catalyzed reporter deposition fluorescence in situ hybridization and confocal microscopy. Additional work is required to understand the general trends in cell abundance as a function of age.
Trends in phylogenetic diversity are generally more complex. Lava flows of different ages ( ~ tens to hundreds of years old) on Fogo Island, Canada28 and on Kīlauea, Hawaiʻi216, were shown to have no correlation between phylogenetic diversity and age, suggesting a greater impact of environmental parameters. Studies along non-vegetated lava flows in Iceland also found that relatively small age differences ( ~ years to decades) do not impact microbial community structure15. Organic C likely correlates with increasing deposit age215 so some studies have suggested that the organic depositional rate is more important than age15,28,219. Conversely, in lava tubes in Hawaiʻi, ~centuries-old sites were generally more phylogenetically diverse than ~decades-old sites102. Over longer timescales (thousands of years or more), phylogenetic diversity and biomass estimates of volcanic deposits increase over time, are similar to environmentally comparable soil environments, and are likely controlled by vascular plant distribution in the area223,224,225. The effect of age may therefore reflect geographical location as well as the rate of plant colonization and rock weathering as the environment becomes increasingly spatially homogenous and nutrient rich (Box 2).
Trends in metabolisms
Phototrophs are abundant in young deglaciated soils4,226. However, the prevailing theory that phototrophs dominate the earliest successional stages in volcanic environments has been challenged with the advent of culture-independent methods14,211. Kelly et al.14 reported few taxa associated with phototrophs and instead found a high proportion of heterotrophs in the phyla Actinomycetota and Pseudomonadota in three-month-old samples in Iceland. They also found potentially N-fixing groups such as Herbaspirillum—which has been reported in volcanic deposits elsewhere227—and the S-oxidizing genus Thiobacillus. However, caution should be taken when making functional inferences using 16S rRNA gene sequencing and next-generation omics techniques are generally required to evaluate metabolic potential228.
Shotgun metagenomic sequencing was used on samples from HTHH and genes associated with S-redox metabolisms, CO oxidation, and H2 oxidation were found. Notably, there was an absence of photosynthetic Cyanobacteriota, but genes associated with anoxygenic photosynthesis in members of the families Beijerinckiaceae and Acetobacteraceae were present, presumably using S as an electron donor211. Trace gasses such as CO and H2 have long been suggested to support chemoautotrophic bacteria in volcanic systems, many of which are capable of N fixation121. Trace gas measurements on Kīlauea, Hawaiʻi, found that CO and H2 consumption by microorganisms supported a large proportion of metabolic activity in unvegetated sites22,215,229. In vegetated sites, leaf litter and contributions from the rhizosphere likely were the primary sources of organic matter. Similarly, culture-based studies on Surtsey found a community predominantly composed of aerobic heterotrophs in fumaroles and subsurface samples85.
Together, these studies indicate that organic C inputs must initially come from meteoritic water, necromass, or from the chemoautotroph groups. Subsequently, biomass likely expands with endogenous organic matter production, photoautotroph colonization, and from additional external inputs. Trace gas oxidizers and chemoautotrophic groups may therefore play a similar functional role in early stages, as photoautotrophs do in deglaciated soils3,220, by providing a source of fixed C. These trends are also likely different in lava tubes, where percolating organic matter often remains unused in older tubes due to the high proportion of chemoautotrophs124.
Lava flows may also be severely N-limited, which could select for the colonization of N-fixers and ammonia oxidizers230. For example, nitrification potential was the highest in the youngest lava flows in the south of Chile231. Similarly, on Mauna Loa, Hawaiʻi, N-fixation rates were generally constant as a function of lava age, whereas the keystone N-fixer changed from lichen in decades-old lavas to rhizosphere-associated bacteria in centuries-old lavas232.
A fixed-site study spanning six years after an eruption on Miyake-jima217, Japan, reported a large population of acidophilic, Fe(II)-oxidizing species, (e.g., Leptospirillum, Acidithiobacillus ferrivorans, and Acidithiobacillus ferrooxidans, which are all capable of N fixation, with the latter also capable of H2 oxidation). In these basaltic pyroclastic materials, the relative proportion of the class Betaproteobacteria (Pseudomonadota) increased as a function of age while the proportion of Fe-oxidizing taxa decreased. Similarly, as systems in Hawaiʻi increased in age and geothermal systems cooled to ambient, Pseudomonadota and Actinomycetota likely became more prevalent102. Studies of older lava flows in Hawaiʻi and in Craters of the Moon, Idaho, did not detect any known Fe-oxidizing taxa and instead were also dominated by Pseudomonadota, Acidobacteriota, and Actinomycetota25, though analysis using metagenomic techniques found genes associated with Fe uptake and Mn oxidation in Craters of the Moon lava caves126.
SO2 exposure from volcanic emissions had a major impact on microbial community assembly on Miyake-jima. No sequences associated with Cyanobacteriota were found in samples younger than 10 years, likely due to acidic pH and SO2 exposure. Despite being younger, sites less exposed to SO2 were more similar to mature forest soils and had a smaller proportion of Fe-oxidizing taxa and N-fixation pathways than heavily exposed sites12. These results again suggest short-term variations in age may be less important than habitat filtering and competitive biological interactions (Box 2)233. Fe-oxidizing taxa are of particular interest to astrobiological studies of Mars due to abundant reduced Fe within the subsurface and their ability to produce biosignatures such as biomineralization products, distinctive Fe-isotope patterns, and morphological biosignatures in a rock121,234. Their presence in low-biomass volcanic terrains is promising in the search for life on Mars.
Conclusions
Bacteria and archaea play crucial roles in the weathering of rocks and the development of soils that lead to the colonization of more complex life. Recent work has shown that the trajectories of assembly are largely influenced by habitat type, environmental filters, and competitive biological interactions (Supplementary Table 2). Still, the microbial ecological succession in young volcanic terrains remains a largely unexplored frontier due to the extreme conditions of these environments. Future research should aim to achieve a definitive mechanistic understanding of the abiotic and biotic factors contributing to primary succession (Box 2) and provide more information about the metabolic capabilities of these microbial communities. Next-generation sequencing is just beginning to be employed in volcanic terrains and analysis of the metatranscriptome has rarely been used. The study of succession in volcanic environments has applications beyond microbial ecology, including astrobiology118,235. Mars has a hydrological and volcanically active past39,40. By examining the microbial communities in analogous environments on Earth, we can gain insights into the possibility of life on Mars53,92. Given that the search for life on Mars has been identified as a high-priority science objective by the planetary science community, studying these systems remains timely and relevant7.
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
Fierer, N., Nemergut, D., Knight, R. & Craine, J. M. Changes through time: Integrating microorganisms into the study of succession. Res. Microbiol. 161, 635–642 (2010).
Tscherko, D., Rustemeier, J., Richter, A., Wanek, W. & Kandeler, E. Functional diversity of the soil microflora in primary succession across two glacier forelands in the Central Alps. Eur. J. Soil Sci. 54, 685–696 (2003).
Schmidt, S. K. et al. The earliest stages of ecosystem succession in high-elevation (5000 meters above sea level), recently deglaciated soils. Proc. R. Soc. B Biol. Sci. 275, 2793–2802 (2008).
Ciccazzo, S., Esposito, A., Borruso, L. & Brusetti, L. Microbial communities and primary succession in high altitude mountain environments. Ann. Microbiol. 66, 43–60 (2016).
Ferrenberg, S. et al. Changes in assembly processes in soil bacterial communities following a wildfire disturbance. ISME J. 7, 1102–1111 (2013).
Schmincke, H.-U. Volcanism. (Berlin, Heidelberg: Springer Berlin Heidelberg, 2004).
National Academies of Sciences, Engineering, and Medicine. Origins, Worlds, and Life: A Decadal Strategy for Planetary Science and Astrobiology 2023-2032. (Washington DC: The National Academies Press, 2022).
Izawa, M. R. M., Banerjee, N. R., Flemming, R. L., Bridge, N. J. & Schultz, C. Basaltic glass as a habitat for microbial life: Implications for astrobiology and planetary exploration. Planet. Space Sci. 58, 583–591 (2010).
Cockell, C. S., Kelly, L. & Summers, S. Microbiology of volcanic environments in Extremophiles handbook (ed. Horikoshi, K.) 917–933 (Springer Japan, 2011).
Cockell, C. S. et al. Sample collection and return from Mars: Optimising sample collection based on the microbial ecology of terrestrial volcanic environments. Space Sci. Rev. 215, 44 (2019).
Diniega, S., Smrekar, S. E., Anderson, S. & Stofan, E. R. The influence of temperature-dependent viscosity on lava flow dynamics. J. Geophys. Res. Earth Surf. 118, 1516–1532 (2013).
Fujimura, R. et al. Unique pioneer microbial communities exposed to volcanic sulfur dioxide. Sci. Rep. 6, 1–9 (2016).
Byloos, B., Maan, H., Van Houdt, R., Boon, N. & Leys, N. The ability of basalt to leach nutrients and support growth of Cupriavidus metallidurans CH34 depends on basalt composition and element release. Geomicrobiol. J. 35, 438–446 (2018).
Kelly, L. C., Cockell, C. S., Thorsteinsson, T., Marteinsson, V. & Stevenson, J. Pioneer microbial communities of the Fimmvörðuháls lava flow, Eyjafjallajökull, Iceland. Microb. Ecol. 68, 504–518 (2014).
Byloos, B. et al. Characterization of the bacterial communities on recent Icelandic volcanic deposits of different ages. BMC Microbiol. 18, 122 (2018).
Herrera, A. & Cockell, C. S. Exploring microbial diversity in volcanic environments: A review of methods in DNA extraction. J. Microbiol. Methods 70, 1–12 (2007).
Eggler, W. A. Plant life of Paricutin Volcano, Mexico, eight years after activity ceased. Am. Midl. Nat. 69, 38–68 (1963).
Englund, B. Algal nitrogen fixation on the lava field of Heimaey, Iceland. Oecologia 34, 45–55 (1978).
Kurina, L. M. & Vitousek, P. M. Nitrogen fixation rates of Stereocaulon vulcani on young Hawaiian lava flows. Biogeochemistry 55, 179–194 (2001).
Shimizu, A. Community structure of lichens in the volcanic highlands of Mt. Tokachi, Hokkaido, Japan. The Bryologist 107, 141–151 (2004).
Kristinsson, H. G. & Heiðmarsson, S. Colonization of lichens on Surtsey 1970–2006. Surtsey Res. 12, 81–104 (2009).
Dunfield, K. E. & King, G. M. Molecular analysis of carbon monoxide-oxidizing bacteria associated with recent Hawaiian volcanic deposits. Appl. Environ. Microbiol. 70, 4242–4248 (2004).
Cockell, C. S. et al. Bacteria in weathered basaltic glass, Iceland. Geomicrobiol. J. 26, 491–507 (2009).
Cockell, C. S., Olsson-Francis, K., Herrera, A. & Meunier, A. Alteration textures in terrestrial volcanic glass and the associated bacterial community. Geobiology 7, 50–65 (2009).
Cockell, C. S. et al. A low-diversity microbiota inhabits extreme terrestrial basaltic terrains and their fumaroles: Implications for the exploration of Mars. Astrobiology 19, 284–299 (2019).
Vellend, M. & Agrawal, A. Conceptual synthesis in community ecology. Q. Rev. Biol. 85, 183–206 (2010).
Memoli, V. et al. Soil element fractions affect phytotoxicity, microbial biomass and activity in volcanic areas. Sci. Total Environ. 636, 1099–1108 (2018).
Biderre-Petit, C. et al. Analysis of bacterial and archaeal communities associated with Fogo volcanic soils of different ages. FEMS Microbiol. Ecol. 96, fiaa104 (2020).
Chase, J. M. & Myers, J. A. Disentangling the importance of ecological niches from stochastic processes across scales. Philos. Trans. R. Soc. B Biol. Sci. 366, 2351–2363 (2011).
Dini-Andreote, F., Stegen, J. C., van Elsas, J. D. & Salles, J. F. Disentangling mechanisms that mediate the balance between stochastic and deterministic processes in microbial succession. Proc. Natl. Acad. Sci. 112, E1326–E1332 (2015).
Corliss, J. B., Baross, J. & Hoffman, S. An hypothesis concerning the relationships between submarine hot springs and the origin of life on Earth. Oceanol. Acta 4, 59–69 (1981).
Burcar, B. T. et al. RNA oligomerization in laboratory analogues of alkaline hydrothermal vent systems. Astrobiology 15, 509–522 (2015).
Spiess, F. N. et al. East Pacific Rise: Hot springs and geophysical experiments. Science 207, 1421–1433 (1980).
Djokic, T., Van Kranendonk, M. J., Campbell, K. A., Walter, M. R. & Ward, C. R. Earliest signs of life on land preserved in ca. 3.5 Ga hot spring deposits. Nat. Commun. 8, 15263 (2017).
Janse van Rensburg, D. J., Heubeck, C. E. & Reimann, S. Volcanoes in the estuaries: Insights into Earth’s oldest (3.22 Ga) terrestrial microbial habitats, Moodies Group, Barberton Greenstone Belt. Precambrian Res. 365, 106325 (2021).
Damer, B. & Deamer, D. The hot spring hypothesis for an origin of life. Astrobiology 20, 429–452 (2020).
Brasier, M. D., Matthewman, R., McMahon, S. & Wacey, D. Pumice as a remarkable substrate for the origin of life. Astrobiology 11, 725–735 (2011).
van Thienen, P. et al. Water, life, and planetary geodynamical evolution. in Geology and habitability of terrestrial planets. (eds. Fishbaugh, K. E., Lognonné, P., Raulin, F., Des Marais, D. J. & Korablev, O.) 167–203 (Springer New York, 2007).
Baker, V. R. et al. Fluvial geomorphology on Earth-like planetary surfaces: A review. Geomorphology 245, 149–182 (2015).
Hamilton, C. W., Fagents, S. A. & Wilson, L. Explosive lava-water interactions in Elysium Planitia, Mars: Geologic and thermodynamic constraints on the formation of the Tartarus Colles cone groups. J. Geophys. Res. E Planets 115, 1–24 (2010).
Hamilton, C. W., Fagents, S. A. & Thordarson, T. Lava-ground ice interactions in Elysium Planitia, Mars: Geomorphological and geospatial analysis of the Tartarus Colles cone groups. J. Geophys. Res. E Planets 116, E03004 (2011).
Mouginis-Mark, P. J., Zimbelman, J. R., Crown, D. A., Wilson, L. & Gregg, T. K. P. Martian volcanism: Current state of knowledge and known unknowns. Geochemistry 82, 125886 (2022).
Byrne, P. K. A comparison of inner Solar System volcanism. Nat. Astron. 4, 321–327 (2020).
Wilson, L. Volcanism in the Solar System. Nat. Geosci. 2, 389–397 (2009).
Voigt, J. R. C. et al. Revealing Elysium Planitia’s young geologic history: Constraints on lava emplacement, areas, and volumes. J. Geophys. Res. Planets 128, e2023JE007947 (2023).
Hauber, E., Brož, P., Jagert, F., Jodłowski, P. & Platz, T. Very recent and wide-spread basaltic volcanism on Mars. Geophys. Res. Lett. 38, L10201 (2011).
Ruff, S. W., Niles, P. B., Alfano, F. & Clarke, A. B. Evidence for a Noachian-aged ephemeral lake in Gusev Crater, Mars. Geology 42, 359–362 (2014).
Mangold, N. et al. Perseverance rover reveals an ancient delta-lake system and flood deposits at Jezero Crater, Mars. Science 374, 711–717 (2021).
Haskin, L. A. et al. Water alteration of rocks and soils on Mars at the Spirit rover site in Gusev Crater. Nature 436, 66–69 (2005).
Horgan, B. H. N., Anderson, R. B., Dromart, G., Amador, E. S. & Rice, M. S. The mineral diversity of Jezero Crater: Evidence for possible lacustrine carbonates on Mars. Icarus 339, 113526 (2020).
Farley, K. A. et al. Aqueously altered igneous rocks sampled on the floor of Jezero Crater, Mars. Science 377, eabo2196 (2022).
Brady, A. L. et al. Microbial community distribution in variously altered basalts: Insights into astrobiology sample site selection. Planet. Space Sci. 194, 105107 (2020).
Duhamel, S., Hamilton, C. W., Pálsson, S. & Björnsdóttir, S. H. Microbial response to increased temperatures within a lava-induced hydrothermal system in Iceland: An analogue for the habitability of volcanic terrains on Mars. Astrobiology 22, 1176–1198 (2022).
Perera, L. J. & Cockell, C. S. Dispersion of bacteria by low-pressure boiling: Life detection in Enceladus’ plume material. Astrobiology 23, 269–279 (2023).
Cousins, C. R. et al. Biogeochemical probing of microbial communities in a basalt-hosted hot spring at Kverkfjöll volcano, Iceland. Geobiology 16, 507–521 (2018).
Moreras-Marti, A. et al. Volcanic controls on the microbial habitability of Mars-analogue hydrothermal environments. Geobiology 19, 489–509 (2021).
Uroz, S., Kelly, L. C., Turpault, M.-P., Lepleux, C. & Frey-Klett, P. The mineralosphere concept: Mineralogical control of the distribution and function of mineral-associated bacterial communities. Trends Microbiol. 23, 751–762 (2015).
Soo, R. M., Wood, S. A., Grzymski, J. J., McDonald, I. R. & Cary, S. C. Microbial biodiversity of thermophilic communities in hot mineral soils of Tramway Ridge, Mount Erebus, Antarctica. Environ. Microbiol. 11, 715–728 (2009).
Costello, E. K., Halloy, S. R. P., Reed, S. C., Sowell, P. & Schmidt, S. K. Fumarole-supported islands of biodiversity within a hyperarid, high-elevation landscape on Socompa Volcano, Puna de Atacama, Andes. Appl. Environ. Microbiol. 75, 735–747 (2009).
Sinha, N., Nepal, S., Kral, T. & Kumar, P. Effects of temperatures and high pressures on the growth and survivability of methanogens and stable carbon isotope fractionation: Implications for deep subsurface life on Mars. Int. J. Astrobiol. 20, 179–185 (2018).
Zeng, X., Alain, K. & Shao, Z. Microorganisms from deep-sea hydrothermal vents. Mar. Life Sci. Technol. 3, 204–230 (2021).
Inskeep, W., Jay, Z., Tringe, S., Herrgard, M. & Rusch, D. The YNP Metagenome Project: Environmental parameters responsible for microbial distribution in the Yellowstone geothermal ecosystem. Front. Microbiol. 4, 67 (2013).
Brock, T. D. Life at high temperatures. Science 158, 1012–1019 (1967).
Des Marais, D. J. & Walter, M. R. Terrestrial hot spring systems: Introduction. Astrobiology 19, 1419–1432 (2019).
Drake, B. D. et al. Evolution of a dynamic paleo-hydrothermal system at Mangatete, Taupo Volcanic Zone, New Zealand. J. Volcanol. Geotherm. Res. 282, 19–35 (2014).
Riding, R. The term stromatolite: Towards an essential definition. Lethaia 32, 321–330 (1999).
Fouke, B. W. Hot-spring systems geobiology: Abiotic and biotic influences on travertine formation at Mammoth Hot Springs, Yellowstone National Park, USA. Sedimentology 58, 170–219 (2011).
Lau, C. Y., Aitchison, J. C. & Pointing, S. B. Early colonization of thermal niches in a silica-depositing hot spring in central Tibet. Geobiology 6, 136–146 (2008).
Starke, V., Kirshtein, J., Fogel, M. L. & Steele, A. Microbial community composition and endolith colonization at an arctic thermal spring are driven by calcite precipitation. Environ. Microbiol. Rep. 5, 648–659 (2013).
Beam, J. P. et al. Assembly and succession of iron oxide microbial mat communities in acidic geothermal springs. Front. Microbiol. 7, 25 (2016).
Kanellopoulos, C., Lamprinou, V., Politi, A., Voudouris, P. & Economou-Amilli, A. Pioneer species of Cyanobacteria in hot springs and their role to travertine formation: The case of Aedipsos hot springs, Euboea (Evia), Greece. Depositional Rec. 8, 1079–1092 (2022).
Jansson, C. & Northen, T. Calcifying Cyanobacteria—The potential of biomineralization for carbon capture and storage. Curr. Opin. Biotechnol. 21, 365–371 (2010).
Della Porta, G., Hoppert, M., Hallmann, C., Schneider, D. & Reitner, J. The influence of microbial mats on travertine precipitation in active hydrothermal systems (Central Italy). Depositional Rec. 8, 165–209 (2022).
Bennett, A. C., Murugapiran, S. K. & Hamilton, T. L. Temperature impacts community structure and function of phototrophic Chloroflexi and Cyanobacteria in two alkaline hot springs in Yellowstone National Park. Environ. Microbiol. Rep. 12, 503–513 (2020).
Bonnefoy, L. E. et al. Landscape evolution associated with the 2014–2015 Holuhraun eruption in Iceland. J. Volcanol. Geotherm. Res. 387, 106652 (2019).
Dundas, C. M. et al. Lava–water interaction and hydrothermal activity within the 2014–2015 Holuhraun lava flow field, Iceland. J. Volcanol. Geotherm. Res. 408, 107100 (2020).
Osinski, G. R., Lee, P., Parnell, J., Spray, J. G. & Baron, M. A case study of impact-induced hydrothermal activity: The Haughton impact structure, Devon Island, Canadian High Arctic. Meteorit. Planet. Sci. 40, 1859–1877 (2005).
Kring, D. A. et al. Probing the hydrothermal system of the Chicxulub impact crater. Sci. Adv. 6, eaaz3053 (2020).
Parnell, J., Lee, P., Cockell, C. S. & Osinski, G. R. Microbial colonization in impact-generated hydrothermal sulphate deposits, Haughton impact structure, and implications for sulphates on Mars. Int. J. Astrobiol. 3, 247–256 (2004).
Thordarson, T. Physical volcanology of lava flows on Surtsey, Iceland: A preliminary report. Surtsey Res. 11, 109–126 (2000).
Cole, P. D., Guest, J. E., Duncan, A. M. & Pacheco, J.-M. Capelinhos 1957–1958, Faial, Azores: Deposits formed by an emergent Surtseyan eruption. Bull. Volcanol. 63, 204 (2001).
Garvin, J. B. et al. Monitoring and modeling the rapid evolution of Earth’s newest volcanic island: Hunga Tonga Hunga Ha’apai (Tonga) using high spatial resolution satellite observations. Geophys. Res. Lett. 45, 3445–3452 (2018).
Óskarsson, B. V., Jónasson, K., Valsson, G. & Belart, J. M. C. Erosion and sedimentation in Surtsey island quantified from new DEMs. Surtsey Res. 14, 63–77 (2020).
Bergsten, P. et al. Basalt-hosted microbial communities in the subsurface of the young volcanic island of Surtsey, Iceland. Front. Microbiol. 12, 728977 (2021).
Bergsten, P., Vannier, P., Frion, J., Mougeolle, A. & Marteinsson, V. Þ Culturable bacterial diversity from the basaltic subsurface of the young volcanic island of Surtsey, Iceland. Microorganisms 10, 1177 (2022).
Marteinsson, V. et al. Microbial colonization in diverse surface soil types in Surtsey and diversity analysis of its subsurface microbiota. Biogeosciences 12, 1191–1203 (2015).
Baker, P. E., Davies, T. G. & Roobol, M. J. Volcanic activity at Deception Island in 1967 and 1969. Nature 224, 553–560 (1969).
Geyer, A. et al. Deciphering the evolution of Deception Island’s magmatic system. Sci. Rep. 9, 373 (2019).
Cameron, R. E. & Benoit, R. E. Microbial and ecological investigations of recent cinder cones, Deception Island, Antarctica–A preliminary report. Ecology 51, 802–809 (1970).
Fermani, P., Mataloni, G. & Van de Vijver, B. Soil microalgal communities on an antarctic active volcano (Deception Island, South Shetlands). Polar Biol. 30, 1381–1393 (2007).
Bendia, A. G. et al. A mosaic of geothermal and marine features shapes microbial community structure on Deception Island volcano, Antarctica. Front. Microbiol. 9, 899 (2018).
Costello, L. J. et al. Habitability of hydrothermal systems at Jezero and Gusev Craters as constrained by hydrothermal alteration of a terrestrial mafic dike. Chem. Erde 80, 125613 (2020).
Ruff, S. W., Campbell, K. A., Van Kranendonk, M. J., Rice, M. S. & Farmer, J. D. The case for ancient hot springs in Gusev Crater, Mars. Astrobiology 20, 475–499 (2019).
Cousins, C. R. et al. Glaciovolcanic hydrothermal environments in Iceland and implications for their detection on Mars. J. Volcanol. Geotherm. Res. 256, 61–77 (2013).
Cousins, C. R. & Crawford, I. A. Volcano-ice interaction as a microbial habitat on Earth and Mars. Astrobiology 11, 695–710 (2011).
Tuffen, H., Pinkerton, H., McGarvie, D. W. & Gilbert, J. S. Melting of the glacier base during a small-volume subglacial rhyolite eruption: Evidence from Bláhnúkur, Iceland. SEdiment. Geol. 149, 183–198 (2002).
Chapman, M. G. et al. Volcanism and ice interactions on Earth and Mars. in Environmental effects on volcanic eruptions: From deep oceans to deep space (eds. Zimbelman, J. R. & Gregg, T. K. P.) 39–73 (Springer US, 2000).
Fraser, C. I., Terauds, A., Smellie, J., Convey, P. & Chown, S. L. Geothermal activity helps life survive glacial cycles. Proc. Natl. Acad. Sci. 111, 5634–5639 (2014).
Travis, B. J., Rosenberg, N. D. & Cuzzi, J. N. On the role of widespread subsurface convection in bringing liquid water close to Mars’ surface. J. Geophys. Res. E Planets 108, 8040 (2003).
García-Lopez, E. et al. Microbial community structure driven by a volcanic gradient in glaciers of the antarctic archipelago South Shetland. Microorganisms 9, 392 (2021).
Wall, K., Cornell, J., Bizzoco, R. W. & Kelley, S. T. Biodiversity hot spot on a hot spot: Novel extremophile diversity in Hawaiian fumaroles. MicrobiologyOpen 4, 267–281 (2015).
Prescott, R. D. et al. Islands within islands: Bacterial phylogenetic structure and consortia in Hawaiian lava caves and fumaroles. Front. Microbiol. 13, 934708 (2022).
Tilling, R. I., Heliker, C. & Swanson, D. A. Eruptions of Hawaiian volcanoes–Past, present, and future. U.S. Geological Survey General Information Product 117, 63 (2010).
Rothschild, L. J. & Mancinelli, R. L. Life in extreme environments. Nature 409, 1092–1101 (2001).
Zhou, Jizhong et al. Spatial and resource factors influencing high microbial diversity in soil. Appl. Environ. Microbiol. 68, 326–334 (2002).
Marlow, J. J. et al. Mapping metabolic activity at single cell resolution in intact volcanic fumarole sediment. FEMS Microbiol. Lett. 367, fnaa031 (2020).
Ellis, D. G., Bizzoco, R. W. & Kelley, S. T. Halophilic archaea determined from geothermal steam vent aerosols. Environ. Microbiol. 10, 1582–1590 (2008).
Benson, C. A., Bizzoco, R. W., Lipson, D. A. & Kelley, S. T. Microbial diversity in nonsulfur, sulfur and iron geothermal steam vents. FEMS Microbiol. Ecol. 76, 74–88 (2011).
Peterson, D. W., Holcomb, R. T., Tilling, R. I. & Christiansen, R. L. Development of lava tubes in the light of observations at Mauna Ulu, Kilauea Volcano, Hawaii. Bull. Volcanol. 56, 343–360 (1994).
Hon, K., Kauahikaua, J., Denlinger, R. & MacKay, K. Emplacement and inflation of pahoehoe sheet flows: Observations and measurements of active lava flows on Kilauea Volcano, Hawaii. GSA Bull. 106, 351–370 (1994).
Sauro, F. et al. Lava tubes on Earth, Moon and Mars: A review on their size and morphology revealed by comparative planetology. Earth-Sci. Rev. 209, 103288 (2020).
Walker, G. P. L. Structure, and origin by injection of lava under surface crust, of tumuli, “lava rises,” “lava-rise pits,” and “lava-inflation clefts” in Hawaii. Bull. Volcanol. 53, 546–558 (1991).
Hamilton, C. W. et al. Lava-rise plateaus and inflation pits in the McCartys lava flow field, New Mexico: An analog for pāhoehoe-like lava flows on planetary surfaces. J. Geophys. Res. Planets 125, e2019JE005975 (2020).
Léveillé, R. J. & Datta, S. Lava tubes and basaltic caves as astrobiological targets on Earth and Mars: A review. Planet. Space Sci. 58, 592–598 (2010).
Gabriel, C. R. & Northup, D. E. Microbial ecology: Caves as an extreme habitat. in Cave microbiomes: A novel resource for drug discovery (ed. Cheeptham, N.) 85–108 (Springer New York, 2013).
Summers Engel, A. et al. Microbial life of cave systems. (De Gruyter, Inc., 2015).
Lavoie, K. H. et al. Comparison of bacterial communities from lava cave microbial mats to overlying surface soils from Lava Beds National Monument, USA. PLOS ONE 12, e0169339 (2017).
Northup, D. E. et al. Lava cave microbial communities within mats and secondary mineral deposits: Implications for life detection on other planets. Astrobiology 11, 601–618 (2011).
Snider, J. R., Goin, C., Miller, R. V., Boston, P. J. & Northup, D. E. Ultraviolet radiation sensitivity in cave bacteria: Evidence of adaptation to the subsurface? Int. J. Speleology 38, 11–22 (2009).
Fishman, C. B. et al. Extreme niche partitioning and microbial dark matter in a Mauna Loa lava tube. J. Geophys. Res. Planets 128, e2022JE007283 (2023).
King, G. M. Chemolithotrophic bacteria: Distributions, functions and significance in volcanic environments. Microbes Environ. 22, 309–319 (2007).
Popa, R., Smith, A. R., Popa, R., Boone, J. & Fisk, M. Olivine-respiring bacteria isolated from the rock-ice interface in a lava-tube cave, a Mars analog environment. Astrobiology 12, 9–18 (2012).
Simon, K. S., Benfield, E. F. & Macko, S. A. Food web structure and the role of epilithic biofilms in cave streams. Ecology 84, 2395–2406 (2003).
Selensky, M. J., Masterson, A. L., Blank, J. G., Lee, S. C. & Osburn, M. R. Stable carbon isotope depletions in lipid biomarkers suggest subsurface carbon fixation in lava caves. J. Geophys. Res. Biogeosciences 126, e2021JG006430 (2021).
Snider, J. Comparison of microbial communities on roots, ceilings and floors of two lava tube caves in New Mexico. M. Sci. Thesis. University of New Mexico. https://digitalrepository.unm.edu/biol_etds/103 (2010).
Weng, M. M. et al. Life underground: Investigating microbial communities and their biomarkers in Mars-analog lava tubes at Craters of the Moon National Monument and Preserve. J. Geophys. Res. Planets 127, e2022JE007268 (2022).
Toju, H., Tanabe, A. S. & Sato, H. Network hubs in root-associated fungal metacommunities. Microbiome 6, 116 (2018).
Zamkovaya, T., Foster, J. S., de Crécy-Lagard, V. & Conesa, A. A network approach to elucidate and prioritize microbial dark matter in microbial communities. ISME J. 15, 228–244 (2021).
Boston, P. J., Ivanov, M. V. & McKay, C. P. On the possibility of chemosynthetic ecosystems in subsurface habitats on Mars. Icarus 95, 300–308 (1992).
Leone, G. A network of lava tubes as the origin of Labyrinthus Noctis and Valles Marineris on Mars. J. Volcanol. Geotherm. Res. 277, 1–8 (2014).
Wagner, R. V. & Robinson, M. S. Distribution, formation mechanisms, and significance of Lunar pits. Icarus 237, 52–60 (2014).
Kempe, S. & Al-Malabeh, A. Newly discovered lava tunnels of the Al-Shaam plateau basalts, Jordan. EGU Geophys. Res. Abstr. 7, 03204 (2005).
Carrier, B. L. et al. Mars extant life: What’s next? Conference report. Astrobiology 20, 785–814 (2020).
O’Brien, S. L. et al. Spatial scale drives patterns in soil bacterial diversity. Environ. Microbiol. 18, 2039–2051 (2016).
Nielsen, M. E. & Fisk, M. R. Surface area measurements of marine basalts: Implications for the subseafloor microbial biomass. Geophys. Res. Lett. 37, L15604 (2010).
Fisk, M. R. & Giovannoni, S. J. Sources of nutrients and energy for a deep biosphere on Mars. J. Geophys. Res. E Planets 104, 11805–11815 (1999).
Schumann, G., Manz, W., Reitner, J. & Lustrino, M. Ancient fungal life in North Pacific Eocene oceanic crust. Geomicrobiol. J. 21, 241–246 (2004).
Peckmann, J., Bach, W., Behrens, K. & Reitner, J. Putative cryptoendolithic life in Devonian pillow basalt, Rheinisches Schiefergebirge, Germany. Geobiology 6, 125–135 (2008).
Staudigel, H. et al. 3.5 billion years of glass bioalteration: Volcanic rocks as a basis for microbial life? Earth-Sci. Rev. 89, 156–176 (2008).
Cousins, C. R., Smellie, J. L., Jones, A. P. & Crawford, I. A. A comparative study of endolithic microborings in basaltic lavas from a transitional subglacial–marine environment. Int. J. Astrobiol. 8, 37–49 (2009).
Cavalazzi, B., Westall, F., Cady, S. L., Barbieri, R. & Foucher, F. Potential fossil endoliths in vesicular pillow basalt, Coral Patch Seamount, Eastern North Atlantic Ocean. Astrobiology 11, 619–632 (2011).
Hon, K., Gansecki, C. & Kauahikaua, J. The transition from ‘a‘ā to pāhoehoe crust on flows emplaced during the Pu’u ‘Ō‘ō-Kūpaianaha eruption. US Geol. Surv. Prof. Pap. 1676, 89–104 (2003).
Voigt, J. R. C. et al. Geomorphological characterization of the 2014–2015 Holuhraun lava flow-field in Iceland. J. Volcanol. Geotherm. Res. 419, 107278 (2021).
Bjarnason, Á. H. Vegetation on lava fields in the Hekla area, Iceland. Acta Phytogeogr. Suec. 77, 1–110 (1991).
McFadden, L. D., Eppes, M. C., Gillespie, A. R. & Hallet, B. Physical weathering in arid landscapes due to diurnal variation in the direction of solar heating. GSA Bull. 117, 161–173 (2005).
Cockell, C. S., Cousins, C., Wilkinson, P. T., Olsson-Francis, K. & Rozitis, B. Are thermophilic microorganisms active in cold environments? Int. J. Astrobiol. 14, 457–463 (2015).
Oppenheimer, C. Volcanic degassing. in Treatise on geochemistry (eds. Holland, H. D. & Turekian, K. K.) 123–166 (Pergamon, 2003).
Jorge Villar, S. E., Edwards, H. G. M. & Benning, L. G. Raman spectroscopic and scanning electron microscopic analysis of a novel biological colonisation of volcanic rocks. Icarus 184, 158–169 (2006).
Kelly, L. C. et al. Bacterial diversity of weathered terrestrial Icelandic volcanic glasses. Microb. Ecol. 60, 740–752 (2010).
Bailey, B., Templeton, A., Staudigel, H. & Tebo, B. M. Utilization of substrate components during basaltic glass colonization by Pseudomonas and Shewanella isolates. Geomicrobiol. J. 26, 648–656 (2009).
McMahon, S., Parnell, J., Ponicka, J., Hole, M. & Boyce, A. The habitability of vesicles in Martian basalt. Astron. Geophys. 54, 1.17–1.21 (2013).
Bagshaw, E. A. et al. The microbial habitability of weathered volcanic glass inferred from continuous sensing techniques. Astrobiology 11, 651–664 (2011).
Rawls, W. J., Pachepsky, Y. A., Ritchie, J. C., Sobecki, T. M. & Bloodworth, H. Effect of soil organic carbon on soil water retention. Geoderma 116, 61–76 (2003).
Davila, A. F., Hawes, I., Ascaso, C. & Wierzchos, J. Salt deliquescence drives photosynthesis in the hyperarid Atacama Desert. Environ. Microbiol. Rep. 5, 583–587 (2013).
Schulze-Makuch, D. et al. Transitory microbial habitat in the hyperarid Atacama Desert. Proc. Natl. Acad. Sci. 115, 2670–2675 (2018).
Herrera, A. et al. Bacterial colonization and weathering of terrestrial obsidian in Iceland. Geomicrobiol. J. 25, 25–37 (2008).
Carrier, B. L., Abbey, W. J., Beegle, L. W., Bhartia, R. & Liu, Y. Attenuation of ultraviolet radiation in rocks and minerals: Implications for Mars science. J. Geophys. Res. Planets 124, 2599–2612 (2019).
Godin, P. J., Moore, C. A., Smith, C. & Moores, J. E. Absorption and scattering of UV and visible light through simulated Martian regoliths and rock samples. Astrobiology 23, 280–290 (2023).
Lovley, D. R. & Phillips, E. J. Novel mode of microbial energy metabolism: Organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 54, 1472–1480 (1988).
Myers, C. R. & Nealson, K. H. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science 240, 1319–1321 (1988).
Stranghoener, M., Schippers, A., Dultz, S. & Behrens, H. Experimental microbial alteration and Fe mobilization from basaltic rocks of the ICDP HSDP2 drill core, Hilo, Hawaii. Front. Microbiol. 9, 1–17 (2018).
Dong, H. et al. A critical review of mineral–microbe interaction and co-evolution: Mechanisms and applications. Natl. Sci. Rev. 9, nwac128 (2022).
Kelly, L. C. et al. Bacterial diversity of terrestrial crystalline volcanic rocks, Iceland. Microb. Ecol. 62, 69–79 (2011).
Chen, J. et al. Distinct effects of volcanic cone types on soil microbiomes: Evidence from cinder cone and spatter cone. CATENA 200, 105180 (2021).
Callac, N. et al. Microbial colonization of basaltic glasses in hydrothermal organic-rich sediments at Guaymas Basin. Front. Microbiol. 4, 250 (2013).
Henri, P. A. et al. Structural iron (II) of basaltic glass as an energy source for Zetaproteobacteria in an abyssal plain environment, off the Mid Atlantic Ridge. Front. Microbiol. 6, 1518 (2016).
Sudek, L. A., Wanger, G., Templeton, A. S., Staudigel, H. & Tebo, B. M. Submarine basaltic glass colonization by the heterotrophic Fe(II)-oxidizing and siderophore-producing deep-sea bacterium Pseudomonas stutzeri VS-10: The potential role of basalt in enhancing growth. Front. Microbiol. 8, 1–12 (2017).
Elser, J. J. et al. Community structure and biogeochemical impacts of microbial life on floating pumice. Appl. Environ. Microbiol. 81, 1542–1549 (2015).
Li, B. & Brett, M. T. The influence of dissolved phosphorus molecular form on recalcitrance and bioavailability. Environ. Pollut. 182, 37–44 (2013).
Adcock, C. T., Hausrath, E. M. & Forster, P. M. Readily available phosphate from minerals in early aqueous environments on Mars. Nat. Geosci. 6, 824–827 (2013).
Webster, J. D. & Piccoli, P. M. Magmatic apatite: A powerful, yet deceptive, mineral. Elements 11, 177–182 (2015).
Busigny, V., Laverne, C. & Bonifacie, M. Nitrogen content and isotopic composition of oceanic crust at a superfast spreading ridge: A profile in altered basalts from ODP Site 1256, Leg 206. Geochem. Geophys. Geosystems 6, Q12O01 (2005).
Zhang, X. et al. Nitrogen stimulates the growth of subsurface basalt-associated microorganisms at the western flank of the Mid-Atlantic Ridge. Front. Microbiol. 7, 633 (2016).
Barton, H. A. et al. The impact of host rock geochemistry on bacterial community structure in oligotrophic cave environments. Int. J. Speleology 36, 93–104 (2007).
Jones, A. A. & Bennett, P. C. Mineral microniches control the diversity of subsurface microbial populations. Geomicrobiol. J. 31, 246–261 (2014).
Markússon, S. H. & Stefánsson, A. Geothermal surface alteration of basalts, Krýsuvík Iceland—Alteration mineralogy, water chemistry and the effects of acid supply on the alteration process. J. Volcanol. Geotherm. Res. 206, 46–59 (2011).
Sánchez-García, L. et al. Fingerprinting molecular and isotopic biosignatures on different hydrothermal scenarios of Iceland, an acidic and sulfur-rich Mars analog. Sci. Rep. 10, 21196 (2020).
McCollom, T., Moskowitz, B., Berquó, T. & Hynek, B. Acid-sulfate alteration of basalt in fumarolic environments on Earth and Mars. 43rd Lunar and Planetary Science Conference, Houston, TX, 1574 (2012).
Dultz, S. et al. Alteration of a submarine basaltic glass under environmental conditions conducive for microorganisms: Growth patterns of the microbial community and mechanism of palagonite formation. Geomicrobiol. J. 31, 813–834 (2014).
Wilmeth, D. T. et al. Environmental and biological influences on carbonate precipitation within hot spring microbial mats in Little Hot Creek, CA. Front. Microbiol. 9, 1464 (2018).
Furnes, H. & Staudigel, H. Biological mediation in ocean crust alteration: How deep is the deep biosphere? Earth Planet. Sci. Lett. 166, 97–103 (1999).
Cuadros, J. Clay minerals interaction with microorganisms: A review. Clay Miner. 52, 235–261 (2017).
Fomina, M. & Skorochod, I. Microbial interaction with clay minerals and its environmental and biotechnological implications. Minerals 10, 861 (2020).
Hausrath, E. M., Navarre-Sitchler, A. K., Sak, P. B., Steefel, C. I. & Brantley, S. L. Basalt weathering rates on Earth and the duration of liquid water on the plains of Gusev Crater, Mars. Geology 36, 67–70 (2008).
Li, G. et al. Temperature dependence of basalt weathering. Earth Planet. Sci. Lett. 443, 59–69 (2016).
Fitch, E. P., Fagents, S. A., Thordarson, T. & Hamilton, C. W. Fragmentation mechanisms associated with explosive lava–water interactions in a lacustrine environment. Bull. Volcanol. 79, 12 (2017).
Hamilton, C. W., Fitch, E. P., Fagents, S. A. & Thordarson, T. Rootless tephra stratigraphy and emplacement processes. Bull. Volcanol. 79, 11 (2017).
Jaeger, W. L. et al. Emplacement of the youngest flood lava on Mars: A short, turbulent story. Icarus 205, 230–243 (2010).
Knights, D. et al. Bayesian community-wide culture-independent microbial source tracking. Nat. Methods 8, 761–765 (2011).
Griffin, D. W., Gonzalez-Martin, C., Hoose, C. & Smith, D. J. Global-scale atmospheric dispersion of microorganisms. in Microbiology of aerosols. (ed. Delort, A., Amato, P.) 155–194 (John Wiley & Sons, Ltd, 2017).
Mayol, E. et al. Long-range transport of airborne microbes over the global tropical and subtropical ocean. Nat. Commun. 8, 201 (2017).
Bak, E. N. et al. Wind-driven saltation: An overlooked challenge for life on Mars. Astrobiology 19, 497–505 (2019).
Hagiwara, K., Matsumoto, T., Tsedendamba, P., Baba, K. & Hoshino, B. Bacterial characteristics of dust particle saltation in Gobi dust sites, Mongolia. Atmosphere 12, 1456 (2021).
Tong, Y. & Lighthart, B. Solar radiation is shown to select for pigmented bacteria in the ambient outdoor atmosphere. Photochem. Photobiol. 65, 103–106 (1997).
Brodie, E. L. et al. Urban aerosols harbor diverse and dynamic bacterial populations. Proc. Natl. Acad. Sci. 104, 299–304 (2007).
Brunet, Y., Wéry, N. & Galès, A. Short-scale transport of bioaerosols. in Microbiology of aerosols. (ed. Delort, A., Amato, P.) 137–154 (John Wiley & Sons, Ltd, 2017).
Amato, P. et al. Survival and ice nucleation activity of bacteria as aerosols in a cloud simulation chamber. Atmos. Chem. Phys. 15, 6455–6465 (2015).
Amato, P. et al. Active microorganisms thrive among extremely diverse communities in cloud water. PLOS ONE 12, e0182869 (2017).
Lazaridis, M. Bacteria as cloud condensation nuclei (CCN) in the atmosphere. Atmosphere 10, 786 (2019).
Woo, C. & Yamamoto, N. Falling bacterial communities from the atmosphere. Environ. Microbiome 15, 22 (2020).
Gat, D., Mazar, Y., Cytryn, E. & Rudich, Y. Origin-dependent variations in the atmospheric microbiome community in Eastern Mediterranean dust storms. Environ. Sci. Technol. 51, 6709–6718 (2017).
Yamaguchi, N., Ichijo, T., Sakotani, A., Baba, T. & Nasu, M. Global dispersion of bacterial cells on Asian dust. Sci. Rep. 2, 525 (2012).
Arnalds, O., Dagsson-Waldhauserova, P. & Olafsson, H. The Icelandic volcanic aeolian environment: Processes and impacts — A review. Aeolian Res. 20, 176–195 (2016).
Hu, W. et al. Abundance and viability of particle-attached and free-floating bacteria in dusty and nondusty air. Biogeosciences 17, 4477–4487 (2020).
Martiny, J. B. H. et al. Microbial biogeography: Putting microorganisms on the map. Nat. Rev. Microbiol. 4, 102–112 (2006).
DeLeon-Rodriguez, N. et al. Microbiome of the upper troposphere: Species composition and prevalence, effects of tropical storms, and atmospheric implications. Proc. Natl. Acad. Sci. 110, 2575–2580 (2013).
Whitaker, R. J., Grogan, D. W. & Taylor, J. W. Geographic barriers isolate endemic populations of hyperthermophilic archaea. Science 301, 976–978 (2003).
Perfumo, A. & Marchant, R. Global transport of thermophilic bacteria in atmospheric dust. Environ. Microbiol. Rep. 2, 333–339 (2010).
Herbold, C. W., Lee, C. K., McDonald, I. R. & Cary, S. C. Evidence of global-scale aeolian dispersal and endemism in isolated geothermal microbial communities of Antarctica. Nat. Commun. 5, 3875 (2014).
Bargagli, R., Broady, P. A. & Walton, D. W. H. Preliminary investigation of the thermal biosystem of Mount Rittmann fumaroles (northern Victoria Land, Antarctica). Antarct. Sci. 8, 121–126 (1996).
Dragone, N. B. et al. The early microbial colonizers of a short-lived volcanic island in the Kingdom of Tonga. mBio 14, e03313–e03322 (2023).
Van Eaton, A. R., Harper, M. A. & Wilson, C. J. N. High-flying diatoms: Widespread dispersal of microorganisms in an explosive volcanic eruption. Geology 41, 1187–1190 (2013).
Morris, C. E. & Sands, D. C. Impacts of microbial aerosols on natural and agro-ecosystems: Immigration, invasions, and their consequences. in Microbiology of Aerosols. (ed. Delort, A., Amato, P.) 269–279 (John Wiley & Sons, Ltd, 2017).
Johnson, E. A. & Miyanishi, K. Testing the assumptions of chronosequences in succession. Ecol. Lett. 11, 419–431 (2008).
King, G. M. Contributions of atmospheric CO and hydrogen uptake to microbial dynamics on recent Hawaiian volcanic deposits. Appl. Environ. Microbiol. 69, 4067–4075 (2003).
Gomez-Alvarez, V., King, G. M. & Nüsslein, K. Comparative bacterial diversity in recent Hawaiian volcanic deposits of different ages. FEMS Microbiol. Ecol. 60, 60–73 (2007).
Fujimura, R. et al. Analysis of early bacterial communities on volcanic deposits on the island of Miyake (Miyake-jima), Japan: A 6-year study at a fixed site. Microbes Environ. 27, 19–29 (2012).
Hernández, M., Calabi, M., Conrad, R. & Dumont, M. G. Analysis of the microbial communities in soils of different ages following volcanic eruptions. Pedosphere 30, 126–134 (2020).
Kerfahi, D. et al. Development of soil bacterial communities in volcanic ash microcosms in a range of climates. Microb. Ecol. 73, 775–790 (2017).
Nemergut, D. R. et al. Microbial community succession in an unvegetated, recently deglaciated soil. Microb. Ecol. 53, 110–122 (2007).
Santelli, C. M., Edgcomb, V. P., Bach, W. & Edwards, K. J. The diversity and abundance of bacteria inhabiting seafloor lavas positively correlate with rock alteration. Environ. Microbiol. 11, 86–98 (2009).
Stegen, J. C. et al. Quantifying community assembly processes and identifying features that impose them. ISME J. 7, 2069–2079 (2013).
Shillam, L., Hopkins, D. W., Badalucco, L. & Laudicina, V. A. Structural diversity and enzyme activity of volcanic soils at different stages of development and response to experimental disturbance. Soil Biol. Biochem. 40, 2182–2185 (2008).
Lathifah, A. N. et al. Comparative characterization of bacterial communities in moss-covered and unvegetated volcanic deposits of Mount Merapi, Indonesia. Microbes Environ. 34, 268–277 (2019).
Chen, J. et al. Differences in microbial communities from Quaternary volcanic soils at different stages of development: Evidence from Late Pleistocene and Holocene volcanoes. CATENA 201, 105211 (2021).
Kaštovská, K., Elster, J., Stibal, M. & Šantrůčková, H. Microbial assemblages in soil microbial succession after glacial retreat in Svalbard (high arctic). Microb. Ecol. 50, 396–407 (2005).
Lu, H. et al. Characterization of Herbaspirillum- and Limnobacter-related strains isolated from young volcanic deposits in Miyake-Jima island, Japan. Microbes Environ. 23, 66–72 (2008).
Bowman, J. S. & Ducklow, H. W. Microbial communities can be described by metabolic structure: A general framework and application to a seasonally variable, depth-stratified microbial community from the coastal West Antarctic Peninsula. PLOS ONE 10, e0135868 (2015).
King, G. M. & Weber, C. F. Interactions between bacterial carbon monoxide and hydrogen consumption and plant development on recent volcanic deposits. ISME J. 2, 195–203 (2008).
Hathaway, J. J. M., Sinsabaugh, R. L., Dapkevicius, M. D. L. N. E. & Northup, D. E. Diversity of ammonia oxidation (amoA) and nitrogen fixation (nifH) genes in lava caves of Terceira, Azores, Portugal. Geomicrobiol. J. 31, 221–235 (2014).
Hernández, M., Dumont, M. G., Calabi, M., Basualto, D. & Conrad, R. Ammonia oxidizers are pioneer microorganisms in the colonization of new acidic volcanic soils from south of Chile. Environ. Microbiol. Rep. 6, 70–79 (2014).
Crews, T. E., Kurina, L. M. & Vitousek, P. M. Organic matter and nitrogen accumulation and nitrogen fixation during early ecosystem development in Hawaii. Biogeochemistry 52, 259–279 (2001).
Tripathi, B. M. et al. Soil pH mediates the balance between stochastic and deterministic assembly of bacteria. ISME J. 12, 1072–1083 (2018).
Price, A., Pearson, V. K., Schwenzer, S. P., Miot, J. & Olsson-Francis, K. Nitrate-dependent iron oxidation: A potential Mars metabolism. Front. Microbiol. 9, 513 (2018).
Tarnas, J. D. et al. Earth-like habitable environments in the subsurface of Mars. Astrobiology 21, 741–756 (2021).
Ármannsson, H. The fluid geochemistry of Icelandic high temperature geothermal areas. Appl. Geochem. 66, 14–64 (2016).
Jaeger, W. L., Keszthelyi, L. P., McEwen, A. S., Dundas, C. M., & Russell, P. S. Athabasca Valles, Mars: A lava-draped channel system. Science 317, 1709–1712 (2007).
Bardabelias, N., Holt, J. & Christoffersen, M. Characterizing morphology of lava tubes in El Malpais National Monument using ground penetrating radar and LIDAR. 52nd Lunar and Planetary Science Conference, Houston, TX, 2548 (2021).
Marlow, J., Peckmann, J. & Orphan, V. Autoendoliths: A distinct type of rock‐hosted microbial life. Geobiology 13, 303–307 (2015).
Philpotts, A. R. & Ague, J. J. Principles of igneous and metamorphic petrology. (Cambridge University Press, 2022).
Napieralski, S. A. et al. Microbial chemolithotrophy mediates oxidative weathering of granitic bedrock. Proc. Natl. Acad. Sci. 116, 26394–26401 (2019).
Dale, V. H., Swanson, F. J. & Crisafulli, C. M. Disturbance, survival, and succession: Understanding ecological responses to the 1980 eruption of Mount St. Helens in Ecological responses to the 1980 eruption of Mount St. Helens (eds. Dale, V. H., Swanson, F. J. & Crisafulli, C. M.) 3–11 (Springer New York, 2005).
Fischer, D. G., Antos, J. A., Grandy, W. G. & Zobel, D. B. A little disturbance goes a long way: 33-year understory successional responses to a thin tephra deposit. For. Ecol. Manag. 382, 236–243 (2016).
Fodor, E. & Németh, K. Spatter cone in Encyclopedia of planetary landforms (eds. Hargitai, H. & Kereszturi, Á.) 2028–2034 (Springer New York, 2015).
Reynolds, P., Brown, R. J., Thordarson, T. & Llewellin, E. W. The architecture and shallow conduits of Laki-type pyroclastic cones: insights into a basaltic fissure eruption. Bull. Volcanol. 78, 36 (2016).
Herzberg, C. & Asimow, P. D. PRIMELT3 MEGA.XLSM software for primary magma calculation: Peridotite primary magma MgO contents from the liquidus to the solidus. Geochem. Geophys. Geosystems 16, 563–578 (2015).
Waterton, P. et al. Age, origin, and thermal evolution of the ultra-fresh ~1.9Ga Winnipegosis Komatiites, Manitoba, Canada. Lithos 268–271, 114–130 (2017).
Green, D. H., Nicholls, I. A., Viljoen, M. & Viljoen, R. Experimental demonstration of the existence of peridotitic liquids in earliest Archean magmatism. Geology 3, 11–14 (1975).
Stern, C. R., Huang, W. L. & Wyllie, P. J. Basalt-andesite-rhyolite-H2O: Crystallization intervals with excess H2O and H2O-undersaturated liquidus surfaces to 35 kolbras, with implications for magma genesis. Earth Planet. Sci. Lett. 28, 189–196 (1975).
Liu, L. & Lowell, R. P. Modeling heat transfer from a convecting, crystallizing, replenished silicic magma chamber at an oceanic spreading center. Geochem. Geophys. Geosystems 12, Q09010 (2011).
Spera, F. J. & Bohrson, W. A. Rejuvenation of crustal magma mush: A tale of multiply nested processes and timescales. Am. J. Sci. 318, 90–140 (2018).
Berga, M., Székely, A. J. & Langenheder, S. Effects of disturbance intensity and frequency on bacterial community composition and function. PLOS ONE 7, e36959 (2012).
Lemoine, N. P. et al. Strong dispersal limitation of microbial communities at Shackleton Glacier, Antarctica. mSystems 8, e01254–22 (2023).
Jackson, C. R., Churchill, P. F. & Roden, E. E. Successional changes in bacterial assemblage structure during epilithic biofilm development. Ecology 82, 555–566 (2001).
Acknowledgements
Funding support was provided by DoD National Defense Science and Engineering Graduate (NDSEG) Fellowship Program and NASA Planetary Science Through Analog Research (PSTAR) Grant # 80NSSC21K0011.
Author information
Authors and Affiliations
Contributions
N.H. led the writing of the manuscript and generated figures; C.W.H. helped write, edit, and generated figures; and S.D. contributed to writing and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks Ricardo Amils, Rebecca L. Mickol and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Erika Buscardo and Joe Aslin. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hadland, N., Hamilton, C.W. & Duhamel, S. Young volcanic terrains are windows into early microbial colonization. Commun Earth Environ 5, 114 (2024). https://doi.org/10.1038/s43247-024-01280-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43247-024-01280-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.