Abstract
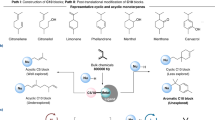
Monoterpenoids are a class of isoprenoids produced from geranyl diphosphate by various monoterpene synthases. Nature has evolved over millions of years to produce various cyclic monoterpenoids. Herein, we present a serendipitous creation of an unnatural monoterpene skeleton through heteroarylative telomerization of isoprene with heterocycles. Under nickel catalysis, a series of cyclic monoterpene derivatives bearing quaternary carbon stereocentre are constructed with up to 98% yield and 97% enantiomeric excess. Preliminary mechanistic studies suggest this atom-economic reaction proceeds through an enantioselective dimerization of isoprene and a sequential C–H alkylation of heterocycles pathway. This work not only contributes an efficient enantioselective transformation of bulk chemical isoprene, but also provides a guide to create an unnatural monoterpene framework that may exhibit different biological activities.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data relating to the characterization data of materials and products, general methods, optimization studies, experimental procedures, mechanistic studies, mass spectrometry, high-performance liquid chromatography, NMR spectra and computational studies are available in the Supplementary Information. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2127531 (3a). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Oldfield, E. & Lin, F. Y. Terpene biosynthesis: modularity rules. Angew. Chem. Int. Ed. 51, 1124–1137 (2012).
Brill, Z. G., Condakes, M. L., Ting, C. P. & Maimone, T. J. Navigating the chiral pool in the total synthesis of complex terpene natural products. Chem. Rev. 117, 11753–11795 (2017).
Tu, H.-F., Zhang, X., Zheng, C., Zhu, M. & You, S.-L. Enantioselective dearomative prenylation of indole derivatives. Nat. Catal. 1, 601–608 (2018).
Sacchettini, J. C. & Poulter, C. D. Creating isoprenoid diversity. Science 277, 1788–1789 (1997).
Hyatt, D. C. et al. Structure of limonene synthase, a simple model for terpenoid cyclase catalysis. Proc. Natl Acad. Sci. USA 104, 5360–5365 (2007).
Gao, Y., Honzatko, R. B. & Peters, R. J. Terpenoid synthase structures: a so far incomplete view of complex catalysis. Nat. Prod. Rep. 29, 1153–1175 (2012).
Chang, W. C., Song, H., Liu, H. W. & Liu, P. Current development in isoprenoid precursor biosynthesis and regulation. Curr. Opin. Chem. Biol. 17, 571–579 (2013).
Nishimura, T., Ebe, Y. & Hayashi, T. Iridium-catalyzed [3 + 2] annulation of cyclic N-sulfonyl ketimines with 1,3-dienes via C-H activation. J. Am. Chem. Soc. 135, 2092–2095 (2013).
Nishimura, T., Nagamoto, M., Ebe, Y. & Hayashi, T. Enantioselective [3 . 2] annulation via C-H activation between cyclic N-acyl ketimines and 1,3-dienes catalyzed by iridium/chiral diene complexes. Chem. Sci 4, 4499–4504 (2013).
Perry, G. J. P., Jia, T. & Procter, D. J. Copper-catalyzed functionalization of 1,3-dienes: hydrofunctionalization, borofunctionalization, and difunctionalization. ACS Catal. 10, 1485–1499 (2019).
Smutny, E. J. Oligomerization and dimerization of butadiene under homogeneous catalysis. Reaction with nucleophiles and the synthesis of 1,3,7-octatriene. J. Am. Chem. Soc. 89, 6793–679 (1967).
Takahashi, S., Shibano, T. & Hagihara, N. The dimerization of butadiene by palladium complex catalysts. Tetrahedron Lett. 8, 2451–2453 (1967).
Faßbach, T. A., Vorholt, A. J. & Leitner, W. The telomerization of 1,3-dienes - a reaction grows up. Chem. Cat. Chem. 11, 1153–1166 (2019).
Keim, W. & Roper, M. Terpene amine synthesis via palladium-catalyzed isoprene telomerization with ammonia. J. Org. Chem. 46, 3702–3707 (1981).
Hidai, M. et al. Palladium-catalyzed asymmetric telomerization of isoprene - preparation of optically-active citronellol. J. Organomet. Chem. 232, 89–98 (1982).
Keim, W., Kurtz, K.-R. & Röper, M. Palladium catalyzed telomerization of isoprene with secondary amines and conversion of the resulting terpene amines to terpenols. J. Mol. Catal. 20, 129–138 (1983).
Maddock, S. M. & Finn, M. G. Palladium-catalyzed head-to-head telomerization of isoprene with amines. Organometallics 19, 2684–2689 (2000).
Leca, F. & Reau, R. 2-Pyridyl-2-phospholenes: new P,N ligands for the palladium-catalyzed isoprene telomerization. J. Catal. 238, 425–429 (2006).
Jackstell, R., Grotevendt, A., Michalik, D., El Firdoussi, L. & Beller, M. Telomerization and dimerization of isoprene by in situ generated palladium-carbene catalysts. J. Organomet. Chem. 692, 4737–4744 (2007).
Gordillo, A., Pachón, L. D., de Jesus, E. & Rothenberg, G. Palladium-catalysed telomerisation of isoprene with glycerol and polyethylene glycol: a facile route to new terpene derivatives. Adv. Synth. Catal. 351, 325–330 (2009).
Maluenda, I. et al. Room temperature, solventless telomerization of isoprene with alcohols using (N-heterocyclic carbene)-palladium catalysts. Catal. Sci. Technol. 5, 1447–1451 (2015).
Colavida, J. et al. Regioselectivity control in Pd-catalyzed telomerization of isoprene enabled by solvent and ligand selection. ACS Catal. 10, 11458–11465 (2020).
Ackermann, L., Vicente, R. & Kapdi, A. R. Transition-metal-catalyzed direct arylation of (hetero)arenes by C-H bond cleavage. Angew. Chem. Int. Ed. 48, 9792–9826 (2009).
Hartwig, J. F. Regioselectivity of the borylation of alkanes and arenes. Chem. Soc. Rev. 40, 1992–2002 (2011).
Gandeepan, P. et al. 3d Transition metals for C-H activation. Chem. Rev. 119, 2192–2452 (2019).
Clement, N. D. & Cavell, K. J. Transition-metal-catalyzed reactions involving imidazolium salt/N-heterocyclic carbene couples as substrates. Angew. Chem. Int. Ed. 43, 3845–3847 (2004).
Nakao, Y., Kashihara, N., Kanyiva, K. S. & Hiyama, T. Nickel-catalyzed hydroheteroarylation of vinylarenes. Angew. Chem. Int. Ed. 49, 4451–4454 (2010).
Shih, W. C. et al. The regioselective switch for amino-NHC mediated C-H activation of benzimidazole via Ni-Al synergistic catalysis. Org. Lett. 14, 2046–2049 (2012).
Wang, Y. X. et al. Enantioselective Ni-Al bimetallic catalyzed exo-selective C-H cyclization of imidazoles with alkenes. J. Am. Chem. Soc. 140, 5360–5364 (2018).
Diesel, J., Grosheva, D., Kodama, S. & Cramer, N. A bulky chiral N-heterocyclic carbene nickel catalyst enables enantioselective C-H functionalizations of indoles and pyrroles. Angew. Chem. Int. Ed. 58, 11044–11048 (2019).
Loup, J., Muller, V., Ghorai, D. & Ackermann, L. Enantioselective aluminum-free alkene hydroarylations through C-H activation by a chiral nickel/JoSPOphos manifold. Angew. Chem. Int. Ed. 58, 1749–1753 (2019).
Hu, Y. C., Ji, D. W., Zhao, C. Y., Zheng, H. & Chen, Q. A. Catalytic prenylation and reverse prenylation of indoles with isoprene: regioselectivity manipulation through choice of metal hydride. Angew. Chem. Int. Ed. 58, 5438–5442 (2019).
Yang, J. et al. Cobalt-catalyzed hydroxymethylarylation of terpenes with formaldehyde and arenes. Chem. Sci. 10, 9560–9564 (2019).
Kuai, C. S. et al. Ligand-regulated regiodivergent hydrosilylation of isoprene under iron catalysis. Angew. Chem. Int. Ed. 59, 19115–19120 (2020).
Li, Y. et al. Acid-catalyzed chemoselective C- and O- prenylation of cyclic 1,3-diketones. Chin. J. Catal. 41, 1401–1409 (2020).
Jiang, W. S. et al. Orthogonal regulation of nucleophilic and electrophilic sites in Pd-catalyzed regiodivergent couplings between indazoles and isoprene. Angew. Chem. Int. Ed. 60, 8321–8328 (2021).
Zhao, C. Y. et al. Bioinspired and ligand-regulated unnatural prenylation and geranylation of oxindoles with isoprene under Pd catalysis. Angew. Chem. Int. Ed. 61, e202207202 (2022).
Janssen-Muller, D., Schlepphorst, C. & Glorius, F. Privileged chiral N-heterocyclic carbene ligands for asymmetric transition-metal catalysis. Chem. Soc. Rev. 46, 4845–4854 (2017).
Zhang, W. B., Yang, X. T., Ma, J. B., Su, Z. M. & Shi, S. L. Regio- and enantioselective C-H cyclization of pyridines with alkenes enabled by a nickel/N-heterocyclic carbene catalysis. J. Am. Chem. Soc. 141, 5628–5634 (2019).
Thongpaen, J., Manguin, R. & Basle, O. Chiral N-heterocyclic carbene ligands enable asymmetric C-H bond functionalization. Angew. Chem. Int. Ed. 59, 10242–10251 (2020).
Duan, C.-L. et al. Acetic acid-promoted rhodium(III)-catalyzed hydroarylation of terminal alkynes. Synlett 30, 932–938 (2019).
Saidi, O. et al. Ruthenium-catalyzed meta sulfonation of 2-phenylpyridines. J. Am. Chem. Soc. 133, 19298–19301 (2011).
Acknowledgements
We thank Y.-G. Zhou (Dalian Institute of Chemical Physics) and Z.-S. Ye (Dalian University of Technology) for helpful discussions and manuscript revisions. Financial support from Dalian Institute of Chemical Physics (grant no. DICPI201902), Dalian Outstanding Young Scientific Talent (grant no. 2020RJ05) and the National Natural Science Foundation of China (grant no. 22071239) is acknowledged.
Author information
Authors and Affiliations
Contributions
Q.-A.C. conceived and supervised the project. Q.-A.C. and G.Z. designed the experiments. G.Z., C.-Y.Z., X.-T.M., Y.L., X.-X.Z., H.L., D.-W.J. and Y.-C.H. performed the experiments and analysed the data. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Mengchun Ye, Wei Guan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7, Tables 1–10, Methods, Discussions, References and Copies of NMR and HPLC for products.
Supplementary Data 1
Compound 3a.
Supplementary Data 2
Structure factor of 3a.
Supplementary Data 3
Computational data.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, G., Zhao, CY., Min, XT. et al. Nickel-catalysed asymmetric heteroarylative cyclotelomerization of isoprene. Nat Catal 5, 708–715 (2022). https://doi.org/10.1038/s41929-022-00825-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-022-00825-z
This article is cited by
-
Nickel-catalyzed divergent Mizoroki–Heck reaction of 1,3-dienes
Nature Communications (2023)
-
Nucleophilic aromatization of monoterpenes from isoprene under nickel/iodine cascade catalysis
Nature Communications (2023)