Abstract
The global loss of heterochromatin during ageing has been observed in eukaryotes from yeast to humans, and this has been proposed as one of the causes of ageing. However, the cause of this age-associated loss of heterochromatin has remained enigmatic. Here we show that heterochromatin markers, including histone H3K9 di/tri-methylation and HP1, decrease with age in muscle stem cells (MuSCs) as a consequence of the depletion of the methyl donor S-adenosylmethionine (SAM). We find that restoration of intracellular SAM in aged MuSCs restores heterochromatin content to youthful levels and rejuvenates age-associated features, including DNA damage accumulation, increased cell death, and defective muscle regeneration. SAM is not only a methyl group donor for transmethylation, but it is also an aminopropyl donor for polyamine synthesis. Excessive consumption of SAM in polyamine synthesis may reduce its availability for transmethylation. Consistent with this premise, we observe that perturbation of increased polyamine synthesis by inhibiting spermidine synthase restores intracellular SAM content and heterochromatin formation, leading to improvements in aged MuSC function and regenerative capacity in male and female mice. Together, our studies demonstrate a direct causal link between polyamine metabolism and epigenetic dysregulation during murine MuSC ageing.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
RNA-seq data have been deposited in the NCBI Gene Expression Omnibus (GEO) with accession no. GSE229853. ATAC-seq data have been deposited in the GEO with accession no. GSE229851. The GRCm39 genome is available in the NCBI RefSeq (GCF_000001635.27-RS_2023_04). All related source data are made available to readers as part of the manuscript files. Source data are provided with this paper.
Code availability
This paper does not report any original code.
References
Benayoun, B. A., Pollina, E. A. & Brunet, A. Epigenetic regulation of ageing: linking environmental inputs to genomic stability. Nat. Rev. Mol. Cell Biol. 16, 593–610 (2015).
Villeponteau, B. The heterochromatin loss model of aging. Exp. Gerontol. 32, 383–394 (1997).
Ljungman, M. & Hanawalt, P. C. Efficient protection against oxidative DNA damage in chromatin. Mol. Carcinog. 5, 264–269 (1992).
Wood, J. G. & Helfand, S. L. Chromatin structure and transposable elements in organismal aging. Front. Genet. 4, 274 (2013).
Larson, K. et al. Heterochromatin formation promotes longevity and represses ribosomal RNA synthesis. PLoS Genet. 8, e1002473 (2012).
Smeal, T., Claus, J., Kennedy, B., Cole, F. & Guarente, L. Loss of transcriptional silencing causes sterility in old mother cells of S. cerevisiae. Cell 84, 633–642 (1996).
Wasserzug-Pash, P. et al. Loss of heterochromatin and retrotransposon silencing as determinants in oocyte aging. Aging Cell 21, e13568 (2022).
Scaffidi, P. & Misteli, T. Lamin A-dependent nuclear defects in human aging. Science 312, 1059–1063 (2006).
Lee, J.-H., Kim, E. W., Croteau, D. L. & Bohr, V. A. Heterochromatin: an epigenetic point of view in aging. Exp. Mol. Med. 52, 1466–1474 (2020).
Bi, S. et al. SIRT7 antagonizes human stem cell aging as a heterochromatin stabilizer. Protein Cell 11, 483–504 (2020).
Liu, L. et al. Hairless regulates heterochromatin maintenance and muscle stem cell function as a histone demethylase antagonist.Proc. Natl Acad. Sci. USA 118, e2025281118 (2021).
Liang, C. et al. Stabilization of heterochromatin by CLOCK promotes stem cell rejuvenation and cartilage regeneration. Cell Res. 31, 187–205 (2021).
Berger, S. L. & Sassone-Corsi, P. Metabolic signaling to chromatin.Cold Spring Harb. Perspect. Biol. 8, a019463 (2016).
Su, X., Wellen, K. E. & Rabinowitz, J. D. Metabolic control of methylation and acetylation. Curr. Opin. Chem. Biol. 30, 52–60 (2016).
Chiang, P. K. et al. S-Adenosylmethionine and methylation. FASEB J. 10, 471–480 (1996).
Mews, P. et al. Acetyl-CoA synthetase regulates histone acetylation and hippocampal memory. Nature 546, 381–386 (2017).
Tsukada, Y. et al. Histone demethylation by a family of JmjC domain-containing proteins. Nature 439, 811–816 (2006).
Carey, B. W., Finley, L. W. S., Cross, J. R., Allis, C. D. & Thompson, C. B. Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature 518, 413–416 (2015).
Shiraki, N. et al. Methionine metabolism regulates maintenance and differentiation of human pluripotent stem cells. Cell Metab. 19, 780–794 (2014).
Yucel, N. et al. Glucose metabolism drives histone acetylation landscape transitions that dictate muscle stem cell function. Cell Rep. 27, 3939–3955 (2019).
Brunet, A., Goodell, M. A. & Rando, T. A. Ageing and rejuvenation of tissue stem cells and their niches. Nat. Rev. Mol. Cell Biol. 24, 45–62 (2023).
Goodell, M. A. & Rando, T. A. Stem cells and healthy aging. Science 350, 1199–1204 (2015).
Liu, B., Qu, J., Zhang, W., Izpisua Belmonte, J. C. & Liu, G.-H. A stem cell aging framework, from mechanisms to interventions. Cell Rep. 41, 111451 (2022).
Conboy, I. M. & Rando, T. A. Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle 4, 407–410 (2005).
Liu, L. et al. Impaired Notch signaling leads to a decrease in p53 activity and mitotic catastrophe in aged muscle stem cells. Cell Stem Cell 23, 544–556 (2018).
Porpiglia, E. et al. Elevated CD47 is a hallmark of dysfunctional aged muscle stem cells that can be targeted to augment regeneration. Cell Stem Cell 29, 1653–1668 (2022).
Hernando-Herraez, I. et al. Ageing affects DNA methylation drift and transcriptional cell-to-cell variability in mouse muscle stem cells. Nat. Commun. 10, 4361 (2019).
Liu, L. et al. Chromatin modifications as determinants of muscle stem cell quiescence and chronological aging. Cell Rep. 4, 189–204 (2013).
Zhang, X. et al. The loss of heterochromatin is associated with multiscale three-dimensional genome reorganization and aberrant transcription during cellular senescence. Genome Res. 31, 1121–1135 (2021).
Benjamin, D. I. et al. Multiomics reveals glutathione metabolism as a driver of bimodality during stem cell aging. Cell Metab. 35, 472–486 (2023).
Liu, F. et al. Discovery of an in vivo chemical probe of the lysine methyltransferases G9a and GLP. J. Med. Chem. 56, 8931–8942 (2013).
Greiner, D., Bonaldi, T., Eskeland, R., Roemer, E. & Imhof, A. Identification of a specific inhibitor of the histone methyltransferase SU(VAR)3-9. Nat. Chem. Biol. 1, 143–145 (2005).
Kikuchi, H. & Sato, S. Fractionation of nonhistone proeins on a column of daunomycin-CH-Sepharose 4B. Biochim. Biophys. Acta 532, 113–121 (1978).
Haws, S. A. et al. Methyl-metabolite depletion elicits adaptive responses to support heterochromatin stability and epigenetic persistence. Mol. Cell 78, 210–223 (2020).
Ohtani, M. et al. Spermidine regulates insulin synthesis and cytoplasmic Ca2+ in mouse beta-TC6 insulinoma cells. Cell Struct. Funct. 34, 105–113 (2009).
Massaro, C., Thomas, J. & Phanstiel Iv, O. Investigation of polyamine metabolism and homeostasis in pancreatic cancers.Med. Sci. 5, 32 (2017).
Tabor, H. The protective effect of spermine and other polyamines against heat denaturation of deoxyribonucleic acid. Biochemistry 1, 496–501 (1962).
Eisenberg, T. et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 11, 1305–1314 (2009).
Agrimi, G. et al. Identification of the human mitochondrial S-adenosylmethionine transporter: bacterial expression, reconstitution, functional characterization and tissue distribution. Biochem. J. 379, 183–190 (2004).
Sun, Y. & Locasale, J. W. Rethinking the bioavailability and cellular transport properties of S-adenosylmethionine. Cell Stress 6, 1–5 (2021).
Wu, S. E., Huskey, W. P., Borchardt, R. T. & Schowen, R. L. Chiral instability at sulfur of S-adenosylmethionine. Biochemistry 22, 2828–2832 (1983).
Albers, E. Metabolic characteristics and importance of the universal methionine salvage pathway recycling methionine from 5′-methylthioadenosine. IUBMB Life 61, 1132–1142 (2009).
Mentch, S. J. et al. Histone methylation dynamics and gene regulation occur through the sensing of one-carbon metabolism. Cell Metab. 22, 861–873 (2015).
Yu, W. et al. One-carbon metabolism supports S-adenosylmethionine and histone methylation to drive Inflammatory macrophages. Mol. Cell 75, 1147–1160 (2019).
Dai, Z., Mentch, S. J., Gao, X., Nichenametla, S. N. & Locasale, J. W. Methionine metabolism influences genomic architecture and gene expression through H3K4me3 peak width. Nat. Commun. 9, 1955 (2018).
Sadhu, M. J. et al. Nutritional control of epigenetic processes in yeast and human cells. Genetics 195, 831–844 (2013).
Toraño, E. G., García, M. G., Fernández-Morera, J. L., Niño-García, P. & Fernández, A. F. The impact of external factors on the epigenome: in utero and over lifetime. BioMed. Res. Int. 2016, 2568635 (2016).
Hyun, K., Jeon, J., Park, K. & Kim, J. Writing, erasing and reading histone lysine methylations. Exp. Mol. Med. 49, e324 (2017).
Pan, Y.-H. et al. The critical roles of polyamines in regulating ColE7 production and restricting ColE7 uptake of the colicin-producing Escherichia coli. J. Biol. Chem. 281, 13083–13091 (2006).
Rato, C., Amirova, S. R., Bates, D. G., Stansfield, I. & Wallace, H. M. Translational recoding as a feedback controller: systems approaches reveal polyamine-specific effects on the antizyme ribosomal frameshift. Nucleic Acids Res. 39, 4587–4597 (2011).
Wang, C. et al. Defining the molecular requirements for the selective delivery of polyamine conjugates into cells containing active polyamine transporters. J. Med. Chem. 46, 5129–5138 (2003).
Fan, J. et al. Spermidine coupled with exercise rescues skeletal muscle atrophy from D-gal-induced aging rats through enhanced autophagy and reduced apoptosis via AMPK-FOXO3a signal pathway. Oncotarget 8, 17475–17490 (2017).
Luchessi, A. D. et al. Involvement of eukaryotic translation initiation factor 5A (eIF5A) in skeletal muscle stem cell differentiation. J. Cell. Physiol. 218, 480–489 (2009).
Machado, L. et al. Tissue damage induces a conserved stress response that initiates quiescent muscle stem cell activation. Cell Stem Cell 28, 1125–1135 (2021).
Park, M. H. The post-translational synthesis of a polyamine-derived amino acid, hypusine, in the eukaryotic translation initiation factor 5A (eIF5A). J. Biochem. 139, 161–169 (2006).
Uchitomi, R. et al. Metabolomic analysis of skeletal muscle in aged mice. Sci. Rep. 9, 10425 (2019).
Nishimura, K., Shiina, R., Kashiwagi, K. & Igarashi, K. Decrease in polyamines with aging and their ingestion from food and drink. J. Biochem. 139, 81–90 (2006).
Minois, N., Carmona-Gutierrez, D. & Madeo, F. Polyamines in aging and disease. Aging 3, 716–732 (2011).
Casero, R. A. Jr., Murray Stewart, T. & Pegg, A. E. Polyamine metabolism and cancer: treatments, challenges and opportunities. Nat. Rev. Cancer 18, 681–695 (2018).
Murray-Stewart, T. R., Woster, P. M. & Casero, R. A. Jr. Targeting polyamine metabolism for cancer therapy and prevention. Biochem. J. 473, 2937–2953 (2016).
Lewandowski, N. M. et al. Polyamine pathway contributes to the pathogenesis of Parkinson disease. Proc. Natl Acad. Sci. USA 107, 16970–16975 (2010).
Polis, B., Karasik, D. & Samson, A. O. Alzheimer’s disease as a chronic maladaptive polyamine stress response. Aging 13, 10770–10795 (2021).
Morrison, L. D., Becker, L., Ang, L. C. & Kish, S. J. Polyamines in human brain: regional distribution and influence of aging. J. Neurochem. 65, 636–642 (1995).
Graham, S. F. et al. Untargeted metabolomic analysis of human plasma indicates differentially affected polyamine and l-arginine metabolism in mild cognitive impairment subjects converting to Alzheimer’s disease. PLoS ONE 10, e0119452 (2015).
Ost, M. et al. Muscle mitohormesis promotes cellular survival via serine/glycine pathway flux. FASEB J. 29, 1314–1328 (2015).
Cosgrove, B. D. et al. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat. Med. 20, 255–264 (2014).
Price, F. D. et al. Inhibition of JAK-STAT signaling stimulates adult satellite cell function. Nat. Med. 20, 1174–1181 (2014).
Liu, L., Cheung, T. H., Charville, G. W. & Rando, T. A. Isolation of skeletal muscle stem cells by fluorescence-activated cell sorting. Nat. Protoc. 10, 1612–1624 (2015).
Charville, G. W. et al. Ex vivo expansion and in vivo self-renewal of human muscle stem cells. Stem Cell Reports 5, 621–632 (2015).
Anders, S. & Huber, W. Differential expression analysis for sequence count data. Genome Biol. 11, R106 (2010).
Brett, J. O. et al. Exercise rejuvenates quiescent skeletal muscle stem cells in old mice through restoration of Cyclin D1. Nat. Metab. 2, 307–317 (2020).
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2019R1A6A3A03031423) to J.K., and by funding from the Glenn Foundation for Medical Research and the National Institutes of Health (NIH) (grant nos. P01AG036695, R01 AG068667, R01 AR073248 and AG071783) to T.A.R. Work using the TEM described in this study was supported, in part, by an NIH S10 Award (1S10OD028536-01), titled ‘OneView 4kX4k sCMOS camera for TEM applications’ from the Office of Research Infrastructure Programs. The RNA-seq data were generated with instrumentation purchased with NIH funds (S10OD025212 and 1S10OD021763). LC–MS-based metabolite quantification was supported by the Vincent Coates Foundation Mass Spectrometry Laboratory, Stanford University Mass Spectrometry. It used the Waters Xevo TQ-XS mass spectrometer system (RRID: SCR_018510), which was purchased with funding from an NIH Shared Instrumentation grant no. S10OD026962. Human biopsy samples were obtained from DNW. We thank the cooperation of DNW and all tissue donors and their families, for giving the gift of life and the gift of knowledge, by their generous donation. Schematic images in the figures were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
J.K. designed the studies and carried out the experiments with assistance from D.I.B., S.K., J.S.S., G.D., R.L., A.G. and J.O.B. with guidance from T.A.R. throughout. J.K. interpreted the results with guidance and input from T.A.R. and L.L. J.K. and T.A.R. wrote the paper and assembled the data with assistance from D.I.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Jason Locasale and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Ashley Castellanos-Jankiewicz, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
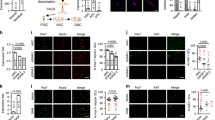
Extended Data Fig. 1 Differential chromatin accessibility and gene expression in young and old MuSCs.
a, Gating strategy for fluorescence-activated cell sorting (FACS) isolation of muscle stem cells (MuSCs). Purity of isolated MuSCs is > 98% as assessed by staining for Pax7 of cells fixed one hour after plating. (Scale bar, 50 μm) b-d, Representative confocal immunofluorescence images of MuSCs of young and old female mice (Scale bar, 5 μm). RFUs of H3K9me3 (b), H3K9me2 (c), or HP1α (d) were normalized to fluorescence intensities of total H3 (n = 4). e, Pearson correlation of ATAC-seq profiles from young and old MuSCs (n = 2). A total of about 60,000 signal summits were found in each sample. f, Representative heat maps of ATAC-seq tag intensity 3 kb around transcription start sites (TSSs) in young and old MuSCs. g, Read density within 3 kb upstream of TSSs and 3 kb downstream of TESs. h, Hierarchical clustering of peak enrichment patterns between young and old MuSCs. i, PCA plot of RNAseq data from freshly isolated young and old MuSCs. j, Volcano plots of differentially expressed genes (DEGs) between young and old MuSCs. Dashed lines indicate fold-change (log2FC > 0.5) and p-value cut-offs (Padj < 0.05). Total 1,641 DEGs were found. Among the DEGs, 866 genes were highly expressed in old MuSCs, and 775 genes were highly expressed in young MuSCs. k, Normalized DESeq read counts of methyltransferases for H3K9 (Suv39h1, Ehmt1, Ehmt2, Setdb1, and Setdb2). (n = 4) l, Normalized DESeq read counts of demethylases for H3K9 (Kdm3a, Kdm3b, Kdm4a, Kdm4b, Kdm4c, and Kdm7b). (n = 4) DESeq normalization was conducted by using median-of-ratio method as described in the Methods. Data are shown as median and quartiles (b-d) and as mean ± SD (k, l). P values were calculated by two-sided unpaired Student’s t-tests (b-d, k, l). *P < 0.05; **P < 0.01; ***P < 0.001. Statistical details are provided in Source Data.
Extended Data Fig. 2 Restoration of intracellular SAM promotes heterochromatin formation and reduces susceptibility to DNA damage and cell death of old MuSCs.
a, Intracellular SAM content of MuSCs measured by SAM ELISA (n = 4). b-d, Representative confocal immunofluorescence images of MuSCs (Scale bar, 5 μm). RFUs of H3K9me3 (b), H3K9me2 (c), or HP1α (d) were normalized to RFUs of total H3 (n = 4). e-f, Representative confocal immunofluorescence images of old MuSCs treated as indicated (Scale bar, 5 μm). RFUs of H3K9me3 (e) or HP1α (f) were normalized to RFUs of total H3 (n = 4). g, Representative heat maps of ATAC-seq tag intensity 3 kb around TSSs in vehicle- or SAM-treated old MuSCs. h, Read density within 3 kb upstream of TSSs and 3 kb downstream of the TESs. i, Hierarchical clustering of peak enrichment patterns. j, (Top) Representative confocal immunofluorescence images of γ-H2AX foci (Scale bar, 5 μm). (Bottom) Quantification of the number of γ-H2AX foci per cell (n = 3). k, (Left) Representative confocal images of γ-H2AX foci. (Scale bar, 5 μm) (Right) The ratio of γ-H2AX foci per cell was quantified from old MuSCs treated as indicated (n = 4). l, FACS analysis of propidium iodide (PI)-positive MuSCs. A day after the administration of 7 Gy γ-irradiation to the hind limbs, MuSCs were isolated from young, old, or old mice treated with SAM, and subsequently cultured for 2 days. m, The ratio of apoptotic cells over total cells was quantified by TUNEL assay and plotted (n = 4). n-p, Relative fluorescence units (RFUs) of H3K9me3 (n), H3K9me2 (o), and HP1α (p) were normalized to RFUs of total H3 (n = 3). q, Human MuSCs were treated with SAM for 2 days, and 0.75 μM of doxorubicin was added and incubated for another day. TUNEL positive cells were quantified (n = 3). Data are shown as mean ± SD (a, j, k, m, q) and as median and quartiles (b-f, n-p). P values were calculated by two-sided unpaired Student’s t-tests (a-f, j, k, m-q). *P < 0.05; **P < 0.01; ***P < 0.001.
Extended Data Fig. 3 Inhibiting spermidine synthesis promotes heterochromatin re-formation and reduces DNA damage in old MuSCs.
a, Diagram of spermidine metabolism. The synthesis of spermidine and spermine requires dcSAM as aminopropyl donor, which is produced from SAM by AMD1. b, Representative Western Blots of SRM, PAOX, SAT1, and α-tubulin in MuSCs which were freshly isolated form young and old mice (n = 3). c-e, Band intensities of SRM (c), PAOX (d), and SAT1 (e) were normalized to the level of α-tubulin (n = 3). f, Quantification of RFUs of intracellular spermidine in MuSCs from vehicle- or MCHA-treated old mice (n = 4). g, Intracellular SAM content of MuSCs measured by SAM ELISA (n = 4). h-j, Representative confocal immunofluorescence images of MuSCs of old female mice treated with vehicle or MCHA (Scale bar, 5 μm). RFUs of H3K9me3 (h), H3K9me2 (i), or HP1α (j) were normalized to RFUs of total H3 (n = 4). k, Representative western blots of H3K9me3, H3K9me2, H3K9me1, HP1α, and total H3 of MuSCs (n = 3). l-o, Band intensities of H3K9me3 (l), H3K9me2 (m), HP1α (n), and H3K9me1 (o) were normalized to the band intensities of total H3 (n = 3). p, (Left) Representative transmission electron microscopy images of MuSCs on EDL sections. (Right) The percentage of heterochromatin cross sectional area over total cross-sectional area of nucleus is quantified (n = 15 cells examined over 3 independent young mice treated with vehicle, n = 14 cells examined over 3 independent young mice treated with MCHA). The box represents the interquartile range, with the lower and upper hinges indicating the 25th and 75th percentiles, respectively. The horizontal line inside the box marks the median score. The whiskers extend to the minimum and maximum values. q, Representative heat maps of ATAC-seq tag intensity 3 kb around TSSs. r, Read density within 3 kb upstream of TSSs and 3 kb downstream of the TESs. s, Hierarchical clustering of peak enrichment patterns. t, (Left) Representative confocal immunofluorescence images of γ-H2AX foci. (Right) Quantification of the number of γ-H2AX foci per cell (n = 3). Data are shown as mean ± SD (c-g, l-o, t) and as median and quartiles (h-j, p). P values were calculated by two-sided unpaired Student’s t-tests (c-j, l-p, t). *P < 0.05; **P < 0.01; ***P < 0.001.
Extended Data Fig. 4 Heterochromatin re-formation induced by restoration of SAM reduces susceptibility to genotoxic stress and cell death of old MuSCs.
a, Quantification of RFUs of intracellular SAM (n = 4). b, Quantification of RFUs of intracellular putrescine. Freshly isolated old MuSCs were treated with vehicle, putrescine, or ornithine for 48 hr (n = 4). c, After treating old MuSCs as described in (b), doxorubicin was added to the media, and the cells were cultured for another day. (Left) Representative confocal images of γ-H2AX foci. (Scale bar, 5 μm) (Right) The ratio of γ-H2AX foci per cell (n = 4). d, Old MuSCs were treated as described in (c). The ratio of apoptotic cells over total cells was quantified by TUNEL assay (n = 4). e, Quantification of mean RFUs of intracellular spermidine (n = 4). f, Quantification of intracellular SAM measured by SAM ELISA (n = 4). g, Representative western blots of SRM and α-tubulin in old MuSCs (n = 3). h, Band intensity of SRM was normalized to band intensity of α-tubulin (n = 3). i-j, Old MuSCs transfected with siControl or siSrm were stained with antibodies against SAM (i) or spermidine (j). Mean RFUs of intracellular SAM or spermidine in each group of cells were quantified (n = 3 for SAM staining, n = 4 for spermidine staining). k, Old MuSCs were transfected with siControl or siSrm and treated with vehicle, UNC0642, or Chaetocin for 24 hr as indicated. (Left) Representative confocal immunofluorescence images of γ-H2AX foci (Scale bar, 5 μm). (Right) Quantification of the number of γ-H2AX foci per cell (n = 4). l, Quantification of the ratio of TUNEL positive cells over total cells (n = 4). m-n, Freshly isolated human MuSCs were treated as indicated for 48 hr. RFUs of H3K9me3 (m) or HP1α (n) were normalized to RFUs of total H3 (n = 3). o, Human MuSCs treated as described in (m-n) were additionally treated with doxorubicin for 24 hr. (Left) Representative confocal images (Scale bar, 5 μm). (Right) Quantification of the number of γ-H2AX foci per cell (n = 3). p, The human MuSCs treated as described in (o) were subjected to TUNEL assay. The ratio of TUNEL positive cells over total cells was quantified (n = 3). Data are shown as mean ± SD (a-d, f, h-l, o, p) and as median and quartiles (e, m, n). P values were calculated by two-sided unpaired Student’s t-tests (a-f, h-p). *P < 0.05; **P < 0.01; ***P < 0.001.
Extended Data Fig. 5 Inhibiting spermidine synthesis does not impair autophagy of aged MuSCs.
a-b, Quantification of intracellular spermidine levels. Violin plots showing RFUs of spermidine in old MuSCs treated with each dose of spermidine (a) or MCHA (b) as indicated (n = 3). c-d, (Left) Representative confocal immunofluorescence images of old MuSCs treated with vehicle or each dose of spermidine (c) or MCHA (d) for 48 hr. In each case, cells were treated with or without 50 μM of chloroquine (CQ) for the last 3 hr of incubation. (Right) Quantification of number of LC3B foci per cell. (Scale bar, 10 μM) Data are shown as median and quartiles (a, b) and mean ± SD (c, d). P values were calculated by two-sided unpaired Student’s t-tests (a-d). *P < 0.05; **P < 0.01; ***P < 0.001.
Extended Data Fig. 6 Restoration of SAM and H3K9 methylation improves in vivo potency of old MuSCs to regenerate new muscle.
a-b, Representative images of bioluminescence of vehicle- or SAM-treated MuSCs (a) and vehicle- or MCHA-treated old MuSCs (b) which had been transplanted into muscles of NSG mice. A day after transplantation is Day 1 (n = 6). c, Representative FACS plots for analysis of RFP-positive MuSCs isolated from recipient NSG mice. Purity of isolated RFP-positive MuSCs is > 98% as assessed by staining for Pax7 of cells (Scale bar, 50 μm). d, Old MuSCs were transduced with shRNA containing lentiviruses as indicated. After 48 hr of culture, the cells were subjected to western blotting (n = 4). e, Band intensity of Srm was normalized to the band intensity of α-tubulin (n = 4). f-g, Old MuSCs transduced with shControl or shSrm were stained with antibodies against SAM (f) or spermidine (g). Mean RFUs of intracellular SAM or spermidine were quantified (n = 3). h, Representative BLI images taken from Day 1 to Day 15. MuSCs transduced with shControl or shSrm were transplanted into muscles of NSG mice (n = 6). i, Paired comparisons of relative bioluminescence flux measured from the right and left muscles of each mouse eight days after transplantation. j, Western blot analysis of SRM and SUV39H1 in MuSCs. Old MuSCs were transduced with shRNA containing lentiviruses as indicated. After 48 hr of transduction, the cells were subjected to western blotting (n = 3). k, Representative BLI images of MuSCs transduced with shSrm or shSrm with shSuv39h1 which had been transplanted into muscles of NSG mice (n = 8). l, Paired comparisons of relative bioluminescence flux measured from the right and left muscles of each mouse ten days after transplantation. m, Schematic of transplantation. n, (Left) Quantified results of BLI measured at different time points from 60 to 71 days following transplantation (n = 6). (Right) Representative images of bioluminescence captured 71 days after transplantation. Data are shown as mean ± SD (e-g). P values were calculated by two-sided unpaired Student’s t-tests (e-g) and by one-sided student’s t test, pairwise between groups (i, l, n). *P < 0.05; **P < 0.01; ***P < 0.001. Schematic images in Fig. 6m were created with BioRender.com.
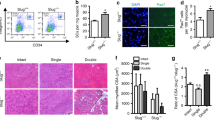
Extended Data Fig. 7 Restoration of SAM is associated with improved muscle regeneration and function of aged mice.
a, MuSCs were isolated from the injured muscles of female mice at 2 days post injury (dpi). (Left) Representative confocal images. (Scale bar, 5 μm) (Right) The ratio of γ-H2AX foci per cell was quantified (n = 4 for young mice, and old mice treated with vehicle or SAM, n = 3 for old mice treated with MCHA). b, (Left) Representative TUNEL assay images of female MuSCs isolated at 2dpi (Scale bar, 50 μm). (Right) The ratio of apoptotic cells over total cells was quantified (n = 4 for young mice, and old mice treated with vehicle or SAM, n = 3 for old mice treated with MCHA). c, (Left) The percentage of PI positive female MuSCs isolated at 2dpi (n = 4 for young mice, and old mice treated with vehicle or SAM, n = 3 for old mice treated with MCHA). (Right) The representative FACS plot. d-e, Frequency histogram of cross-sectional areas (CSAs) of centrally nucleated myofibers in TA muscle sections. TA muscles were harvested from old mice treated with vehicle, MCHA (d) or SAM (e) at 7 dpi (n = 3). f, (Left) Representative immunostaining of regenerating muscle fibers from female mice (Scale bar, 100 μm). (Right) Quantification of the mean CSAs of myofibers with centrally located nuclei in TA muscle sections (n = 4 for young and old female mice treated with vehicle, n = 3 for old female mice treated with MCHA or SAM). g, (Left) Representative immunostaining of non-regenerating muscle fibers (Scale bar, 100 μm). (Right) Quantification of the mean CSAs of myofibers in TA muscle sections (n = 3). h, (Top) Representative immunostaining of regenerating muscle fibers (Scale bar, 100 μm). (Bottom) Quantification of the mean CSAs of myofibers with centrally located nuclei in TA muscle sections (n = 3). i, Representative tissue immunostaining images of TA muscle sections from young mice collected at 7 dpi (n = 4, Scale bar, 100 μm). j, Quantification of the number of Pax7-positive MuSCs per each tissue section (n = 4). k, Old mice were given vehicle or MTA in drinking water, and MuSCs from each mouse were subjected to SAM ELISA (n = 4). l, (Top) Representative immunostaining of regenerating muscle fibers from old mice treated with vehicle or MTA (Scale bar, 100 μm). (Bottom) Quantification of the mean CSAs of myofibers with centrally located nuclei in TA muscle sections (n = 3). m, Grip force measured without injury (n = 4). n-o, Stance time (n) and stride time (o) were measured at 14 dpi (n = 8 for young mice, n = 7 for old mice treated with vehicle or SAM, n = 6 for old mice treated with MCHA). Data are shown as mean ± SD (a-c, f-h, j-o). P values were calculated by two-sided unpaired Student’s t-tests (e-g) and by one-sided student’s t test, pairwise between groups (a-c, f-h, j-o). *P < 0.05; **P < 0.01; ***P < 0.001.
Supplementary information
Supplementary Information
Supplementary Tables 1–3
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed western blots. Statistical source data.
Source Data Extended Data Fig. 4
Unprocessed western blots. Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Unprocessed western blots. Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, J., Benjamin, D.I., Kim, S. et al. Depletion of SAM leading to loss of heterochromatin drives muscle stem cell ageing. Nat Metab 6, 153–168 (2024). https://doi.org/10.1038/s42255-023-00955-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-023-00955-z