Abstract
Salmonella enterica persist in the chicken gut by suppressing inflammatory responses via expansion of intestinal regulatory T cells (Tregs). In humans, T cell activation is controlled by neurochemical signaling in Tregs; however, whether similar neuroimmunological signaling occurs in chickens is currently unknown. In this study, we explore the role of the neuroimmunological axis in intestinal Salmonella resistance using the drug reserpine, which disrupts intracellular storage of catecholamines like norepinephrine. Following reserpine treatment, norepinephrine release was increased in both ceca explant media and Tregs. Similarly, Salmonella killing was greater in reserpine-treated explants, and oral reserpine treatment reduced the level of intestinal Salmonella Typhimurium and other Enterobacteriaceae in vivo. These antimicrobial responses were linked to an increase in antimicrobial peptide and IL-2 gene expression as well as a decrease in CTLA-4 gene expression. Globally, reserpine treatment led to phosphorylative changes in epidermal growth factor receptor (EGFR), mammalian target of rapamycin (mTOR), and the mitogen-associated protein kinase 2(MEK2). Exogenous norepinephrine treatment alone increased Salmonella resistance, and reserpine-induced antimicrobial responses were blocked using beta-adrenergic receptor inhibitors, suggesting norepinephrine signaling is crucial in this mechanism. Furthermore, EGF treatment reversed reserpine-induced antimicrobial responses, whereas mTOR inhibition increased antimicrobial activities, confirming the roles of metabolic signaling in these responses. Finally, MEK1/2 inhibition suppressed reserpine, norepinephrine, and mTOR-induced antimicrobial responses. Overall, this study demonstrates a central role for MEK1/2 activity in reserpine induced neuro-immunometabolic signaling and subsequent antimicrobial responses in the chicken intestine, providing a means of reducing bacterial colonization in chickens to improve food safety.
Similar content being viewed by others
Introduction
Poultry products are the primary vehicle for broad-host, nontyphoidal Salmonella enterica contamination and foodborne disease in the United States1,2, causing 1.35 million infections and costing approximately $400 million annually3. Although extensive efforts have been made to minimize Salmonella incidence in poultry via antimicrobials, the spread of resistance genes has caused an emergence of Salmonella isolates resistant to essential antibiotics3,4. Furthermore, live Salmonella vaccines and probiotics are commonly implemented as prophylactics in commercial poultry to reduce Salmonella load, however, their individual efficacies against Salmonella resistance are inconsistent5,6,7. Altogether, current methods are insufficient in the reduction of Salmonella in chickens, suggesting that a deeper understanding of biological factors affecting Salmonella colonization is needed to develop more successful treatments.
In chickens, broad host Salmonella serovars induce an immunotolerant state in the chicken intestine via increased regulatory T cells (Tregs), which suppress the inflammatory immune responses necessary to clear Salmonella8,9. Thus, interfering with Treg activities in the gut may improve antibacterial responses against Salmonella. A largely-understudied field in chicken biology is neuroimmunology, or the interactions between the nervous and immune systems10. The intestine is highly-innervated with neurons and immune cell populations, which can then interact via neurochemical signaling11. In mammals, Tregs synthesize their own stores of catecholamine neurochemicals like norepinephrine, and disrupting these intracellular stores via reserpine inhibits Treg function12. However, whether chicken Tregs have similar neurochemical stores and if they too are affected by reserpine have not yet been investigated.
In this report, we found that reserpine causes the release of intracellular norepinephrine stores from chicken ceca explants and intestinal Tregs, driving increased antimicrobial responses against Salmonella. These ex vivo antimicrobial responses were recapitulated in vivo, as birds orally treated with reserpine exhibited reduced gut Enterobacteriaceae and Salmonella post-challenge compared to control birds. Furthermore, we found that reserpine treatment induced T cell activation, reduced CTLA-4 gene expression, and deactivated metabolic pathways like epidermal growth factor receptor (EGFR) signaling and mammalian target of rapamycin (mTOR) signaling, which were linked to antimicrobial responses. Lastly, we found that MEK1/2 activation plays a central role in reserpine-induced antimicrobial activities.
Results
Reserpine treatment induces norepinephrine release from intestinal cells
In an intestinal explant model13 (Supplementary Fig. 1), we demonstrated neurochemical release in ceca tissues at 1 h post-reserpine treatment (1 µM) using ultra-high-performance liquid chromatography (UHPLC). Culture media from reserpine-treated explants had increased levels of norepinephrine and no changes in serotonergic metabolites compared to controls (Fig. 1a). However, this norepinephrine release did not induce inflammatory damage in the explants, as pathological scores were statistically identical between groups (Supplementary Fig. 2a). Using flow cytometry to sort lymphocyte populations (Fig. 1b) potentially responsible for norepinephrine release in the ceca, Tregs (i.e., CD4+CD25+) had significantly greater intracellular norepinephrine stores versus naïve T helper (TH) cells (i.e., CD4+CD25−), and reserpine treatment reduced intracellular norepinephrine levels in Tregs alone (Fig. 1c). However, intracellular stores of serotonergic metabolites were unaffected by reserpine treatment (Supplementary Fig. 2b, c).
Neurochemical release from explants (a) and sorted T cells (b, c) was evaluated via UHPLC. Reserpine treatment (1 µM) increased bactericidal responses against Salmonella in explants (d) regardless of serovar. Significant differences indicated by asterisks: *P < 0.05; **P < 0.01; ***P < 0.001. Error bars indicate the standard deviation above and below the mean.
Reserpine treatment increases Salmonella resistance in ex vivo and in vivo conditions
In ceca explants from 21-day-old birds, supernatant from the reserpine-treated group had higher killing ability against Salmonella compared to that of control explants regardless of strains tested, e.g., Salmonella Typhimurium and Salmonella Kentucky (Fig. 1d). However, reserpine itself was not bactericidal (Supplementary Fig. 2d), confirming that Salmonella killing was mediated by host factors. To test in vivo reserpine-induced antimicrobial responses, we orally treated chickens with 0, 0.5, or 5 mg reserpine/kg body weight from 1 to 3 days post-hatch (dph). Reserpine treatment at either concentration did not affect the chicken weight gain at pre- (Supplementary Fig. 3a) nor post-Salmonella challenge (Supplementary Fig. 3b), nor did oral reserpine treatment induce the significant release of any neurochemicals systemically (Supplementary Fig. 3c). Given that reserpine induced antimicrobial responses ex vivo, we predicted reserpine may affect the commensal gut microbiota. However, 16S rRNA sequencing showed that reserpine treatment did not affect the levels of the majority of commensal bacteria in the ceca (Fig. 2a and Supplementary Figs. 4, 5). Nevertheless, antimicrobial responses were clearly observed after birds were challenged with Salmonella Typhimurium UK-1. At two days post-Salmonella challenge, fecal shedding of total Enterobacteriaceae and Salmonella was significantly reduced by reserpine treatment regardless of concentration (Fig. 2b). Similarly, total Enterobacteriaceae and Salmonella CFUs in ceca content were reduced by reserpine treatment at four days post-challenge (Fig. 2c). In addition to colonizing the chicken intestine, broad host Salmonella strains like UK-1 have the capacity to invade internal organs in young birds14. Here, Salmonella Typhimurium UK-1 was detected in ceca, spleen, and bursa but not in the liver of challenged birds. Although Salmonella levels were statistically identical between groups in the bursa, reserpine treatment significantly reduced Salmonella levels in the spleen (Supplementary Fig. 6). Furthermore, reserpine treatment did not induce pathological inflammation at any concentration in the small intestine nor ceca (Supplementary Fig. 7), and ceca goblet cell numbers were significantly increased by reserpine treatment (Fig. 3). This is in line with a previous study demonstrating that, in mammals, reserpine treatment increases the production of intestinal mucus15,16, which is synthesized by goblet cells in the epithelium17.
Oral reserpine treatment did not dramatically affect the composition of the commensal ceca microbiome at the genera level (a). However, post-Salmonella Typhimurium UK-1 challenge, reserpine treatment reduced total Enterobacteriaceae and S. Typhimurium UK-1 (b, c). Significant differences indicated by asterisks: *P < 0.05; **P < 0.01; ***P < 0.001. Error bars indicate the standard deviation above and below the mean.
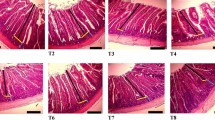
Representative images of Alcian blue staining in ceca tissues (a). Total calculations of goblet cells/villus edge length (mm) (b). Scale is indicated by white bar (bottom right corner per image; 50 µm). Significant differences indicated by asterisks: **P < 0.01; ***P < 0.001. Error bars indicate the standard deviation above and below the mean.
Reserpine treatment increases antimicrobial peptide expression while decreasing CTLA-4 expression
To determine underlying mechanisms responsible for improved antimicrobial responses upon reserpine treatment, we measured genes expression through transcriptional changes via RT-qPCR. Expression of the regulatory cytokine IL-1018 was unchanged (Fig. 4a); however, the expression of CTLA-4, a surface-bound protein associated with Tregs that downregulates immune responses19, was downregulated in reserpine-treated explants versus controls (Fig. 4A). In line with this downregulated immunosuppressive factor, reserpine treatment increased gene expression of antimicrobial peptides (AMPs) like beta defensin 12 (BD-12), BD-14, and fowlicidin 1 (Fowl-1) versus controls (Fig. 4a). Furthermore, the expression of IL-2, a cytokine released by activated T cells20,21, was also increased by reserpine treatment versus control (Fig. 4a).
AMP and IL-2 gene expression was increased by reserpine treatment while CTLA-4 gene expression was decreased (a). Norepinephrine treatment alone increased anti-Salmonella responses in explants (b), and the effect of reserpine was blocked using beta-adrenergic receptor inhibitors ICI-118551 (β2) or L-748337 (β3) (c). Reserpine-induced antibacterial activities were inhibited by MEK1/2 kinase inactivation and EGF treatment (d), and rapamycin-induced bactericidal responses are partially dependent on MEK1/2 signaling (e). Finally, norepinephrine-induced bactericidal responses are dependent on MEK1/2 signaling (f). Significant differences indicated by asterisks: *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Error bars indicate the standard deviation above and below the mean.
Reserpine-treated explants undergo large immunometabolic shifts
To determine what global immunometabolic pathways were affected by reserpine, we used a chicken-specific kinome peptide array, which measures changes in phosphorylation activities within several signaling pathways22. Overall, reserpine treatment altered several immunological and metabolic pathways (Table 1). In total, 414 proteins from the top 25 KEGG pathways were differentially phosphorylated upon reserpine treatment (Table 1). Within these pathways, several were involved in the EGFR signaling pathway and T cell receptor (TCR) signaling pathway, and these pathways were further analyzed. EGFR was dephosphorylated at the Tyr869 residue (Table 2). Furthermore, in the EGFR signaling pathway, mTOR was phosphorylated at Ser2448 and Thr2446 but was dephosphorylated at Ser2481 (Table 2). Uniquely, mitogen-activated protein (MAP) kinase 2 (MEK2), a component of the MEK1/2 signaling pathway23, was phosphorylated at the Ser306 residue (Table 2), important for MEK2 activation24. Similarly, MEK2 is also involved in the TCR signaling pathway, in which CD28, a T cell co-receptor crucial for T cell activation25, was phosphorylated (Table 2).
Reserpine-induced antimicrobial responses are dependent on norepinephrine and metabolic signaling
Given that reserpine treatment (1) increased intracellular norepinephrine release and (2) induced changes in EGFR and mTOR phosphorylation, we investigated the roles of these pathways in antimicrobial responses. Explants treated with norepinephrine alone similarly induced antibacterial responses in a dose-dependent manner (Fig. 4b), which was blocked by inhibiting beta-adrenergic receptors 2 and 3 (Fig. 4c). Treatment of explants with recombinant EGF alone prevented reserpine-induced antimicrobial responses (Fig. 4d). However, treatment with EGFR inhibitor AG1478 alone did not trigger any antimicrobial responses (Fig. 4d). Additionally, treatment of explants with rapamycin, an inhibitor of the mTOR pathway, increased bactericidal responses (Fig. 4e). Overall, these findings demonstrate that reserpine treatment induces antimicrobial responses through multiple signaling pathways.
MEK1/2 signaling plays a central role in reserpine-induced antimicrobial responses
In our kinome analyses, we found that these immunometabolic signaling changes were associated with MEK2 phosphorylation, suggesting MEK1/2 signaling plays a vital role in these responses. Using the MEK1/2 signaling inhibitor U0126, MEK1/2 signaling inhibition reversed the antimicrobial response induced by reserpine (Fig. 4d). Similarly, antimicrobial responses in rapamycin-treated explants were partially reversed upon MEK1/2 inhibition (Fig. 4e). Finally, antimicrobial responses in norepinephrine-treated explants were reversed upon MEK1/2 inhibition (Fig. 4f). Overall, these data demonstrate a central role for MEK1/2 signaling in the antimicrobial response induced by reserpine and other neuro-immunometabolic signaling pathways.
Discussion
Chicken products like meat and eggs are primary vehicles for salmonellosis1,2. Reducing Salmonella colonization in the chicken intestine is paramount to mitigating salmonellosis in humans. In this study, we demonstrate that reserpine treatment releases intracellular stores of norepinephrine and induces significant changes in chicken ceca immunometabolism, resulting in increased antibacterial responses against Salmonella. The ex vivo explant model used in this study allows for preserving the totality of intestinal cell populations present in vivo while maintaining spatial organization, which provides a more accurate representation of in vivo conditions13. In support of the utility of this model, we found that reserpine treatment induces antimicrobial responses against Salmonella ex vivo and in vivo. In our study, reserpine treatment increased the expression of several AMPs, including beta-defensins 12 and 14 as well as fowlicidin-1. Beta-defensins are crucial to regulating the gut microbiota and homeostasis26. Thus, strategies that increase host beta-defensin production are viable replacements for antibiotic treatment27. Although these molecules are directly bactericidal, they have additional functions as well. For example, fowlicidin-1 can neutralize bacterial lipopolysaccharide (LPS)28, a microbe-associated molecular pattern that potently induces inflammation29. Furthermore, beta-defensins reduce intestinal apoptotic signals in LPS-treated animals30. Thus, improving the production of these AMPs may both increase resistance against bacterial pathogens, as well as mitigate host damage induced by these antibacterial responses. In support of this, we found no differences in pathological scores between groups despite a clear elevation in immunological responses in reserpine-treated explants. However, the transcriptional factors responsible for reserpine-induced antimicrobial peptide production are unclear at this time. Activation of the transcription factor c-FOS increases antimicrobial responses in macrophages31 while suppressing excessive inflammatory responses32,33,34. Given these findings were reflected in our study, reserpine-induced c-FOS activation may be driving these antimicrobial responses, although this remains to be determined.
This reserpine-driven increase in AMP production was associated with increased IL-2 expression and reduced CTLA-4 expression. Upon activation of naïve T cells, IL-2 production is increased, which induces further T cell proliferation, promotes CD4+ differentiation, and facilitates effector and memory CD8+ T cell formation20. This activation process is dependent on the interaction between costimulatory ligand CD28, expressed on naïve T cells, and CD80/86, expressed on antigen-presenting cells (APCs)35. However, Tregs can interfere with this interaction via CTLA-4, which outcompetes CD28 for CD80/86 binding, inhibiting IL-2 accumulation and thus preventing T cell activation25,36. One of the mechanisms in which Salmonella persists in the chicken gut is by increasing intestinal Tregs, which prevents the inflammatory responses necessary to clear Salmonella9. Thus, we hypothesized that reserpine treatment could inactivate chicken Tregs as shown in human Tregs12, which would permit anti-Salmonella responses in the gut. As expected, reserpine decreased CTLA-4 expression, which is constitutively expressed on Tregs37. We found that CD28 was phosphorylated in reserpine-treated explants, suggesting that CD28 activation and IL-2 production were occurring due to reduced CTLA-4 levels. Furthermore, NFATC1 (but not NFATC2) was phosphorylated upon reserpine treatment. Activation of these transcription factors has been linked to IL-2 production in memory CD4+ T cells38, suggesting that reserpine is increasing IL-2 gene expression through NFATC1 activation.
One notable observation is that reserpine treatment in vivo did not dramatically change the resident gut microbiota in young birds. The gut microbiota is crucial to proper animal development, driving immune and physiological maturation39,40. Furthermore, antibiotic treatment in young animals causes dramatic changes in their gut microbiota41, which can predispose them to bacterial infection and physiological dysfunction later in life by depleting populations of gut microbes crucial for normal development42,43. Thus, oral reserpine treatment in day-old birds is a feasible way to promote resistance against Salmonella without negatively affecting the developing gut microbiota, although the long-term effects of early-age reserpine treatment on the gut microbiota are unclear. Although changes in Fusobacteria, Lactobacillaceae, and Erysipelotrichaceae were induced by reserpine treatment, these changes were not consistent between reserpine-treated groups and did not appear to be associated with any biological parameter measured in this study (antimicrobial responses, inflammation, mucus production, etc). Thus, the biological impact of these specific shifts in the commensal microbiota is unclear and does not contribute to the host responses investigated in this study. Still, this lack of antimicrobial activity may appear to contrast the reserpine-induced antimicrobial responses seen in our ex vivo explant model. In birds, innately-produced gallinacins are the primary AMPs produced in the intestine at post-hatch, peaking at days 1–3 post-hatch and begin to drop by day 4 post-hatch, in which AMPs controlled through the adaptive immune system become dominant in the chicken intestine44. In our study, explants from 21-day-old birds were used to assess reserpine efficacy, in which these intestinal explants would have a more-mature adaptive immune system. Thus, reserpine-induced antimicrobial responses appear to be dependent on the adaptive immune system. This is supported by our finding that reserpine induces norepinephrine release in chicken intestinal Tregs, which coincidentally migrate from the thymus to the chicken intestine around day four post-hatch45,46,47. Dhawan and colleagues (2016) determined that specific subsets of intestinal Tregs are crucial for regulating AMP responses48, although the subset of Tregs responsible for this mechanism in chickens is still unclear and warrant further investigation. In humans, reserpine inhibits intracellular vesicle storage of catecholamines such as norepinephrine, which induce autocrine/paracrine signaling loops that suppress Treg function and stimulate immune activation12. In this study on chickens, reserpine treatment increased norepinephrine release from both explants and intestinal Tregs. Thus, Tregs at least partially contribute to the total pool of norepinephrine released by intestinal cells. However, in our hands and due to limited reagents and methods for primary chicken cell cultures, we could not culture chicken intestinal Tregs for longer than six hours, preventing any direct examination of reserpine on Treg immunosuppressive function. However, we did find that treatment with norepinephrine alone at the physiological concentration released after one hour of reserpine treatment could stimulate antibacterial responses, which was dependent on beta-adrenergic receptors. Norepinephrine is a well-known mediator of neuroimmunological responses, inducing cytokine production, cell proliferation, and antibody secretion by lymphocytes49,50 and has been demonstrated to improve antibacterial responses via cross-talk between sympathetic ganglia and resident tissue macrophages51. Overall, the intracellular release of norepinephrine drives antimicrobial responses via autocrine/paracrine signaling of intestinal cell populations. Future work should determine which specific cellular populations (i.e., enterocytes, enteric neurons, APCs) interact with the regulatory T cells involved in this mechanism.
Given the clear immunological stimulation induced by reserpine treatment, we hypothesized that several metabolic pathways might also be affected due to the interplay between host metabolism and the immune system10. To this end, we used the chicken kinome peptide array, which measures immunometabolic signaling at the post-translational level22 and thus enables a more accurate evaluation of which processes are affected by reserpine. EGFR signaling is crucial for goblet cell-associated antigen passage (GAP) formation in the mammalian intestine52, and inhibiting EGFR increases beta-defensin production in intestinal cells in vitro53. In this study, we found that EGFR was dephosphorylated in reserpine-treated explants and using recombinant EGF reversed reserpine-induced antimicrobial responses in vitro, demonstrating the importance of EGFR signaling in this system. However, EGFR inhibition alone did not trigger antimicrobial responses, suggesting that EGFR signaling alone is not sufficient to induce antimicrobial responses. Additionally, the mTOR pathway is conserved among eukaryotic organisms and has received vast attention due to its diverse involvement in nutrient sensing, immunity, and aging in animals54. Rapamycin, originally derived from the soil bacterium Streptomyces hygroscopicus, is commonly used as an mTOR inhibitor40. In this study, reserpine induced differential mTOR phosphorylation at multiple sites upon reserpine treatment. Phosphorylation of S2448 and T2446 is carried out by the kinase S6K55, and pS2448 drives mTORC1 activation56. In this study, mTORC1 may have been activated upon reserpine treatment, as these two mTOR sites, S6K, and raptor (i.e., RPTOR) were all phosphorylated. However, mTORC1 activation does not play a role in these antimicrobial responses, as deactivating mTOR via rapamycin treatment induced similar antimicrobial responses as reserpine treatment. Although these mTOR sites were phosphorylated, S2481 was uniquely dephosphorylated upon reserpine treatment. The sole site for mTOR autophosphorylation57, S2481 has been the only site determined to regulate intrinsic mTOR activities58,59. Thus, S2481 dephosphorylation deactivates mTOR function, and our study finds that mTOR inhibition increases antimicrobial responses in this ceca explant model. This finding is supported by previous work demonstrating rapamycin treatment increases anti-Campylobacter responses in the murine intestine and directly stimulates antimicrobial responses in splenocytes60. Thus, in addition to inducing norepinephrine signaling, reserpine also deactivates EGFR and mTOR, and all three of these pathways contribute to antimicrobial responses in chickens. Given that numerous mTOR sites were phosphorylated and dephosphorylated by reserpine treatment, future studies should look at the individual roles of these sites in antimicrobial responses, which could serve as drug targets to promote bacterial resistance.
Although we identified several pathways that differed in phosphorylation patterns, MEK1/2 signaling is well-established as an essential component of beta-defensin production at mucosal barriers53,61,62. However, MEK1/2 signaling has never been previously described to be involved in reserpine activity. Here, upon reserpine treatment, MEK2 was phosphorylated at S306. Using the inhibitor U0126, we found that inhibiting MEK1/2 signaling reversed reserpine induced antimicrobial responses, as well as those induced by norepinephrine and rapamycin treatment alone, suggesting that pS306 is a central component of this signaling pathway induced by reserpine and is critical to achieving an antimicrobial response.
In summary, we found that reserpine increases AMP production and immune activation in the chicken intestine by inducing norepinephrine release and beta-adrenergic receptor activation. These changes are correlated with reduced CTLA-4 expression, as well as EGFR and mTOR deactivation, and these antimicrobial responses were dependent on MEK1/2 activation. Thus, we propose that targeting the neuroimmunological axis via oral reserpine treatment could be a viable strategy for increasing Salmonella resistance in poultry animals. Furthermore, since oral reserpine treatment also increased resistance against total Enterobacteriaceae populations, this treatment may also increase resistance against other bacterial pathogens.
Materials and methods
Ethics statement
Animal experiments were approved by Iowa State University Institutional Animal Care and Use Committee, Log # 18-386. Animal enrichments were added to open floor pens to minimize stress during experimental procedures. Euthanasia techniques (CO2 asphyxiation) followed the American Veterinary Medical Association Guidelines (2013).
Ceca explant model and treatment
Methods for chicken ceca explant cultures were adapted from an ex vivo colon explant model for mice13 and are summarized in Supplementary Fig. 1. Briefly, 0.1 g tissue pieces from the ceca of 21-day-old chickens were incubated in antibiotic-treated Dulbecco’s modified eagle medium (DMEM) for 30 min at 39.5 °C (5% CO2). Explant tissues were then washed with antibiotic-free DMEM to remove residual antibiotics, individually transferred to 24-well plates, and incubated in DMEM with 0 or 1 µM reserpine for six hours at 39.5 °C (5% CO2). Alternatively, to confirm reserpine-mediated signaling pathways, tissues were incubated with norepinephrine (1.32 mg/ml or 1.32 µg/ml), beta-adrenergic receptor inhibitors ICI-118551 (β2; 1 µM; MedChemExpress, LLC) or L-748337 (β3; 1 µM; R&D Systems), U0126 (MEK1/2 inhibitor; 20 µM; Invivogen), human recombinant EGF (200 ng/ml; Biotang Inc), AG-1478 (EGFR tyrosine kinase inhibitor; 1 µM; BioVision), or rapamycin (mTOR pathway inhibitor; 10 ng/ml or 1000 ng/ml).
Ultra-high-performance liquid chromatography
To assess neurochemical release from explants, media from explant cultures were centrifuged at 12,000 × g for 5 min at 4 °C, and supernatants were pre-treated with 2 M perchloric acid (1:10 dilution), flash-frozen, and stored at −80 °C. Upon thawing, UHPLC with electrochemical detection (UHPLC-EC) was performed on media supernatants as previously described63. To assess neurochemical release from lymphocyte populations, regulatory T cells (CD4+CD25+) or naïve T cells (CD4+CD25−) were sorted via flow cytometry (see methods section for lymphocyte extraction) and treated with 0 or 1 µM reserpine for 30 min at 39.5 °C. Cells were then pelleted via centrifugation at 300 × g for 10 min at 4 °C, and 10 mg pellets were resuspended in 0.2 M perchloric acid, flash-frozen, and stored at −80 °C. Upon thawing, UHPLC-EC was performed on cellular as described earlier.
Intestinal lymphocyte extraction and flow cytometry
T cells were extracted from the chicken lamina propria as previously described64,65. To sort for specific T cell populations, 106−7 cells were resuspended in Zombie violet dye (1:100 solution) and incubated for 20 min at room temperature in the dark. Cells were then centrifuged at 300 × g for 5 min at room temperature, and pellets were resuspended via sorting buffer (PBS with 1% FBS) and incubated for 10 min at 4 °C for a blocking step. Thereafter, cells were centrifuged for 5 min at 300 × g, and then resuspended with 10 µg/ml anti-CD4 and 10 µg/ml anti-CD25 manually conjugated with Alexa-555 or Alexa-488 fluorophores, respectively. Following a 30 min incubation in the dark at 4 °C, cells were then washed with sorting buffer, and viable CD4+CD25+ and CD4+CD25− populations were sorted via FACSAria III (BD Biosciences) at Iowa State University’s core facility. The gating strategy is exemplified in Supplementary File 1.
Bactericidal assays against Salmonella
Following explant incubation, media from individual explants were centrifuged at 12,000 × g for 5 min at 4 °C, and the supernatant was stored at −80 °C until ready for use. S. enterica strains (Supplementary Table 1) were grown overnight on LB agar (0.1% glucose), and individual colonies were added to PBS until OD600 0.1. This inoculum was subsequently diluted in PBS until 102 CFU/100 µl was reached. Explant supernatants were added to Salmonella inoculum at 1:1 ratio and incubated for six hours at 39.5 °C. Solutions were then serially diluted and plated on MacConkey for bacterial enumeration. All bactericidal assays were run in triplicate.
In vivo reserpine treatment and Salmonella challenge
One-day-old white leghorn chicks (Valo BioMedia, IA) were orally treated daily with 0, 0.5, or 5 mg reserpine per kg body weight (100 µl) for three days. At four days old, chicks were orally inoculated with 100 µl (109 CFU) Salmonella Typhimurium strain UK-1 (Supplementary Table 1). Prior to reserpine treatment and Salmonella challenge, birds were fasted from food and water for at least 4 h, and food and water were returned to pens 30 min post-treatment and challenge, respectively. Two days post-challenge, feces were serially diluted and plated onto MacConkey agar for Enterobacteriaceae and Salmonella enumeration. Four days post-challenge, ceca contents, spleen, liver, and bursa were collected from each bird, homogenized, and plated onto MacConkey agar. Chicken weights were collected daily throughout the study.
DNA isolation and 16S rRNA sequencing
Total DNA was isolated from ceca contents (homogenized from both cecal loops per bird; n = 9 or 10 per treatment group) using the DNeasy Powerlyzer PowerSoil Kit (Qiagen). Extracted DNAs were determined for quality via NanoDrop 2000 spectrophotometer (A260/A280). Concentrations were then determined via Qubit dsDNA broad range kit (Thermo Fisher Scientific. DNAs were used for library preparation using the MiSeq and HiSeq2500 kit (Illumina) following all the manufacturer’s instructions with 151 × 151 paired-end MiSeq sequencing (Illumina). 16S rRNA sequencing was performed at the Iowa State DNA facility using Illumina MiSeq (v3). For sequence analysis, using the QIIME2 (version 2019.10) pipeline, sequences were demultiplexed using the demux emp-paired function and denoised using the plugin DADA2. SILVA database at the 99% operational taxonomic units (OTUs) spanning the V4-V5 16S rRNA region (806R: CAAGCAGAAGACGGCATACGAGATAGTCAGCCAGCCGGACTACNVGGGTWTCTAAT; 515F: AATGATACGGCGACCACCGAGATCTACACGCTXXXXXXXXXXXXTATGGTAATTGTGTGYCAGCMGCCGCGGTAA) was used to classify each of the reads using QIIME2’s feature-classifier function. For more details, please refer to the GitHub repository at ISUgenomics/2021_Aug_MelhaMellata_reserpine: reserpine study (github.com). The 16S rRNA dataset is available in the NCBI Sequence Read Archive (SRA) repository with accession BioProject ID PRJNA755726.
Intestinal pathology scoring and goblet cell enumeration
Explants were placed into 4% paraformaldehyde (PFA) and stored at RT. Subsequently, 5 μm paraffin-embedded cross-sections were stained with hematoxylin and eosin (H&E) to assess gut inflammation. Parameters measuring inflammation (i.e., focal, multifocal, diffuse), infiltrate (i.e., presence of heterophils, lymphocytes, macrophages as well as hemorrhages), necrosis (i.e., focal, multifocal, diffuse), and location (i.e., lamina propria, villous lamina propria, crypt lamina propria) were used. All analyses were performed by a certified pathologist at Iowa State University. To enumerate goblet cells in ceca tissue, sections were stained with Alcian blue to enumerate goblet cells. To quantify goblet cell numbers per villus edge length, the length of the intestinal epithelium was measured using computer software. Goblet cells were then individually counted and divided by villus edge length. Counting on five replicate sections was performed per bird, and 8–10 birds were analyzed per treatment group.
RT-qPCR
Total RNA was extracted from explant tissues using the PureLink RNA Mini Kit (Life Technologies), and high quality RNAs (A260/A280 ratios ~ 2.0) were assessed via Nanodrop 2000 and quantified via Qubit 2.0 Fluorometer. Reverse transcription assays were performed via High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher) to attain cDNA. Thereafter, SYBR Green (Thermo Scientific) three-step cycling qPCR reactions were performed on StepOnePlus for individual genes (Supplementary Table 2) for 45 cycles. Differences in gene expression were assessed via 2−ΔΔCt method using the housekeeping gene encoding glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a control66.
Chicken-specific immunometabolic kinome peptide array
Following incubation, ceca explants were flash-frozen, stored at −80 °C, and transported overnight on dry ice to the University of Delaware. Peptide array protocol and analyses were carried out as previously described22. The resulting data output was then used in downstream applications such as STRING67 and KEGG54 databases used to pinpoint changes in the protein−protein interactions and signal transduction pathways.
Statistics and reproducibility
Statistical comparisons for UHPLC and Salmonella resistance data were performed via Student’s t-test or one-way ANOVA on GraphPad Prism software. For the kinome array, signal intensities from scanned array images were arranged into the PIIKA2 input format in Excel, and resultant data were subsequently analyzed via PIIKA2 peptide array analysis software (http://saphire.usask.ca/saphire/piika/index.html). After normalizing these data, we performed comparisons between reserpine-treated and un-treated explants, calculating fold change (= treatment/control) and a significance P-value, which was calculated by conducting a one-sided paired t-test between treatment and control values for a given peptide. The resultant fold change and significance values were used to generate optional pathway analysis via standard R statistical functions or online analysis platforms. All in vivo experiments were done in duplicate, and in vitro experiments were performed in triplicate.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The 16S rRNA dataset is available in the NCBI Sequence Read Archive (SRA) repository with accession BioProject ID PRJNA755726. Raw kinome data are provided in Supplementary Data 1.
References
Batz, M. B., Hoffmann, S. & Morris, J. G. Ranking the disease burden of 14 pathogens in food sources in the United States using attribution data from outbreak investigations and expert elicitation. J. Food Prot. 75, 1278–1291 (2012).
Antunes, P., Mourao, J., Campos, J. & Peixe, L. Salmonellosis: the role of poultry meat. Clin. Microbiol. Infect. 22, 110–121 (2016).
Center for Disease Control and Prevention. Antibiotic Resistance Threats Report. https://www.hhs.gov/sites/default/files/michael-craig-cdc-talk-thursday-am-508.pdf (2019).
Fricke, W. F. et al. Antimicrobial resistance-conferring plasmids with similarity to virulence plasmids from avian pathogenic Escherichia coli strains in Salmonella enterica Serovar Kentucky isolates from poultry. Appl. Environ. Microbiol. 75, 5963–5971 (2009).
Redweik, G. A. J. R., Stromberg, Z. R., Van Goor, A. & Mellata, M. Protection against avian pathogenic Escherichia coli and Salmonella Kentucky exhibited in chickens given both probiotics and live Salmonella vaccine. Poult. Sci. 99, 752–762 (2002).
McWhorter, A. R. & Chousalkar, K. K. A long-term efficacy trial of a live, attenuated Salmonella Typhimurium vaccine in layer hens. Front. Microbiol. 9, 1380 (2018).
Redweik, G. A. J. R., Jochum, J. & Mellata, M. Live bacterial prophylactics in modern poultry. Front. Vet. Sci. 7, 592312 (2020).
Kogut, M. H. & Arsenault, R. J. Immunometabolic phenotype alterations associated with the induction of disease tolerance and persistent asymptomatic infection of Salmonella in the chicken intestine. Front. Immunol. 8, 372 (2017).
Shanmugasundaram, R. et al. Effect of Salmonella infection on cecal tonsil regulatory T cell properties in chickens. Poult. Sci. 94, 1828–1835 (2015).
Nutma, E., Willison, H., Martino, G. & Amor, S. Neuroimmunology—the past, present, and future. Clin. Exp. Immunol. 197, 278–293 (2019).
Brinkman, D. J., Ten Hove, A. S., Vervoordeldonk, M. J., Luyer, M. D. & de Jonge, W. J. Neuroimmune interactions in the gut and their significance for intestinal immunity. Cells 8, 670 (2019).
Cosentino, M. et al. Human CD4+CD25+ regulatory T cells selectively express tyrosine hydroxylase and contain endogenous catecholamines subserving an autocrine/paracrine inhibitory functional loop. Blood 109, 632–642 (2007).
Udden, S. M., Waliullah, S., Harris, M. & Zaki, H. The ex vivo colon organ culture and its use in antimicrobial host defense studies. J. Vis. Exp. 120, 55347 (2017).
Hassan, J. O. & Curtiss, R. Virulent Salmonella typhimurium-induced lymphocyte depletion and immunosuppression in chickens. Infect. Immun. 62, 2027–2036 (1994).
Forstner, J., Roomi, N., Khorasani, R., Kuhns, W. & Forstner, G. Effect of reserpine on the histochemical and biochemical properties of rat intestinal mucus. Exp. Mol. Pathol. 54, 129–143 (1991).
Park, C., Reid, P. E., Owen, D. A., Sanker, J. M. & Applegarth, D. A. Morphological and histochemical changes in intestinal mucus in the reserpine-treated rat model of cystic fibrosis. Exp. Mol. Pathol. 47, 1–12 (1987).
Kim, Y. S. & Ho, S. B. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr. Gastroenterol. Rep. 12, 319–330 (2010).
Couper, K. N., Blount, D. G. & Riley, E. M. IL-10: the master regulator of immunity to infection. J. Immunol. 180, 5771–5777 (2008).
Wing, K. et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science 322, 271–275 (2008).
Liao, W., Lin, J. X. & Leonard, W. J. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 38, 13–25 (2013).
Pol, J. G., Caudana, P., Paillet, J., Piaggio, E. & Kroemer, G. Effects of interleukin-2 in immunostimulation and immunosuppression. J. Exp. Med. 217, e20191247 (2020).
Arsenault, R. J., Trost, B. & Kogut, M. H. A comparison of the chicken and turkey proteomes and phosphoproteomes in the development of poultry-specific immuno-metabolism kinome peptide arrays. Front. Vet. Sci. 1, 22 (2014).
Zheng, C. F. & Guan, K. L. Cloning and characterization of two distinct human extracellular signal-regulated kinase activator kinases, MEK1 and MEK2. J. Biol. Chem. 268, 11435–11439 (1993).
Procaccia, S., Ordan, M., Cohen, I., Bendetz-Nezer, S. & Seger, R. Direct binding of MEK1 and MEK2 to AKT induces Foxo1 phosphorylation, cellular migration, and metastasis. Sci. Rep. 7, 43078 (2017).
Rudd, C. E., Taylor, A. & Schneider, H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol. Rev. 229, 12–26 (2009).
Meade, K. G. & O’Farrelly, C. β-Defensins: farming the microbiome for homeostasis and health. Front. Immunol. 9, 3072 (2018).
Sechet, E., Telford, E., Bonamy, C., Sansonetti, P. J. & Sperandio, B. Natural molecules induce and synergize to boost expression of the human antimicrobial peptide β-defensin-3. Proc. Natl Acad. Sci. USA 115, 9869–9878 (2018).
Xiao, Y. et al. Identification and functional characterization of three chicken cathelicidins with potent antimicrobial activity. J. Biol. Chem. 281, 2858–2867 (2006).
Kogut, M. H., He, H. & Kaiser, P. Lipopolysaccharide binding protein/CD14/TLR4-dependent recognition of Salmonella LPS induces the functional activation of chicken heterophils and up-regulation of pro-inflammatory cytokine and chemokine gene expression in these cells. Anim. Biotechnol. 16, 165–181 (2005).
Xie, K. et al. β-Defensin 129 attenuates bacterial endotoxin-induced inflammation and intestinal epithelial cell apoptosis. Front. Immunol. 10, 2333 (2019).
Hop, H. et al. The key role of c-FOS for immune regulation and bacterial dissemination in Brucella infected macrophage. Front. Cell. Infect. Microbiol. 8, 287 (2018).
Ray, N. et al. C-Fos suppresses systemic inflammatory response to endotoxin. Int. Immunol. 18, 671–677 (2006).
Okada, S., Obata, S., Hatano, M. & Tokuhisa, T. Dominant negative effect of the c-fos family gene products on inducible NO synthase expression in macrophages. Int. Immunol. 15, 1275–1282 (2003).
Maruyama, K. et al. c-Fos-deficient mice are susceptible to Salmonella enterica serovar Typhimurium infection. Infect. Immun. 75, 1520–1523 (2007).
Appleman, L. J., Berezovskaya, A., Grass, I. & Boussiotis, V. A. CD28 costimulation mediates T cell expansion via IL-2-independent and IL-2-dependent regulation of cell cycle progression. J. Immunol. 164, 144–151 (2000).
Krummel, M. F. & Allison, J. P. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J. Exp. Med. 183, 2533–2540 (1996).
Walker, L. S. Treg and CTLA-4: two intertwining pathways to immune tolerance. J. Autoimmun. 45, 49–57 (2013).
Dienz, O. et al. Accumulation of NFAT mediates IL-2 expression in memory, but not naïve, CD4+ T cells. Proc. Natl Acad. Sci. USA 104, 7175–7180 (2007).
Wall, R. et al. Role of gut microbiota in early infant development. Clin. Med. Pediatr. 3, 45–54 (2009).
Bauer, E., Williams, B. A., Smidt, H., Verstegen, M. W. A. & Mosenthin, R. Influence of the gastrointestinal microbiota on development of the immune system in young animals. Curr. Issues Intest. Microbiol. 7, 35–51 (2006).
Videnska, P. et al. Chicken faecal microbiota and disturbances induced by single or repeated therapy with tetracycline and streptomycin. BMC Vet. Res. 9, 30 (2013).
Lynn, M. A. et al. Early-life antibiotic-driven dysbiosis leads to dysregulated vaccine immune responses in mice. Cell Host Microbe 23, 653–660 (2018).
Gasparrini, A. J. et al. Persistent metagenomic signatures of early-life hospitalization and antibiotic treatment in the infant gut microbiota and resistome. Nat. Microbiol. 4, 2285–2297 (2019).
Crhanova, M. et al. Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar enteritidis infection. Infect. Immun. 79, 2755–2763 (2011).
Bar-Shira, E., Sklan, D. & Friedman, A. Establishment of immune competence in the avian GALT during the immediate post-hatch period. Dev. Comp. Immunol. 27, 147–157 (2003).
Lillehoj, H. S. & Chung, K. S. Postnatal development of T-lymphocyte subpopulations in the intestinal intraepithelium and lamina propria in chickens. Vet. Immunol. Immunopathol. 31, 347–360 (1992).
Van Immerseel, F. Dynamics of immune cell infiltration in the caecal lamina propria of chickens after neonatal infection with a Salmonella enteritidis strain. Dev. Comp. Immunol. 26, 355–364 (2002).
Dhawan, S. et al. Acetylcholine-producing T cells in the intestine regulate antimicrobial peptide expression and microbial diversity. Am. J. Physiol. Gastrointest. Liver Physiol. 311, G920–G933 (2016).
Kasprowicz, D. J. et al. Stimulation of the B cell receptor, CD86 (B7-2), and the beta 2-adrenergic receptor intrinsically modulates the level of IgG1 and IgE produced per B cell. J. Immunol. 165, 680–690 (2000).
Kohm, A. P. & Sanders, V. M. Norepinephrine and beta 2-adrenergic receptor stimulation regulate CD4+ T and B lymphocyte function in vitro and in vivo. Pharmacol. Rev. 53, 487–525 (2001).
Gabanyi, I. et al. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell 164, 378–391 (2016).
Knoop, K. A. et al. Maternal activation of the EGFR prevents translocation of gut-residing pathogenic Escherichia coli in a model of late-onset neonatal sepsis. Proc. Natl Acad. Sci. USA 117, 7941–7949 (2020).
Bonamy, C. et al. Expression of the human antimicrobial peptide β-defensin-1 is repressed by the EGFR-ERK-MYC axis in colonic epithelial cells. Sci. Rep. 8, 18043 (2018).
Saxton, R. & Sabatini, D. mTOR signaling in growth, metabolism, and disease. Cell 168, 960–976 (2017).
Chiang, G. G. & Abraham, R. T. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J. Biol. Chem. 280, 25485–25490 (2010).
Rosner, M. et al. mTOR phosphorylated at S2448 binds to raptor and rictor. Amino Acids 38, 223–228 (2010).
Foster, K. G. & Fingar, D. C. Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony. J. Biol. Chem. 285, 14071–14077 (2010).
Soliman, G. A., Acosta-Jaquez, H. A. & Dunlop, E. A. mTOR Ser-2481 autophosphorylation monitors mTORC-specific catalytic activity and clarifies rapamycin mechanism of action. J. Biol. Chem. 285, 7866–7879 (2010).
Caron, E. et al. A comprehensive map of the mTOR signaling network. Mol. Syst. Biol. 6, 435 (2010).
Sun, X., Threadgill, D. & Jobin, C. Campylobacter jejuni induces colitis through activation of mammalian target of rapamycin signaling. Gastroenterol 142, 86–95.e85 (2012).
Krisanaprakornkit, S., Kimball, J. R. & Dale, V. A. Regulation of human beta-defensin-2 in gingival epithelial cells: the involvement of mitogen-activated protein kinase pathways, but not the NF-kappaB transcription factor family. J. Immunol. 168, 316–324 (2002).
Moon, S. K. et al. Activation of a Src-dependent Raf-MEK1/2-ERK signaling pathway is required for IL-1alpha-induced upregulation of beta-defensin 2 in human middle ear epithelial cells. Biochem. Biophys. Acta 1590, 41–51 (2002).
Villageliú, D. N., Rasmussen, S., & Lyte, M. A microbial endocrinology-based simulated small intestinal medium for the evaluation of neurochemical production by gut microbiota. FEMS Microbiol. Ecol. 94, fiy096 (2018).
Qiu, Z. & Sheridan, B. S. Isolating lymphocytes from the mouse small intestinal immune system. J. Vis. Exp. 132, 57281 (2018).
Trapecar, M. et al. An optimized and validated method for isolation and characterization of lymphocytes from HIV+ human gut biopsies. AIDS Res. Hum. Retroviruses 33, S31–S39 (2017).
Achanta, M. et al. Tissue expression and developmental regulation of chicken cathelicidin antimicrobial peptides. J. Anim. Sci. Biotechnol. 3, 15 (2012).
Szklarczyk, D. et al. STRING v10: protein−protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 43, D447–D452 (2015).
Acknowledgements
This research was supported by Iowa State University Start-up funding and the United States Department of Agriculture (USDA) Hatch project IOW03902 (MM), USDA-National Institute of Food and Agriculture project #021069-00001 (GR); W.E. Lloyd Chair in Toxicology funding (ML), and USDA-Agricultural Research Service Project #3091-32000-034-00 (MK). The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication. Mention of commercial products is for the sole purpose of providing specific information, not a recommendation nor endorsement by the USDA. Additionally, the authors thank Jack Peterson and Sasha Celada (Iowa State University undergraduates) and the Genome Informatics Facility (Iowa State University) for technical assistance in this study.
Author information
Authors and Affiliations
Contributions
G.R. and M.M. conceived and designed the experiments, as well as wrote the manuscript. G.R., M.K., R.A., and M.M. performed the experiments, analyzed the data, and revised the manuscript. R.A., M.K., M.L., and M.M. contributed reagents, materials, and analysis tools. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review information
Communications Biology thanks Dipshikha Chakravortty and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Sridhar Mani and Karli Montague-Cardoso.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Redweik, G.A.J., Kogut, M.H., Arsenault, R.J. et al. Reserpine improves Enterobacteriaceae resistance in chicken intestine via neuro-immunometabolic signaling and MEK1/2 activation. Commun Biol 4, 1359 (2021). https://doi.org/10.1038/s42003-021-02888-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-021-02888-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.