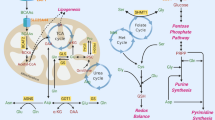
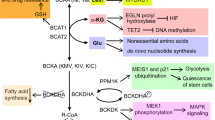
Abstract
Few metabolites can claim a more central and versatile role in cell metabolism than acetyl coenzyme A (acetyl-CoA). Acetyl-CoA is produced during nutrient catabolism to fuel the tricarboxylic acid cycle and is the essential building block for fatty acid and isoprenoid biosynthesis. It also functions as a signalling metabolite as the substrate for lysine acetylation reactions, enabling the modulation of protein functions in response to acetyl-CoA availability. Recent years have seen exciting advances in our understanding of acetyl-CoA metabolism in normal physiology and in cancer, buoyed by new mouse models, in vivo stable-isotope tracing approaches and improved methods for measuring acetyl-CoA, including in specific subcellular compartments. Efforts to target acetyl-CoA metabolic enzymes are also advancing, with one therapeutic agent targeting acetyl-CoA synthesis receiving approval from the US Food and Drug Administration. In this Review, we give an overview of the regulation and cancer relevance of major metabolic pathways in which acetyl-CoA participates. We further discuss recent advances in understanding acetyl-CoA metabolism in normal tissues and tumours and the potential for targeting these pathways therapeutically. We conclude with a commentary on emerging nodes of acetyl-CoA metabolism that may impact cancer biology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Walsh, C. T., Tu, B. P. & Tang, Y. Eight kinetically stable but thermodynamically activated molecules that power cell metabolism. Chem. Rev. 118, 1460–1494 (2018).
Sugden, M. C. & Holness, M. J. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am. J. Physiol. Endocrinol. Metab. 284, E855–E862 (2003).
Adina-Zada, A. et al. Allosteric regulation of the biotin-dependent enzyme pyruvate carboxylase by acetyl-CoA. Biochem. Soc. Trans. 40, 567–572 (2012).
Martin, W. F. Older than genes: the acetyl CoA pathway and origins. Front. Microbiol. 11, 817 (2020).
Currie, E., Schulze, A., Zechner, R., Walther, T. C. & Farese, R. V. Jr Cellular fatty acid metabolism and cancer. Cell Metab. 18, 153–161 (2013).
Batchuluun, B., Pinkosky, S. L. & Steinberg, G. R. Lipogenesis inhibitors: therapeutic opportunities and challenges. Nat. Rev. Drug Discov. 21, 283–305 (2022).
Longo, J., van Leeuwen, J. E., Elbaz, M., Branchard, E. & Penn, L. Z. Statins as anticancer agents in the era of precision medicine. Clin. Cancer Res. 26, 5791–5800 (2020).
Vasan, K., Werner, M. & Chandel, N. S. Mitochondrial metabolism as a target for cancer therapy. Cell Metab. 32, 341–352 (2020).
Stacpoole, P. W. Therapeutic targeting of the pyruvate dehydrogenase complex/pyruvate dehydrogenase kinase (PDC/PDK) axis in cancer. J. Natl Cancer Inst. https://doi.org/10.1093/jnci/djx071 (2017).
Jonas, M. C., Pehar, M. & Puglielli, L. AT-1 is the ER membrane acetyl-CoA transporter and is essential for cell viability. J. Cell Sci. 123, 3378–3388 (2010).
Sivanand, S., Viney, I. & Wellen, K. E. Spatiotemporal control of acetyl-CoA metabolism in chromatin regulation. Trends Biochem. Sci. 43, 61–74 (2018).
Rohrig, F. & Schulze, A. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer 16, 732–749 (2016).
Campbell, S. L. & Wellen, K. E. Metabolic signaling to the nucleus in cancer. Mol. Cell 71, 398–408 (2018).
Srere, P. A. & Bhaduri, A. Incorporation of radioactive citrate into fatty acids. Biochim. Biophys. Acta 59, 487–489 (1962).
Bhaduri, A. & Srere, P. A. The incorporation of citrate carbon into fatty acids. Biochim. Biophys. Acta 70, 221–230 (1963).
Metallo, C. M. et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 481, 380–384 (2011).
Mullen, A. R. et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 481, 385–388 (2011).
Wise, D. R. et al. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc. Natl Acad. Sci. USA 108, 19611–19616 (2011).
Kumar, A. et al. NaCT/SLC13A5 facilitates citrate import and metabolism under nutrient-limited conditions. Cell Rep. 36, 109701 (2021).
Liu, X. et al. Acetate production from glucose and coupling to mitochondrial metabolism in mammals. Cell 175, 502–513.e13 (2018).
Vysochan, A., Sengupta, A., Weljie, A. M., Alwine, J. C. & Yu, Y. ACSS2-mediated acetyl-CoA synthesis from acetate is necessary for human cytomegalovirus infection. Proc. Natl Acad. Sci. USA 114, E1528–E1535 (2017).
Trefely, S., Lovell, C. D., Snyder, N. W. & Wellen, K. E. Compartmentalised acyl-CoA metabolism and roles in chromatin regulation. Mol. Metab. 38, 100941 (2020).
Zhao, S. et al. ATP-citrate lyase controls a glucose-to-acetate metabolic switch. Cell Rep. 17, 1037–1052 (2016).
Zhao, S. et al. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature 579, 586–591 (2020).
Choudhary, C., Weinert, B. T., Nishida, Y., Verdin, E. & Mann, M. The growing landscape of lysine acetylation links metabolism and cell signalling. Nat. Rev. Mol. Cell Biol. 15, 536–550 (2014).
Wellen, K. E. et al. ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324, 1076–1080 (2009).
Lee, J. V. et al. Akt-dependent metabolic reprogramming regulates tumor cell histone acetylation. Cell Metab. 20, 306–319 (2014). This study demonstrates that AKT-dependent phosphorylation of ACLY at S455 promotes the maintenance of acetyl-CoA production and histone acetylation under glucose limitation and that phosphorylated AKT S473 correlates with histone acetylation levels in human tumours.
Takahashi, H., McCaffery, J. M., Irizarry, R. A. & Boeke, J. D. Nucleocytosolic acetyl-coenzyme a synthetase is required for histone acetylation and global transcription. Mol. Cell 23, 207–217 (2006).
Sivanand, S. et al. Nuclear acetyl-CoA production by ACLY promotes homologous recombination. Mol. Cell 67, 252–265.e6 (2017).
Mews, P. et al. Acetyl-CoA synthetase regulates histone acetylation and hippocampal memory. Nature 546, 381–386 (2017).
Bulusu, V. et al. Acetate recapturing by nuclear acetyl-CoA synthetase 2 prevents loss of histone acetylation during oxygen and serum limitation. Cell Rep. 18, 647–658 (2017).
Huang, Z. et al. ACSS2 promotes systemic fat storage and utilization through selective regulation of genes involved in lipid metabolism. Proc. Natl Acad. Sci. USA 115, E9499–E9506 (2018).
Li, X. et al. Nucleus-translocated ACSS2 promotes gene transcription for lysosomal biogenesis and autophagy. Mol. Cell 66, 684–697.e9 (2017).
Mendoza, M. et al. Enzymatic transfer of acetate on histones from lysine reservoir sites to lysine activating sites. Sci. Adv. 8, eabj5688 (2022).
Ye, C. & Tu, B. P. Sink into the epigenome: histones as repositories that influence cellular metabolism. Trends Endocrinol. Metab. 29, 626–637 (2018).
Sutendra, G. et al. A nuclear pyruvate dehydrogenase complex is important for the generation of acetyl-CoA and histone acetylation. Cell 158, 84–97 (2014).
Nagaraj, R. et al. Nuclear localization of mitochondrial TCA cycle enzymes as a critical step in mammalian zygotic genome activation. Cell 168, 210–223.e11 (2017).
Chen, J. et al. Compartmentalized activities of the pyruvate dehydrogenase complex sustain lipogenesis in prostate cancer. Nat. Genet. 50, 219–228 (2018).
Zervopoulos, S. D. et al. MFN2-driven mitochondria-to-nucleus tethering allows a non-canonical nuclear entry pathway of the mitochondrial pyruvate dehydrogenase complex. Mol. Cell 82, 1066–1077.e7 (2022).
Madiraju, P., Pande, S. V., Prentki, M. & Madiraju, S. R. Mitochondrial acetylcarnitine provides acetyl groups for nuclear histone acetylation. Epigenetics 4, 399–403 (2009).
Duvel, K. et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 39, 171–183 (2010).
Porstmann, T. et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 8, 224–236 (2008).
DeBose-Boyd, R. A. & Ye, J. SREBPs in lipid metabolism, insulin signaling, and beyond. Trends Biochem. Sci. 43, 358–368 (2018).
Gouw, A. M. et al. The MYC oncogene cooperates with sterol-regulated element-binding protein to regulate lipogenesis essential for neoplastic growth. Cell Metab. 30, 556–572.e5 (2019).
Schug, Z. T. et al. Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell 27, 57–71 (2015). This work demonstrates that ACSS2 is upregulated under lipid-deprived and oxygen-deprived conditions to promote acetate-dependent DNL and support tumour growth.
Kondo, A. et al. Extracellular acidic pH activates the sterol regulatory element-binding protein 2 to promote tumor progression. Cell Rep. 18, 2228–2242 (2017).
Li, Z. et al. Acetyl-CoA synthetase 2: a critical linkage in obesity-induced tumorigenesis in myeloma. Cell Metab. 33, 78–93.e7 (2021).
Ning, Z. et al. USP22 regulates lipidome accumulation by stabilizing PPARgamma in hepatocellular carcinoma. Nat. Commun. 13, 2187 (2022).
Odera, J. O. et al. NRF2/ACSS2 axis mediates the metabolic effect of alcohol drinking on esophageal squamous cell carcinoma. Biochem. J. 477, 3075–3089 (2020).
Comerford, S. A. et al. Acetate dependence of tumors. Cell 159, 1591–1602 (2014). This study finds that ACSS2 is crucial for acetate capturing for both lipid synthesis and histone acetylation and that mice lacking ACSS2 exhibited reduced liver tumour burden.
Mashimo, T. et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell 159, 1603–1614 (2014). This tracing study in human glioblastoma reveals acetate oxidation in tumours.
Zhang, S., He, J., Jia, Z., Yan, Z. & Yang, J. Acetyl-CoA synthetase 2 enhances tumorigenesis and is indicative of a poor prognosis for patients with renal cell carcinoma. Urol. Oncol. 36, 243.e9–243.e20 (2018).
Sun, L. et al. Decreased expression of acetyl-CoA synthase 2 promotes metastasis and predicts poor prognosis in hepatocellular carcinoma. Cancer Sci. 108, 1338–1346 (2017).
Bae, J. M. et al. Downregulation of acetyl-CoA synthetase 2 is a metabolic hallmark of tumor progression and aggressiveness in colorectal carcinoma. Mod. Pathol. 30, 267–277 (2017).
Chen, Y. et al. ACLY: A biomarker of recurrence in breast cancer. Pathol. Res. Pract. 216, 153076 (2020).
Xu, Y. et al. Identification and integrative analysis of ACLY and related gene panels associated with immune microenvironment reveal prognostic significance in hepatocellular carcinoma. Cancer Cell Int. 21, 409 (2021).
Wei, X. et al. Targeting ACLY attenuates tumor growth and acquired cisplatin resistance in ovarian cancer by inhibiting the PI3K-AKT pathway and activating the AMPK-ROS pathway. Front. Oncol. 11, 642229 (2021).
Wang, J. et al. Low expression of ACLY associates with favorable prognosis in acute myeloid leukemia. J. Transl. Med. 17, 149 (2019).
White, P. J. et al. The BCKDH kinase and phosphatase integrate BCAA and lipid metabolism via regulation of ATP-citrate lyase. Cell Metab. 27, 1281–1293.e7 (2018).
Berwick, D. C., Hers, I., Heesom, K. J., Moule, S. K. & Tavare, J. M. The identification of ATP-citrate lyase as a protein kinase B (Akt) substrate in primary adipocytes. J. Biol. Chem. 277, 33895–33900 (2002).
Guy, P. S., Cohen, P. & Hardie, D. G. Rat mammary gland ATP-citrate lyase is phosphorylated by cyclic AMP-dependent protein kinase. FEBS Lett. 109, 205–208 (1980).
Potapova, I. A., El-Maghrabi, M. R., Doronin, S. V. & Benjamin, W. B. Phosphorylation of recombinant human ATP:citrate lyase by cAMP-dependent protein kinase abolishes homotropic allosteric regulation of the enzyme by citrate and increases the enzyme activity. Allosteric activation of ATP:citrate lyase by phosphorylated sugars. Biochemistry 39, 1169–1179 (2000).
Guy, P. S., Cohen, P. & Hardie, D. G. Purification and physicochemical properties of ATP citrate (pro-3S) lyase from lactating rat mammary gland and studies of its reversible phosphorylation. Eur. J. Biochem. 114, 399–405 (1981).
Pentyala, S. N. & Benjamin, W. B. Effect of oxaloacetate and phosphorylation on ATP-citrate lyase activity. Biochemistry 34, 10961–10969 (1995).
Ranganathan, N. S., Srere, P. A. & Linn, T. C. Comparison of phospho- and dephospho-ATP citrate lyase. Arch. Biochem. Biophys. 204, 52–58 (1980).
Houston, B. & Nimmo, H. G. Effects of phosphorylation on the kinetic properties of rat liver ATP-citrate lyase. Biochim. Biophys. Acta 844, 233–239 (1985).
Carrer, A. et al. Acetyl-CoA metabolism supports multistep pancreatic tumorigenesis. Cancer Discov. 9, 416–435 (2019). This study shows that mice lacking ACLY in the pancreas display impaired acinar-to-ductal metaplasia and reduced pancreatic tumour formation in a genetic model of pancreatic cancer.
Martinez Calejman, C. et al. mTORC2-AKT signaling to ATP-citrate lyase drives brown adipogenesis and de novo lipogenesis. Nat. Commun. 11, 575 (2020).
Covarrubias, A. J. et al. Akt-mTORC1 signaling regulates Acly to integrate metabolic input to control of macrophage activation. Elife 5, e11612 (2016).
Senapati, P. et al. Hyperinsulinemia promotes aberrant histone acetylation in triple-negative breast cancer. Epigenetics Chromatin 12, 44 (2019).
Basappa, J. et al. ACLY is the novel signaling target of PIP2/PIP3 and Lyn in acute myeloid leukemia. Heliyon 6, e03910 (2020).
McCubrey, J. A. et al. GSK-3 as potential target for therapeutic intervention in cancer. Oncotarget 5, 2881–2911 (2014).
Hughes, K., Ramakrishna, S., Benjamin, W. B. & Woodgett, J. R. Identification of multifunctional ATP-citrate lyase kinase as the alpha-isoform of glycogen synthase kinase-3. Biochem. J. 288, 309–314 (1992).
Lin, R. et al. Acetylation stabilizes ATP-citrate lyase to promote lipid biosynthesis and tumor growth. Mol. Cell 51, 506–518 (2013).
Zhang, C. et al. Cullin3-KLHL25 ubiquitin ligase targets ACLY for degradation to inhibit lipid synthesis and tumor progression. Genes Dev. 30, 1956–1970 (2016).
Hallows, W. C., Lee, S. & Denu, J. M. Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc. Natl Acad. Sci. USA 103, 10230–10235 (2006).
Akella, N. M., Ciraku, L. & Reginato, M. J. Fueling the fire: emerging role of the hexosamine biosynthetic pathway in cancer. BMC Biol. 17, 52 (2019).
Ciraku, L. et al. O-GlcNAc transferase regulates glioblastoma acetate metabolism via regulation of CDK5-dependent ACSS2 phosphorylation. Oncogene 41, 2122–2136 (2022).
Hardie, D. G. & Pan, D. A. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem. Soc. Trans. 30, 1064–1070 (2002).
Pinkosky, S. L. et al. Long-chain fatty acyl-CoA esters regulate metabolism via allosteric control of AMPK beta1 isoforms. Nat. Metab. 2, 873–881 (2020).
Lally, J. S. V. et al. Inhibition of acetyl-CoA carboxylase by phosphorylation or the inhibitor ND-654 suppresses lipogenesis and hepatocellular carcinoma. Cell Metab. 29, 174–182.e5 (2019). This study uses mouse models in which ACC is constitutively active and an ACC inhibitor to reveal that ACC-dependent lipogenesis promotes HCC.
Fullerton, M. D. et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 19, 1649–1654 (2013).
German, N. J. et al. PHD3 loss in cancer enables metabolic reliance on fatty acid oxidation via deactivation of ACC2. Mol. Cell 63, 1006–1020 (2016).
Brownsey, R. W., Boone, A. N., Elliott, J. E., Kulpa, J. E. & Lee, W. M. Regulation of acetyl-CoA carboxylase. Biochem. Soc. Trans. 34, 223–227 (2006).
Longo, J. et al. The mevalonate pathway is an actionable vulnerability of t(4;14)-positive multiple myeloma. Leukemia 35, 796–808 (2021).
Moon, S. H. et al. p53 represses the mevalonate pathway to mediate tumor suppression. Cell 176, 564–580.e19 (2019).
Freed-Pastor, W. A. et al. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell 148, 244–258 (2012).
Trefely, S. et al. Subcellular metabolic pathway kinetics are revealed by correcting for artifactual post harvest metabolism. Mol. Metab. 30, 61–71 (2019).
Trefely, S. et al. Quantitative subcellular acyl-CoA analysis reveals distinct nuclear metabolism and isoleucine-dependent histone propionylation. Mol. Cell 82, 447–462.e6 (2022). This article reports the development of a rigorous method for the quantification of acyl-CoA esters in subcellular compartments, including mitochondria, the cytosol and the nucleus.
Storz, P. Acinar cell plasticity and development of pancreatic ductal adenocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 14, 296–304 (2017).
McGregor, G. H. et al. Targeting the metabolic response to statin-mediated oxidative stress produces a synergistic antitumor response. Cancer Res. 80, 175–188 (2020).
Longo, J. et al. An actionable sterol-regulated feedback loop modulates statin sensitivity in prostate cancer. Mol. Metab. 25, 119–130 (2019).
Pandyra, A. et al. Immediate utility of two approved agents to target both the metabolic mevalonate pathway and its restorative feedback loop. Cancer Res. 74, 4772–4782 (2014).
Jiang, W., Hu, J. W., He, X. R., Jin, W. L. & He, X. Y. Statins: a repurposed drug to fight cancer. J. Exp. Clin. Cancer Res. 40, 241 (2021).
Bauer, D. E., Hatzivassiliou, G., Zhao, F., Andreadis, C. & Thompson, C. B. ATP citrate lyase is an important component of cell growth and transformation. Oncogene 24, 6314–6322 (2005).
Hatzivassiliou, G. et al. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell 8, 311–321 (2005). This study demonstrates that targeting ACLY either using RNA interference or an inhibitor could suppress tumour growth.
Migita, T. et al. ATP citrate lyase: activation and therapeutic implications in non-small cell lung cancer. Cancer Res. 68, 8547–8554 (2008).
Miller, K. D. et al. Targeting ACSS2 with a transition-state mimetic inhibits triple-negative breast cancer growth. Cancer Res. 81, 1252–1264 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03808558 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02223247 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02980029 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03032484 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03179904 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05118776 (2022).
Stockwell, B. R. et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171, 273–285 (2017).
Lei, G., Zhuang, L. & Gan, B. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer 22, 381–396 (2022).
Bersuker, K. et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575, 688–692 (2019).
Mao, C. et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 593, 586–590 (2021).
Garcia-Bermudez, J. et al. Squalene accumulation in cholesterol auxotrophic lymphomas prevents oxidative cell death. Nature 567, 118–122 (2019).
Rysman, E. et al. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res. 70, 8117–8126 (2010).
Talebi, A. et al. Sustained SREBP-1-dependent lipogenesis as a key mediator of resistance to BRAF-targeted therapy. Nat. Commun. 9, 2500 (2018).
Jiang, X., Stockwell, B. R. & Conrad, M. Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 22, 266–282 (2021).
Liu, J. Y. & Wellen, K. E. Advances into understanding metabolites as signaling molecules in cancer progression. Curr. Opin. Cell Biol. 63, 144–153 (2020).
Izzo, L. T., Affronti, H. C. & Wellen, K. E. The bidirectional relationship between cancer epigenetics and metabolism. Annu. Rev. Cancer Biol. 5, 235–257 (2021).
Li, X., Egervari, G., Wang, Y., Berger, S. L. & Lu, Z. Regulation of chromatin and gene expression by metabolic enzymes and metabolites. Nat. Rev. Mol. Cell Biol. 19, 563–578 (2018).
Gao, X. et al. Acetate functions as an epigenetic metabolite to promote lipid synthesis under hypoxia. Nat. Commun. 7, 11960 (2016).
Torrini, C. et al. Lactate is an epigenetic metabolite that drives survival in model systems of glioblastoma. Mol. Cell 82, 3061–3076.e6 (2022).
Rios Garcia, M. et al. Acetyl-CoA carboxylase 1-dependent protein acetylation controls breast cancer metastasis and recurrence. Cell Metab. 26, 842–855.e5 (2017).
Lu, M. et al. ACOT12-dependent alteration of acetyl-CoA drives hepatocellular carcinoma metastasis by epigenetic induction of epithelial-mesenchymal transition. Cell Metab. 29, 886–900.e5 (2019).
Lee, J. V. et al. Acetyl-CoA promotes glioblastoma cell adhesion and migration through Ca2+-NFAT signaling. Genes Dev. 32, 497–511 (2018).
Geeraerts, X. et al. Macrophages are metabolically heterogeneous within the tumor microenvironment. Cell Rep. 37, 110171 (2021).
Baardman, J. et al. Macrophage ATP citrate lyase deficiency stabilizes atherosclerotic plaques. Nat. Commun. 11, 6296 (2020).
Noe, J. T. et al. Lactate supports a metabolic-epigenetic link in macrophage polarization. Sci. Adv. 7, eabi8602 (2021).
de Goede, K. E. et al. Myeloid-specific Acly deletion alters macrophage phenotype in vitro and in vivo without affecting tumor growth. Cancers 13, 3054 (2021).
Liu, M. et al. Metabolic rewiring of macrophages by CpG potentiates clearance of cancer cells and overcomes tumor-expressed CD47-mediated ‘don’t-eat-me’ signal. Nat. Immunol. 20, 265–275 (2019).
Chang, C. H. et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153, 1239–1251 (2013).
Peng, M. et al. Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Science 354, 481–484 (2016).
Chowdhury, S. et al. Intracellular acetyl CoA potentiates the therapeutic efficacy of antitumor CD8+ T cells. Cancer Res. 82, 2640–2655 (2022).
Reinfeld, B. I. et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature 593, 282–288 (2021).
Qiu, J. et al. Acetate promotes T cell effector function during glucose restriction. Cell Rep. 27, 2063–2074.e5 (2019).
Lee, J. et al. Regulator of fatty acid metabolism, acetyl coenzyme a carboxylase 1, controls T cell immunity. J. Immunol. 192, 3190–3199 (2014).
Mayers, J. R. & Vander Heiden, M. G. Nature and nurture: what determines tumor metabolic phenotypes? Cancer Res. 77, 3131–3134 (2017).
Shah, P. A., Patil, R. & Harrison, S. A. NAFLD-related hepatocellular carcinoma: the growing challenge. Hepatology https://doi.org/10.1002/hep.32542 (2022).
Broadfield, L. A. et al. Fat induces glucose metabolism in nontransformed liver cells and promotes liver tumorigenesis. Cancer Res. 81, 1988–2001 (2021).
Sangineto, M. et al. Lipid metabolism in development and progression of hepatocellular carcinoma. Cancers 12, 1419 (2020).
Calvisi, D. F. et al. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology 140, 1071–1083 (2011).
Lambert, J. E., Ramos-Roman, M. A., Browning, J. D. & Parks, E. J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 146, 726–735 (2014).
Park, G., Jung, S., Wellen, K. E. & Jang, C. The interaction between the gut microbiota and dietary carbohydrates in nonalcoholic fatty liver disease. Exp. Mol. Med. 53, 809–822 (2021).
Todoric, J. et al. Fructose stimulated de novo lipogenesis is promoted by inflammation. Nat. Metab. 2, 1034–1045 (2020).
Zhang, Z. et al. Serine catabolism generates liver NADPH and supports hepatic lipogenesis. Nat. Metab. 3, 1608–1620 (2021).
Yenilmez, B. et al. Paradoxical activation of transcription factor SREBP1c and de novo lipogenesis by hepatocyte-selective ATP-citrate lyase depletion in obese mice. J. Biol. Chem. 298, 102401 (2022).
Morrow, M. R. et al. Inhibition of ATP-citrate lyase improves NASH, liver fibrosis, and dyslipidemia. Cell Metab. 34, 919–936.e8 (2022).
Wang, Q. et al. Abrogation of hepatic ATP-citrate lyase protects against fatty liver and ameliorates hyperglycemia in leptin receptor-deficient mice. Hepatology 49, 1166–1175 (2009).
Beigneux, A. P. et al. ATP-citrate lyase deficiency in the mouse. J. Biol. Chem. 279, 9557–9564 (2004).
Abu-Elheiga, L. et al. Mutant mice lacking acetyl-CoA carboxylase 1 are embryonically lethal. Proc. Natl Acad. Sci. USA 102, 12011–12016 (2005).
Harada, N. et al. Hepatic de novo lipogenesis is present in liver-specific ACC1-deficient mice. Mol. Cell Biol. 27, 1881–1888 (2007).
Kim, C. W. et al. Acetyl CoA carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: a bedside to bench investigation. Cell Metab. 26, 576 (2017).
Savage, D. B. et al. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J. Clin. Invest. 116, 817–824 (2006).
Harriman, G. et al. Acetyl-CoA carboxylase inhibition by ND-630 reduces hepatic steatosis, improves insulin sensitivity, and modulates dyslipidemia in rats. Proc. Natl Acad. Sci. USA 113, E1796–E1805 (2016).
Chow, J. D. et al. Genetic inhibition of hepatic acetyl-CoA carboxylase activity increases liver fat and alters global protein acetylation. Mol. Metab. 3, 419–431 (2014).
Nelson, M. E. et al. Inhibition of hepatic lipogenesis enhances liver tumorigenesis by increasing antioxidant defence and promoting cell survival. Nat. Commun. 8, 14689 (2017).
Neinast, M., Murashige, D. & Arany, Z. Branched chain amino acids. Annu. Rev. Physiol. 81, 139–164 (2019).
Neinast, M. D. et al. Quantitative analysis of the whole-body metabolic fate of branched-chain amino acids. Cell Metab. 29, 417–429.e4 (2019).
Mayers, J. R. et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat. Med. 20, 1193–1198 (2014).
Li, J. T. et al. BCAT2-mediated BCAA catabolism is critical for development of pancreatic ductal adenocarcinoma. Nat. Cell Biol. 22, 167–174 (2020).
Sakers, A., De Siqueira, M. K., Seale, P. & Villanueva, C. J. Adipose-tissue plasticity in health and disease. Cell 185, 419–446 (2022).
Lengyel, E., Makowski, L., DiGiovanni, J. & Kolonin, M. G. Cancer as a matter of fat: the crosstalk between adipose tissue and tumors. Trends Cancer 4, 374–384 (2018).
Zhu, Q. et al. Adipocyte mesenchymal transition contributes to mammary tumor progression. Cell Rep. 40, 111362 (2022).
Seki, T. et al. Brown-fat-mediated tumour suppression by cold-altered global metabolism. Nature 608, 421–428 (2022).
Fernandez, S. et al. Adipocyte ACLY facilitates dietary carbohydrate handling to maintain metabolic homeostasis in females. Cell Rep. 27, 2772–2784.e6 (2019).
Smith, U. & Kahn, B. B. Adipose tissue regulates insulin sensitivity: role of adipogenesis, de novo lipogenesis and novel lipids. J. Intern. Med. 280, 465–475 (2016).
Takehara, M. et al. Cancer-associated adipocytes promote pancreatic cancer progression through SAA1 expression. Cancer Sci. 111, 2883–2894 (2020).
Bochet, L. et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 73, 5657–5668 (2013).
Watson, J. A., Fang, M. & Lowenstein, J. M. Tricarballylate and hydroxycitrate: substrate and inhibitor of ATP: citrate oxaloacetate lyase. Arch. Biochem. Biophys. 135, 209–217 (1969).
Onakpoya, I., Hung, S. K., Perry, R., Wider, B. & Ernst, E. The use of garcinia extract (hydroxycitric acid) as a weight loss supplement: a systematic review and meta-analysis of randomised clinical trials. J. Obes. 2011, 509038 (2011).
Jena, B. S., Jayaprakasha, G. K., Singh, R. P. & Sakariah, K. K. Chemistry and biochemistry of (-)-hydroxycitric acid from Garcinia. J. Agric. Food Chem. 50, 10–22 (2002).
Heymsfield, S. B. et al. Garcinia cambogia (hydroxycitric acid) as a potential antiobesity agent: a randomized controlled trial. JAMA 280, 1596–1600 (1998).
Madeo, F., Carmona-Gutierrez, D., Hofer, S. J. & Kroemer, G. Caloric restriction mimetics against age-associated disease: targets, mechanisms, and therapeutic potential. Cell Metab. 29, 592–610 (2019).
Marino, G. et al. Regulation of autophagy by cytosolic acetyl-coenzyme A. Mol. Cell 53, 710–725 (2014). This study shows that nutrient deprivation-induced depletion of acetyl-CoA activates autophagy in a p300-regulated manner.
Longo, V. D. & Panda, S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 23, 1048–1059 (2016).
Lien, E. C. et al. Low glycaemic diets alter lipid metabolism to influence tumour growth. Nature 599, 302–307 (2021).
Pomatto-Watson, L. C. D. et al. Daily caloric restriction limits tumor growth more effectively than caloric cycling regardless of dietary composition. Nat. Commun. 12, 6201 (2021).
Pietrocola, F. et al. Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell 30, 147–160 (2016).
Michaud, M. et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 334, 1573–1577 (2011).
Amaravadi, R. K., Kimmelman, A. C. & Debnath, J. Targeting autophagy in cancer: recent advances and future directions. Cancer Discov. 9, 1167–1181 (2019).
Yamamoto, K. et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature 581, 100–105 (2020).
Zheng, Y. et al. ATP citrate lyase inhibitor triggers endoplasmic reticulum stress to induce hepatocellular carcinoma cell apoptosis via p-eIF2alpha/ATF4/CHOP axis. J. Cell Mol. Med. 25, 1468–1479 (2021).
Shah, S. et al. Targeting ACLY sensitizes castration-resistant prostate cancer cells to AR antagonism by impinging on an ACLY-AMPK-AR feedback mechanism. Oncotarget 7, 43713–43730 (2016).
Pearce, N. J. et al. The role of ATP citrate-lyase in the metabolic regulation of plasma lipids. Hypolipidaemic effects of SB-204990, a lactone prodrug of the potent ATP citrate-lyase inhibitor SB-201076. Biochem. J. 334, 113–119 (1998).
Burke, A. C. & Huff, M. W. ATP-citrate lyase: genetics, molecular biology and therapeutic target for dyslipidemia. Curr. Opin. Lipidol. 28, 193–200 (2017).
Pinkosky, S. L. et al. Liver-specific ATP-citrate lyase inhibition by bempedoic acid decreases LDL-C and attenuates atherosclerosis. Nat. Commun. 7, 13457 (2016).
Tang, Z. et al. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 45, W98–W102 (2017).
Gu, L. et al. The IKKbeta-USP30-ACLY axis controls lipogenesis and tumorigenesis. Hepatology 73, 160–174 (2021).
Qiao, C. et al. IGF1-mediated HOXA13 overexpression promotes colorectal cancer metastasis through upregulating ACLY and IGF1R. Cell Death Dis. 12, 564 (2021).
Pinkosky, S. L. et al. AMP-activated protein kinase and ATP-citrate lyase are two distinct molecular targets for ETC-1002, a novel small molecule regulator of lipid and carbohydrate metabolism. J. Lipid Res. 54, 134–151 (2013).
Huang, X. et al. Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem. J. 412, 211–221 (2008).
Houde, V. P. et al. AMPK beta1 reduces tumor progression and improves survival in p53 null mice. Mol. Oncol. 11, 1143–1155 (2017).
Dai, X. et al. Energy status dictates PD-L1 protein abundance and anti-tumor immunity to enable checkpoint blockade. Mol. Cell 81, 2317–2331.e6 (2021).
Wei, J. et al. An allosteric mechanism for potent inhibition of human ATP-citrate lyase. Nature 568, 566–570 (2019).
Wei, X., Schultz, K., Bazilevsky, G. A., Vogt, A. & Marmorstein, R. Molecular basis for acetyl-CoA production by ATP-citrate lyase. Nat. Struct. Mol. Biol. 27, 33–41 (2020).
Verschueren, K. H. G. et al. Structure of ATP citrate lyase and the origin of citrate synthase in the Krebs cycle. Nature 568, 571–575 (2019). Along with Wei et al. (2019) and Wei et al. (2020), this article reports for the first time the structure of the ACLY homotetramer.
Svensson, R. U. et al. Inhibition of acetyl-CoA carboxylase suppresses fatty acid synthesis and tumor growth of non-small-cell lung cancer in preclinical models. Nat. Med. 22, 1108–1119 (2016). This study demonstrate that ACC inhibition suppresses tumour growth in genetically engineered lung cancer mouse models.
Liefwalker, D. F. et al. Metabolic convergence on lipogenesis in RAS, BCR-ABL, and MYC-driven lymphoid malignancies. Cancer Metab. 9, 31 (2021).
Alkhouri, N., Lawitz, E., Noureddin, M., DeFronzo, R. & Shulman, G. I. GS-0976 (firsocostat): an investigational liver-directed acetyl-CoA carboxylase (ACC) inhibitor for the treatment of non-alcoholic steatohepatitis (NASH). Expert. Opin. Investig. Drugs 29, 135–141 (2020).
Lee, H. et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 22, 225–234 (2020).
Ringel, A. E. et al. Obesity shapes metabolism in the tumor microenvironment to suppress anti-tumor immunity. Cell 183, 1848–1866.e26 (2020).
Wellen, K. E. & Snyder, N. W. Should we consider subcellular compartmentalization of metabolites, and if so, how do we measure them? Curr. Opin. Clin. Nutr. Metab. Care 22, 347–354 (2019).
Dieterich, I. A. et al. Acetyl-CoA flux regulates the proteome and acetyl-proteome to maintain intracellular metabolic crosstalk. Nat. Commun. 10, 3929 (2019).
He, A. et al. Acetyl-CoA derived from hepatic peroxisomal beta-oxidation inhibits autophagy and promotes steatosis via mTORC1 activation. Mol. Cell 79, 30–42.e4 (2020).
Huber, K. et al. N-acetylaspartate pathway is nutrient responsive and coordinates lipid and energy metabolism in brown adipocytes. Biochim. Biophys. Acta Mol. Cell Res. 1866, 337–348 (2019).
Jung, S. M. et al. In vivo isotope tracing reveals the versatility of glucose as a brown adipose tissue substrate. Cell Rep. 36, 109459 (2021).
Levy, M. J. et al. A systems chemoproteomic analysis of acyl-CoA/protein interaction networks. Cell Chem. Biol. 27, 322–333.e5 (2020).
Montgomery, D. C. et al. Global profiling of acetyltransferase feedback regulation. J. Am. Chem. Soc. 138, 6388–6391 (2016).
Goncalves, M. D. et al. High-fructose corn syrup enhances intestinal tumor growth in mice. Science 363, 1345–1349 (2019).
Acknowledgements
K.E.W. and D.A.G. are supported by the NIDDK of the NIH (R01DK116005). K.E.W. is also supported by the NCI (R01CA228339, R01CA174761, R01CA248315 and R01CA262055) and the Ludwig Institute. D.A.G. is also supported by the NIDDK of the NIH (R01DK094004 and R01DK127175).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cancer thanks G. Steinberg, R. Perry and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Adipokines
-
Adipocyte-derived factors released into the blood that can function in an autocrine or paracrine manner to regulate metabolism.
- Coenzyme Q
-
(CoQ). Also known as ubiquinone, an enzyme comprising a redox-active quinone head group and an isoprenoid tail synthesized in the mevalonate pathway that functions as an electron carrier as part of the electron transport chain and as an antioxidant.
- De novo lipogenesis
-
(DNL). The process of building fatty acids from the non-lipid precursor acetyl coenzyme A, which can be generated by a variety of pathways but most commonly from carbohydrates.
- Mevalonate pathway
-
Named after its key intermediate, the five-carbon molecule mevalonate, this pathway generates precursors to a large family of isoprenoids, including cholesterol and coenzyme Q.
- M1 and M2 macrophage phenotypes
-
A classification system in which heterogeneous macrophages are grouped as either M1, which defines activated pro-inflammatory macrophages associated with protection against bacteria and viruses, and M2, which are alternatively activated macrophages that express different markers and are associated with wound healing and immune suppression.
- Positron emission tomography tracers
-
Chemicals than contain a positron-emitting radioisotope that are used to image tumours, for example [18F]fluorodeoxyglucose, a non-metabolizable analogue of glucose that can image tumours with high glucose uptake rates.
- Reductive carboxylation
-
A reductive pathway of glutamine metabolism in which isocitrate dehydrogenase 1 (IDH1) and IDH2 operate in reverse to generate isocitrate and citrate from α-ketoglutarate and CO2.
- Stable-isotope tracing
-
A technique used to follow the metabolic fate of a tracer molecule delivered to cells or tissues, such as [13C]glucose or [13C–15N]glutamine, in which one or more of the abundant and naturally occurring elements — usually C, H or N — are replaced with less abundant, non-radioactive isotopes that can be distinguished in mass by a mass spectrometer.
- Statins
-
Cholesterol-lowering drugs that target 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR).
- Thioester bond
-
A chemical bond that can be summarized as R–CO–S–R′, in which a sulfur instead of oxygen connects the carboxylate ester.
- Ubiquitylation
-
The post-translational modification process of attaching a ubiquitin protein to a lysine residue, which can function as a regulator signal or form polyubiquitin chains targeting a protein for degradation.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guertin, D.A., Wellen, K.E. Acetyl-CoA metabolism in cancer. Nat Rev Cancer 23, 156–172 (2023). https://doi.org/10.1038/s41568-022-00543-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-022-00543-5
This article is cited by
-
Mechanism of intestinal microbiota disturbance promoting the occurrence and development of esophageal squamous cell carcinoma——based on microbiomics and metabolomics
BMC Cancer (2024)
-
TMEM120A-mediated regulation of chemotherapy sensitivity in colorectal cancer cells
Cancer Chemotherapy and Pharmacology (2024)
-
Metabolic regulation of epigenetic drug resistance
Nature Chemical Biology (2023)
-
Metabolic interventions combined with CTLA-4 and PD-1/PD-L1 blockade for the treatment of tumors: mechanisms and strategies
Frontiers of Medicine (2023)
-
Unpacking the complexity of nuclear IL-33 (nIL-33): a crucial regulator of transcription and signal transduction
Journal of Cell Communication and Signaling (2023)