Abstract
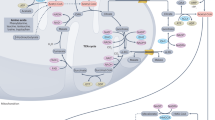
Acetate, a precursor of acetyl-CoA, is instrumental in energy production, lipid synthesis and protein acetylation. However, whether acetate reprogrammes tumour metabolism and plays a role in tumour immune evasion remains unclear. Here, we show that acetate is the most abundant short-chain fatty acid in human non-small cell lung cancer tissues, with increased tumour-enriched acetate uptake. Acetate-derived acetyl-CoA induces c-Myc acetylation, which is mediated by the moonlighting function of the metabolic enzyme dihydrolipoamide S-acetyltransferase. Acetylated c-Myc increases its stability and subsequent transcription of the genes encoding programmed death-ligand 1, glycolytic enzymes, monocarboxylate transporter 1 and cell cycle accelerators. Dietary acetate supplementation promotes tumour growth and inhibits CD8+ T cell infiltration, whereas disruption of acetate uptake inhibits immune evasion, which increases the efficacy of anti-PD-1-based therapy. These findings highlight a critical role of acetate promoting tumour growth beyond its metabolic role as a carbon source by reprogramming tumour metabolism and immune evasion, and underscore the potential of controlling acetate metabolism to curb tumour growth and improve the response to immune checkpoint blockade therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All other data that support the findings of this study are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Schug, Z. T., Vande Voorde, J. & Gottlieb, E. The metabolic fate of acetate in cancer. Nat. Rev. Cancer 16, 708–717 (2016).
Bose, S., Ramesh, V. & Locasale, J. W. Acetate metabolism in physiology, cancer and beyond. Trends Cell Biol. 29, 695–703 (2019).
Nuutinen, H., Lindros, K., Hekali, P. & Salaspuro, M. Elevated blood acetate as indicator of fast ethanol elimination in chronic alcoholics. Alcohol 2, 623–626 (1985).
Mashimo, T. et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell 159, 1603–1614 (2014).
Comerford, S. A. et al. Acetate dependence of tumors. Cell 159, 1591–1602 (2014).
Li, X. et al. Nucleus-translocated ACSS2 promotes gene transcription for lysosomal biogenesis and autophagy. Mol. Cell 66, 684–697 (2017).
Li, X., Qian, X. & Lu, Z. Local histone acetylation by ACSS2 promotes gene transcription for lysosomal biogenesis and autophagy. Autophagy 13, 1790–1791 (2017).
Schug, Z. T. et al. Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell 27, 57–71 (2015).
Gao, X. et al. Acetate functions as an epigenetic metabolite to promote lipid synthesis under hypoxia. Nat. Commun. 7, 11960 (2016).
Zhao, S. et al. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature 579, 586–591 (2020).
Jeon, J. Y. et al. Regulation of acetate utilization by monocarboxylate transporter 1 (MCT1) in hepatocellular carcinoma (HCC). Oncol. Res. 26, 71–81 (2018).
Wang, Y. P. & Lei, Q. Y. Metabolic recoding of epigenetics in cancer. Cancer Commun. 38, 25 (2018).
Smolle, E. et al. Distribution and prognostic significance of gluconeogenesis and glycolysis in lung cancer. Mol. Oncol. 14, 2853–2867 (2020).
Masin, M. et al. GLUT3 is induced during epithelial-mesenchymal transition and promotes tumor cell proliferation in non-small cell lung cancer. Cancer Metab. 2, 11 (2014).
Younes, M., Brown, R. W., Stephenson, M., Gondo, M. & Cagle, P. T. Overexpression of Glut1 and Glut3 in stage I non-small cell lung carcinoma is associated with poor survival. Cancer 80, 1046–1051 (1997).
Li, X., Egervari, G., Wang, Y., Berger, S. L. & Lu, Z. Regulation of chromatin and gene expression by metabolic enzymes and metabolites. Nat. Rev. Mol. Cell Biol. 19, 563–578 (2018).
Shan, C. et al. Lysine acetylation activates 6-phosphogluconate dehydrogenase to promote tumor growth. Mol. Cell 55, 552–565 (2014).
Vervoorts, J. et al. Stimulation of c-MYC transcriptional activity and acetylation by recruitment of the cofactor CBP. EMBO Rep. 4, 484–490 (2003).
Bonen, A. The expression of lactate transporters (MCT1 and MCT4) in heart and muscle. Eur. J. Appl. Physiol. 86, 6–11 (2001).
Dang, C. V., Kim, J. W., Gao, P. & Yustein, J. The interplay between MYC and HIF in cancer. Nat. Rev. Cancer 8, 51–56 (2008).
Koppenol, W. H., Bounds, P. L. & Dang, C. V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 11, 325–337 (2011).
Casey, S. C. et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science 352, 227–231 (2016).
Kress, T. R., Sabo, A. & Amati, B. MYC: connecting selective transcriptional control to global RNA production. Nat. Rev. Cancer 15, 593–607 (2015).
Gan, L. et al. Metabolic targeting of oncogene MYC by selective activation of the proton-coupled monocarboxylate family of transporters. Oncogene 35, 3037–3048 (2016).
Kitajima, S. et al. Suppression of STING associated with LKB1 loss in KRAS-driven lung cancer. Cancer Discov. 9, 34–45 (2019).
Richer, A. L. et al. WEE1 kinase inhibitor AZD1775 has preclinical efficacy in LKB1-deficient non-small cell lung cancer. Cancer Res. 77, 4663–4672 (2017).
Miller, K. D. et al. Acetate acts as a metabolic immunomodulator by bolstering T-cell effector function and potentiating antitumor immunity in breast cancer. Nat. Cancer 4, 1491–1507 (2023).
Wu, F. et al. Single-cell profiling of tumor heterogeneity and the microenvironment in advanced non-small cell lung cancer. Nat. Commun. 12, 2540 (2021).
Chan, J. M. et al. Signatures of plasticity, metastasis and immunosuppression in an atlas of human small cell lung cancer. Cancer Cell 39, 1479–1496 (2021).
Johnson, A. M. et al. Cancer cell-intrinsic expression of MHC class II regulates the immune microenvironment and response to anti-PD-1 therapy in lung adenocarcinoma. J. Immunol. 204, 2295–2307 (2020).
Feng, Y. et al. IL-9 stimulates an anti-tumor immune response and facilitates immune checkpoint blockade in the CMT167 mouse model. Lung Cancer 174, 14–26 (2022).
Liu, J. et al. Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell 147, 223–234 (2011).
Geman, D., Ochs, M., Price, N. D., Tomasetti, C. & Younes, L. An argument for mechanism-based statistical inference in cancer. Hum. Genet. 134, 479–495 (2015).
Scafoglio, C. R. et al. Sodium-glucose transporter 2 is a diagnostic and therapeutic target for early-stage lung adenocarcinoma. Sci. Transl. Med. 10, eaat5933 (2018).
Ye, L. et al. Repressed Blautia-acetate immunological axis underlies breast cancer progression promoted by chronic stress. Nat. Commun. 14, 6160 (2023).
Qiu, J. et al. Acetate promotes T cell effector function during glucose restriction. Cell Rep. 27, 2063–2074 (2019).
Muto, T. et al. TRAF6 functions as a tumor suppressor in myeloid malignancies by directly targeting MYC oncogenic activity. Cell Stem Cell 29, 298–314 (2022).
Tang, H. Y., Goldman, A. R., Zhang, X., Speicher, D. W. & Dang, C. V. Measuring MYC-mediated metabolism in tumorigenesis. Methods Mol. Biol. 2318, 231–239 (2021).
Yang, W. et al. ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat. Cell Biol. 14, 1295–1304 (2012).
Wang, J. et al. A non-metabolic function of hexokinase 2 in small cell lung cancer: promotes cancer cell stemness by increasing USP11-mediated CD133 stability. Cancer Commun. 42, 1008–1027 (2022).
Wang, W. et al. METTL3 promotes tumour development by decreasing APC expression mediated by APC mRNA N6-methyladenosine-dependent YTHDF binding. Nat. Commun. 12, 3803 (2021).
Tang, G. H. et al. A simple and rapid automated radiosynthesis of [18F]fluoroacetate. J. Labelled Comp. Radiopharm. 51, 297–301 (2008).
Wagner, J. et al. A single-cell atlas of the tumor and immune ecosystem of human breast cancer. Cell 177, 1330–1345 (2019).
Xu, D. et al. The gluconeogenic enzyme PCK1 phosphorylates INSIG1/2 for lipogenesis. Nature 580, 530–535 (2020).
Shao, F. et al. Silencing EGFR-upregulated expression of CD55 and CD59 activates the complement system and sensitizes lung cancer to checkpoint blockade. Nat. Cancer 3, 1192–1210 (2022).
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (82188102, to Z.L. and J.H.; 82030074, to Z.L.; 82303100, to J.W.; 32100574 and 82372812, to F.S.), the Ministry of Science and Technology of the People’s Republic of China (2020YFA0803300, to Z.L.), the National Key R&D Program of China (2020AAA0109500, to J.H.; 2023YFC3503200, to F.S.), the Zhejiang Natural Science Foundation-Key Project (LD21H160003, to Z.L.), the Beijing Municipal Science & Technology Commission (Z191100006619115, to J.H.), R&D Program of Beijing Municipal Education Commission (KJZD20191002302, to J.H.), Aiyou Foundation (KY201701, to J.H.), the CAMS Initiative for Innovative Medicine (2021-I2M-1-012, 2021-I2M-1-015, to J.H.), the China Postdoctoral Science Foundation (2023M730327, to J.W.) and the Natural Science Foundation of Shandong Province (ZR2020QH191, to F.S.). Z.L. is the Kuancheng Wang Distinguished Chair. We thank R. Gao and A. Yang (Department of Nuclear Medicine, The First Affiliated Hospital of Xi’an Jiaotong University) for technical assistance of 18F-acetate PET-CT imaging. The MS of isotope tracing was supported by the Metabolomics Facility Center of Metabolomics and Lipidomics at the National Protein Science Technology Center of Tsinghua University.
Author information
Authors and Affiliations
Contributions
Z.L. conceived the concept of the study. Z.L. and J.W. designed the study. J.H. provided critical scientific input. J.W., Y.Y., F.S., D.G. and Y.M. performed the experiments. J.H. established the human cohort and biobanking of specimens for the tissue microarray. Z.L. and J.W. wrote the manuscript with input from all the authors.
Corresponding authors
Ethics declarations
Competing interests
Z.L. owns shares in Signalway Biotechnology, which supplied the rabbit antibodies that recognize c-Myc Lys148 acetylation. Z.L.’s interest in this company had no bearing on its selection as the supplier of these reagents. The other authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Dean Felsher, Zihai Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alfredo Giménez-Cassina, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
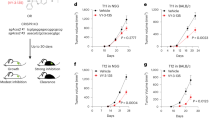
Extended Data Fig. 1 Dietary acetate counteracts energy stress and promotes tumor growth in an MCT1-dependent manner.
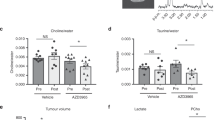
a, LLC cells expressing luciferase were injected orthotopically into the left lung of C56BL/6 mice. The mice were injected intraperitoneally with [U-13C2]-acetate (n = 5) or 3D-acetate (n = 7), and acetate were extracted and measured. b, c, A549 and H1299 cells were cultured in low-glucose medium and were then treated with or without [U-13C2]-acetate or unlabeled acetate. Intracellular acetate (b) and acetyl-CoA (c) were extracted and measured. d, e, A549 and H1299 cells expressing different shRNAs targeting MCT1, MCT2, MCT3, MCT4, SMCT1, or SMCT2 were generated. Immunoblot analyses were performed with the indicated antibodies (d). Cells were cultured in low-glucose medium and were then treated with acetate. Intracellular acetate was extracted and quantified (e). f, A549 cells with or without MCT1 depletion were incubated with [U-13C2]-acetate in low-glucose medium. Fractional abundance of labeled carbons in saponified fatty acids was measured. g, Kaplan–Meier plots of the overall survival rates of patients with human lung adenocarcinoma (LUAD) in the TCGA database with high (n = 131) or low (n = 369) MCT1 expression, as determined by the optimal cutoff. Two-tailed log-rank test. h, LLC cells with or without MCT1 knockout were orthotopically injected into the left lungs of mice (n = 4). Mice were injected with [U-13C2]-acetate or 3D-acetate, and acetate were extracted and measured. i, A549 expressing shRNAs targeting both GLUT1 and GLUT3 were generated. Immunoblot analyses were performed with the indicated antibodies. j, Mice (n = 6) were fasted for 8 h and supplemented with or without acetate in the drinking water. Serum acetate were extracted and measured. k-n, Immunodeficient mice were subcutaneously inoculated with A549 cells with or without MCT1 shRNA expression. Mice were intraperitoneally injected with or without 2-DG and provided with drinking water with or without acetate supplementation. k, Tumor sizes were measured (left), and tumor volumes (right) were calculated. l-n, Acetyl-CoA levels (l), triglyceride levels (m), and cholesterol levels (n) in the indicated tumor tissues were quantified and normalized to the weight of tumor tissues. In (b, c, d, e, f, i, l-n), n = 3. The data are presented as the means ± s.d.; unpaired, two-tailed t test (a-c, f, h, j) or one-way ANOVA (e, k-n). ns, not significant.
Extended Data Fig. 2 Acetate treatment increases c-Myc stability.
a, A549 cells were treated with acetate for 1 h in low-glucose medium, and cell lysates were subjected to immunoprecipitation with an anti-acetylated lysine antibody. Proteins in the immunoprecipitate were separated by SDS-PAGE and stained with Coomassie brilliant blue. The area of the gel near 55 kDa was excised and analyzed by mass spectrometry. b, A549 and H1299 cells with or without acetate treatment in low-glucose medium were incubated with CHX for the indicated durations. c, RNA was extracted from A549 and H1299 cells with or without acetate treatment in low-glucose medium for the indicated durations. RT-qPCR analyses were performed. The data are presented as the means ± s.d. d, e, A549 and H1299 cells with or without depletion of MCT1 (d) or ACSS2 (e) in low-glucose medium were treated with CHX for the indicated periods of time in the presence of acetate. f, A549 and H1299 cells with or without ACSS1 depletion were cultured in low-glucose medium in the presence or absence of acetate. Immunoblot analyses were performed with the indicated antibodies (left, b, d, e, f). The quantification of c-Myc protein levels relative to β-actin levels is shown (right, b, d, e). The representative results are from three independent experiments (b, d, e, f), and the data are presented as the means ± s.d. (b, d, e).
Extended Data Fig. 3 DLAT acetylates c-Myc at K148 in cytosol and maintains high levels of c-Myc.
a, A549 cells were treated with acetate in low-glucose medium, and cell lysates were subjected to immunoprecipitation with an anti-c-Myc antibody. The immunoprecipitated proteins were analyzed by mass spectrometry, and peptide hits associated with c-Myc are shown. b, A549 cells were separated into the cytosolic (C), mitochondrial (M), and nuclear (N) fractions. c, A549 and H1299 cells with or without DLAT depletion were cultured in low-glucose medium in the presence of acetate. Whole cell lysates and cytosolic fractions were prepared, and cytosolic acetyl-CoA was measured (bottom). d, A549 and H1299 cells with or without DLAT depletion in low-glucose medium were treated with CHX for the indicated periods of time in the presence of acetate. e, Purified GST-c-Myc was incubated with purified His-DLAT in the presence of acetyl-CoA. Mass spectrometry analysis was performed, and the results suggested that K148 was acetylated. f, Alignment of protein sequences spanning c-Myc K148 in different species. The conserved lysine residues are in red. g, IHC staining of human lung adenocarcinoma tissues (top) or anti-c-Myc immunoprecipitates from A549 cells with or without acetate (bottom) were analyzed with an anti-c-Myc K148ac antibody in the presence or absence of a blocking peptide for acetylated c-Myc. h, A549 cells with or without p300 depletion were lysed to extract cytosolic (C) and nuclear (N) fractions. i, Cytosolic and nuclear fractions of A549 cells expressing the indicated Flag-c-Myc (∆NLS) protein, which contains the NLS deletion, in the presence or absence of DLAT depletion were prepared. j, A549 and H1299 cells with or without lactate treatment were lysed. k, Sequencing of two individual clones of parental A549 cells with knockin of c-Myc K148R or c-Myc K148Q. The blue arrows indicate mutated nucleotides. The mutated amino acid is indicated by the solid red box. l, A549 cells with or without knockin of c-Myc K148R or c-Myc K148Q mutants were treated with CHX for the indicated durations. C2, clone 2. Immunoprecipitation (b, h-j) and immunoblot (b, c, d, g, h-j, l) analyses were performed with indicated antibodies, and representative results are from three independent experiments. The data are presented as the means ± s.d. (c, d, l). ac indicates an acetylated residue. WCL, whole cell lysate.
Extended Data Fig. 4 c-Myc K148 acetylation promotes the binding of USP10 for deubiquitylation and stabilization of c-Myc.
a, A549 cells were treated with acetate in the condition of glucose starvation. Immunoprecipitation analyses were performed with an anti-c-Myc antibody. The immunoprecipitated proteins were analyzed by mass spectrometry, and peptide hits associated with c-Myc are shown. b, A549 and H1299 cells expressing control shRNA or two different shRNAs targeting USP10 in low-glucose medium were treated with or without MG-132 for 8 h in the presence of acetate. c, H1299 cells with or without expression of two different USP10 shRNAs in low-glucose medium were treated with MG-132 in the presence or absence of acetate. d, H1299 cells with or without USP10 shRNA expression in low-glucose medium were treated with CHX for the indicated periods of time in the presence of acetate. e, A549 and H1299 cells with or without reconstituted expression WT Flag-USP10 or Flag-USP10 C428A in low-glucose medium were treated with CHX for the indicated durations in the presence of acetate. f, A549 and H1299 cells with or without USP10 depletion and reconstituted expression of shRNA-resistant Flag-rUSP10 WT or Flag-rUSP10 C428A were cultured in the presence or absence of acetate in low-glucose medium. Immunoprecipitation (c, f) and immunoblot (b-f) analyses were performed with the indicated antibodies, and representative results are from three independent experiments. The data are presented as the means ± s.d. (d, e). WCL, whole cell lysate. c-Myc-(Ub)n indicates polyubiquitinated c-Myc.
Extended Data Fig. 5 Acetate supplementation promotes glycolysis and proliferation in NSCLC cells and PD-L1-dependent inhibition of CD8+ T-cell activation.
a-c, H1299 cells with or without depletion of MCT1, ACSS2, DLAT, or USP10 were treated with or without acetate in low-glucose medium. Proteins expression was evaluated by immunoblotting with the indicated antibodies (a). The lactate production levels (b) and colony formation ability (c) were analyzed. d, e, A549 cells with or without knockin of c-Myc K148R or c-Myc K148Q were cultured with or without acetate in low-glucose medium. The lactate production levels (d) and colony formation ability (e) were analyzed. f, H358 and H2009 (KP) cells, A549 and H2122 (KL) cells, and H2030 and H358-LKB1-knockout (KPL) cells with or without acetate treatment were lysed for immunoprecipitation and immunoblot analyses with the indicated antibodies. g, A549 cells with or without knockin of c-Myc K148R or c-Myc K148Q were cultured with acetate in low-glucose medium. Intracellular acetate was extracted and quantified. h, c-Myc-depleted LLC cells with reconstituted expression of WT c-Myc, c-Myc K148R, or c-Myc K148Q were generated. Immunoblot analyses were performed with the indicated antibodies. i, PD-L1 shRNA was expressed in c-Myc-depleted LLC cells with reconstituted expression of c-Myc K148Q. Immunoblot analyses were performed with the indicated antibodies. Representative results are from three independent experiments (a, f, h, i). n = 3; mean ± s.d.; one-way ANOVA (b-e, g).
Extended Data Fig. 6 Acetate-induced c-Myc K148 acetylation in tumor cells contributes to the immunosuppressive tumor microenvironment.
a-d, Reduced-dimensionality (UMAP) visualization of SLC16A1 (encoding MCT1) expression (a, c) in clusters representing major cell types (b, d) in NSCLC or SCLC single-cell RNA sequencing (scRNA-seq) data. UMAP, Uniform Manifold Approximation and Projection. e, f, The single-cell transcriptome data analyses of SLC16A1 and MKI67 (encoding ki-67) mRNA expression in the epithelium-cancer cells from NSCLC tumors of 42 patients (e) or SCLC tumors of 31 patients (f). g, CMT167 cells were orthotopically injected into C57BL/6 mice (n = 3) and treated with or without acetate supplementation in drinking water for 20 days. The soluble factors were detected in the tumor tissue lysate by a semiquantitative cytokine array. Significant changes are summarized in a heat map. h, CMT167 c-Myc-depleted cells with or without reconstituted expression of WT c-Myc or c-Myc K148R were performed immunoblot analyses with the indicated antibodies. Representative results are from three independent experiments. i, CMT167 c-Myc-depleted cells with reconstituted expression of WT c-Myc or c-Myc K148R were orthotopically injected into the left lung of C57BL/6 mice (n = 5). Two weeks after cells inoculation, mice were intraperitoneally injected with 2-DG with or without acetate supplementation in the drinking water for one month. The intratumoral infiltration of CD4+ Th1 cells, CD4+ Th2 cells, M1 macrophages, M2 macrophages and MDSCs were quantified using flow cytometry. The data are presented as the means ± s.d.; one-way ANOVA. ns, not significant.
Extended Data Fig. 7 Acetate supplementation of NSCLC cells promotes lung tumor growth and tumor immune evasion.
a, c-Myc-depleted CMT167 cells with reconstituted expression of WT c-Myc and with or with depletion of MCT1, ACSS2, DLAT, or USP10 were treated with or without acetate in low-glucose medium. Proteins expression was evaluated by immunoblot analyses with the indicated antibodies. b, LLC c-Myc-depleted cells with reconstituted expression of WT c-Myc and with or with depletion of ACSS2, DLAT, or USP10 were performed immunoblot analyses with the indicated antibodies. c-j, LLC c-Myc-depleted cells with reconstituted expression of WT c-Myc, c-Myc K148R, or c-Myc K148Q and with or without depletion of ACSS2, DLAT, or USP10 were subcutaneously injected into the flank regions of C57BL/6 mice (n = 6). Mice were intraperitoneally injected with 2-DG with or without acetate supplementation in the drinking water. c, Representative images of tumors are shown (top). Tumor sizes were measured, and tumor volumes were calculated (bottom). d-i, IHC staining of mouse tumor tissues was performed with the indicated antibodies (d, f, h), and IHC scores were calculated (e, g, i). Representative images of staining are shown (d, f, h). Scale bars: 100 μm. j, Tumor-bearing mice (n = 3) were injected intraperitoneally with [U-13C2]-acetate. The tumors were harvested, and acetate in the tumor tissues was extracted and measured. k-o, LLC cells were subcutaneously injected into C57BL/6 mice. The mice were divided into five groups (n = 5 each group) and treated with 2-DG and treated with the indicated monotherapies and combinations of spautin-1 (20 mg/kg), an anti-PD-1 antibody (10 mg/kg), or rat IgG (10 mg/kg). Acetate was supplemented in the drinking water. k, Representative images of tumors are shown (left). Tumor sizes were measured, and tumor volumes were calculated (right). l-o, IHC staining of tumor tissues was performed with the indicated antibodies. Representative images of staining are shown (left), and IHC scores were calculated (right). The proportion of CD8+ T and GzmB+ cells were assessed (o). Scale bars: 100 μm. Representative results are from three independent experiments (a, b). In (c, e, g, i-m, o), mean ± s.d.; one-way ANOVA. ac indicates an acetylated residue.
Extended Data Fig. 8 c-Myc K148 acetylation-mediated enhancement c-Myc expression promotes tumor immune evasion and clinical progression in NSCLC.
a, b, The correlations between the mRNA levels of SLC16A1 (a) or MYC (b) and that of CD274 in the LUAD samples in the TCGA data set were analyzed by Spearman correlation analysis. c-f, The correlations between the mRNA levels of SLC16A1 (c, d) or MYC (e, f) and CD8+ T-cell infiltration in the LUAD samples in the GEO data set were analyzed by the Spearman correlation analysis. g, Human NSCLC specimens (n = 90) were analyzed by IHC with the indicated antibodies. Representative images are shown. Scale bars: 100 µm. h, IHC analyses of tumor samples from Fig. 1n were performed with the indicated antibodies. Representative images are shown (top), and the IHC scores were calculated (bottom). The data are presented as the means ± s.d.; n = 5; one-way ANOVA.
Supplementary information
Supplementary Information
Supplementary Methods and Figs. 1 and 2.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 6
Unprocessed western blots.
Source Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 8
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, J., Yang, Y., Shao, F. et al. Acetate reprogrammes tumour metabolism and promotes PD-L1 expression and immune evasion by upregulating c-Myc. Nat Metab (2024). https://doi.org/10.1038/s42255-024-01037-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42255-024-01037-4