Abstract
This study aimed to establish an astaxanthin-rich strain of the calanoid copepod Pseudodiaptomus annandalei, through selective breeding based on RGB (red, green and blue) value, a parameter indicating color intensity. We evaluated the RGB value frequency distributions of the copepod populations, and selected individuals with the highest 10% and the lowest 10% RGB value over six generations. The RGB value, nauplii production, clutch interval and clutch number were assessed, and the genetic gain was calculated across generations (G0-G5). Two strains of copepods were selected and defined as dark body copepod strain (DBS) and light body copepod strain (LBS) at the end of experiment. Results revealed significantly lower RGB values (male: 121.5 ± 14.1; female: 108.8 ± 15) in the G5 DBS population compared to the G0 (male: 163.9 ± 13.1; female: 162.2 ± 14.6), with higher genetic gains of RGB values during G0 to G2. While DBS females exhibited longer clutch intervals in the G3 and G4, there was no significant difference in nauplii production between the two strains across all generations. Significantly higher astaxanthin content was found in the DBS copepods (0.04 μg/ ind.) compared to the LBS copepods (0.01 μg/ ind.) and the non-selective copepods (0.02 μg/ ind.) 20 months post selective breeding, validating the stability of the desired trait in the DBS strain. This study successfully established an astaxanthin-rich strain of P. annandalei, which provides implications for enhancing marine and brackish larviculture production.
Similar content being viewed by others
Introduction
Copepods, a group of small zooplankton inhabiting diverse aquatic environments, play a pivotal role in coastal ecosystems, often constituting over 75% of the total zooplankton community1. As an essential component of marine mesozooplankton, copepods substantially influence marine trophic systems. Studies investigating gut contents of several fish larvae, including silver pomfret (Pampus argenteus), bluefin tuna (Thunnus thynnus), marbled rockfish (Sebastiscus marmoratus), and sand lance (Ammodytes japonicus), underscored the significant role of copepods as natural prey items for fish larvae2,3,4. Copepods have been employed as live feed for aquatic larvae since the 1980s5, leading to enhanced aquaculture performance in larval rearing, and supporting the cultivation of many emerging marine fish species6,7,8.
Copepods offer distinct advantages over traditional live feeds like Artemia and rotifers. Notably, their captivating “jerking” swimming pattern fosters higher larval predation9,10. Their small body size (< 100 μm) renders them suitable for feeding small-mouthed fish larvae11,12. Most significantly, copepods are nutritionally rich in long-chain polyunsaturated fatty acids (LC-PUFAs), amino acids, pigments, and various micronutrients13,14. LC-PUFAs, particularly eicosapentaenoic acid (EPA) and docosahexaenoic acid15, are pivotal for the survival, growth, development, and stress resilience of many marine larvae16,17. Certain copepod species demonstrate the capacity to convert short-chain fatty acid into LC-PUFA through biochemical precursors from dietary microalgae. The species Pseudodiaptomus annandalei, a prevalent calanoid copepod in the aquaculture industry, was noted for its LC-PUFA bioconversion ability even under PUFA-deficient dietary conditions18,19. Other copepod species, such as Apocyclops royi20 and Tigriopus californicus21, also exhibit similar LC-PUFA bioconversion capacity.
Astaxanthin, the most dominant form of carotenoid pigments in copepods14,22, is correlated with their body color23,24. RGB value has been utilized as an indicator for carotenoid content in aquatic animals25,26. Although the function and causation of astaxanthin accumulation in copepods are not fully understood, studies suggested its role in protecting against oxidative stresses induced by ultraviolet radiation27 and metal toxicants28. Additionally, high astaxanthin content in copepods might serve to safeguard their abundant LC-PUFAs content in lipid droplets29. As a potent antioxidant, astaxanthin has several nutritional benefits to aquatic animals30. Dietary supplement of astaxanthin could improve the immunity, reproduction and pigmentation of juvenile and adult fish31,32,33. For larviculture, supplement of dietary astaxanthin could also support higher larval survival, growth rate, and stress resistance34,35. However, the deficiency in astaxanthin in rotifers and Artemia, necessitates additional enrichment protocols for feeding fish larvae36. Such enrichment, often relying on microalgae based or synthetic astaxanthin, can be cumbersome and costly, with the bioavailability of astaxanthin diminishing over time during storage and digestion within the rotifer and Artermia. In contrast, esterified astaxanthin accumulated in copepods remains relatively stable and bioavailable37,38. Copepod species or strains with high astaxanthin contents, especially when coupled with their high LC-PUFAs content, may offer the potential to significantly enhance marine larviculture production.
This study concentrates on the calanoid copepod P. annandalei, prevalent in tropical and subtropical coastal ecosystems across the Indo-Pacific region39,40,41. This euryhaline egg-bearing species of Pseudodiaptomus, as well as its congener species, is commonly cultivated on a commercial scale15,42,43,44, and harvested for larval feeding in aquaculture industry45,46. However, the inter-individual variability of color-associated traits has rarely been investigated. Moreover, no study has specifically focused on selective breeding for traits associated with astaxanthin content in copepods. In this context, we aimed to establish an astaxanthin-rich strain of P. annandalei through selective breeding, evaluating the interrelations among body color, astaxanthin content, and copepod productivity.
Materials and methods
Microalgae and copepod stock culture
The pure strain of microalga and copepod employed in this study were obtained from the Tungkang Biotechnology Research Center, Fisheries Institute of Taiwan. The stock cultures of microalgae and copepod were maintained following the protocol described by Beyrend-Dur et al39. The microalga Isochrysis galbana was cultivated in 16 L polycarbonate carboys, utilizing 1-μm filtered, sterilized, and aerated seawater maintained at a salinity of 35 ppt. The Walne medium47served as the nutritional source, with cultures re-inoculated every 10–14 days. The stock culture of the copepod P. annandalei was maintained in 20 L polycarbonate carboys, using 1-μm filtered and aerated seawater at a salinity of 20 ppt, achieved through a mixture of natural seawater and distilled water. The copepods were fed with microalga I. galbana every two days at an approximate cell concentration of 105 cells/ mL. A complete water exchange of the stock culture was conducted every two weeks to maintain the water quality. The microalga and copepod cultures were maintained at a constant temperature of 28 ± 1 °C. The photoperiod employed was a balanced 12-h light: 12-h dark cycle, with light intensity set at 4000 lx for microalgae and at 400 lx for copepod.
Identification of astaxanthin in P. annandalei
To establish the protocols for the identification and quantification of astaxanthin content in P. annandalei, a comprehensive series of tests was conducted. To mitigate potential overestimation due to gut residues, all copepods were subjected to gut evacuation prior to extractions. Lyophilized copepods (approximately 1 mg) were placed in a 15 mL centrifugation tube containing 90% acetone, followed by a 2-min sonication and overnight storage at 4 °C in the dark. After extraction, 10 mM NaOH was introduced into the tube for a 2-h saponification process at room temperature. The resultant extractant underwent filtration via a 0.22 μm syringe filter before further analysis. To evaluate the astaxanthin content per individual copepod, 10 adult copepods were carefully sorted and placed in the microcentrifuge tube with 200 uL 90% acetone, and subjected to the extraction and saponification as aforementioned. The astaxanthin extractant was collected from the supernatant after 10-min centrifugation at 8,000 rpm.
The optimal wavelength for detecting copepod astaxanthin and the free astaxanthin standard was examined by conducting a spectrum scan ranging from 380 to 520 nm using the spectrophotometer CT-2200 (ChromTech, Singapore). Subsequently, serial dilutions of astaxanthin standard solutions (0.1, 0.25, 0.5, 0.75, 1, 1.5, 2 μg/mL, 3S 3′S-astaxanthin standard, Sigma-Aldrich, USA) were prepared and measured at the optimal wavelength to establish a correlation between absorbance and astaxanthin concentration.
The extract was injected to High Performance Liquid Chromatography (HPLC) system (Jasco Inc, Tokyo, Japan) equipped with Agilent XDB-C18 column (5 μm, 250 × 4.6 mm, Agilent Technologies, California, USA). A flow rate of 1 mL min−1 was employed, following a linear gradient from 100% A (80:20, methanol: 0.5 M ammonium acetate) to 100% B (90:10, acetonitrile: H2O) over 4 min, followed by a transition to 20% B: 80% C (ethyl acetate) over 14 min. The gradient then returned to 100% B over 3 min before ultimately returning to 100% A over 5 min, and held for 6 minutes48. Signal of the absorbance was measured at 482 nm using an ultraviolet UV–visible detector. The chromatographic characteristic of the copepod extract was compared to the (3S, 3’S)-astaxanthin standard.
Imagery analysis of copepod body color
For the purpose of selective breeding on color-associated traits, the value of RGB (red, green and blue) composition was evaluated through a meticulous analysis of copepod digital images. To prevent any potential bias from gut content coloration, all copepods underwent a 24-h gut evacuation treatment prior to photographing. Subsequently, copepods were placed individually on cavity slides and photographed using a Motic BA210 compound microscope (Motic, China), maintaining consistent optical settings (magnification 100X, fixed condenser and light intensity). After photography, the copepod individuals were immediately transferred to a 12-well culture plate (3.5 mL/ well), where it was temperately cultivated for further selection. Simultaneously, the RGB value corresponding to the prosome and urosome areas, where the pigments primarily accumulated, was analyzed using Adobe Photoshop CS6 software (Adobe Systems, CA, USA) following the protocol described by Díaz‐Jiménez et al25. This approach enabled a rapid assessment of the RGB values for living copepod individuals with no mortality caused, serving as an indicator of the astaxanthin-associated trait for selective breeding in the present study.
Selective breeding protocol based on copepod body color
The stepwise procedure for the selective breeding protocol is visually presented in Fig. 1. To initiate the process, a representative cohort of 40–50 adult copepods was randomly collected from the stock culture (G0), and their body colors were subjected to imagery analysis as described in section "Imagery analysis of copepod body color". Frequency distributions of RGB value were constructed immediately after imagery analyses for males and females, respectively. Based on the RGB frequency distributions of the G0 population, 25 females and 10 males with the highest 10% RGB values (i.e. the 10% lightest) and the lowest 10% RGB values (i.e. the 10% darkest) were then selected, and transferred to 2L beakers to initiate the cultures of the first selective generation (G1) for light body strain (LBS) and dark body strain (DBS), respectively.
The selected G1 copepods were cultivated in 2L beakers (4 replicates) containing diluted seawater at 20 ppt and maintained at 28 ± 1 °C. After a 7-day spawning duration (approximately 3 clutches), the G1 adults were removed from the beakers through sieving using a 500 μm mesh. The nauplii and copepodites of the subsequent generation (G2) were cultivated until maturity. The cultures were observed daily, and the subsequent selection was conducted 24 h after the first appearance of ovigerous females in the populations. A group of 40–50 G2 adult copepod was collected from every population, and subjected to the imagery analysis, and the selection of G2 population was conducted as aforementioned. The multigenerational selective breeding protocol was carried out repetitively for 5 successive generations (G1 to G5).
To assess the variation of female productivities between the LBS and DBS strains across generations, eight ovigerous females per replicate were collected at random from the removed adults after the 7-day spawning duration. These females were independently cultivated in 12-well culture plates containing 3.5 ml of culture water. The nauplii production and clutch interval were documented daily under a stereomicroscope (SZX9, OLYMPUS, Tokyo, Japan) over a 7-day span. In this study, all copepod individuals were gently transferred using wide-bore pipettes, and no mortality was observed during the manipulations.
Genetic gain, a measure of proportional phenotypic alteration during selective breeding, was calculated as the percentage change in the evaluated biological trait between successive generations. The genetic gain in RGB value was quantified using the formula:
where RGB(Gx) represents the RGB value of the present generation; RGB(Gx−1) represents the RGB value of the preceding generation.
The actual astaxanthin concentrations (per copepod biomass and individual) and RGB values of both LBS and DBS copepods were ascertained 50 days post selective breeding (PSB). In addition, the actual astaxanthin concentrations of the LBS, DBS and stock culture (the non-selective population) were analyzed 20 months post the selective breeding program. These assessments served to determine the stability of the desired trait within the selective copepod strains.
Data analysis
To evaluate the differences between the two selective strains at the same generation, comparisons of RGB values, astaxanthin content, and productivity were performed using the student’s t-test. Furthermore, for analyzing data among the five generations and three populations (LBS, DBS and stock culture), a one-way ANOVA was employed. Subsequent to the identification of significant differences, the Tukey multiple comparison test was conducted to elucidate their pairwise differences between groups. All statistical analyses were executed utilizing SPSS 18.0 (SPSS, Chicago, IL, USA), with the predetermined significant level set at p < 0.05.
Results
Identification and quantification of astaxanthin in P. annandalei
The maximum absorbance for both copepod astaxanthin and the astaxanthin standard was recorded at 482 nm (Fig. 2A). A clear and consistent linear relationship was observed between astaxanthin concentration and absorbance (Fig. 2B). The equation coefficient of determination value (R2) was calculated to be 0.9902.
(A) Absorbance of the astaxanthin standard (left axis) and P. annandalei astaxanthin extract (right axis) in the wavelength spectrum of 380–520 nm; (B) Correlation between absorbance at 482 nm and concentration of astaxanthin standard; (C) HPLC chromatograms of astaxanthin standard (left axis) and P. annandalei astaxanthin extract (right axis). ast.: astaxanthin.
The HPLC chromatogram of the copepod pigment extract displayed a prominent peak at a retention time of 11.7 min (Fig. 2C). The chromatographic characteristic and retention time of the extracted astaxanthin were identical to those of the free (3S, 3′S)-astaxanthin standard (Fig. 2C).
Selective breeding based on copepod body color
RGB value of copepod body
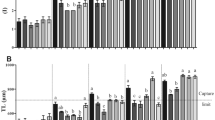
Within the LBS copepods, the RGB values of the G5 and 50-d PSB populations demonstrated no significant deviation from those of the G0 population (Table 1). Regarding the frequency distribution, individuals with RGB values spanning from 160 to 180 occurred frequently and consistently in the populations of all generations (Figs. 3 and 4).
Conversely, the DBS copepods showed notable shifts in RGB values. The G5 and 50-d PSB populations exhibited significantly lower RGB values in comparison to the G0 population (Table 1). Remarkable changes were evident in the frequency distribution of RGB values among the DBS populations across successive generations. The range of the top 10% distributed RGB value reduced from 170 to180 at G0 to 110 to140 at G5 and 50-d PSB (Figs. 3 and 4). The genetic gain values elucidate the trend within the DBS population (Table 2). Notably, higher genetic gains were recorded in the first two generations (− 10.49% to − 15.17%), whereas the three last generations exhibited comparatively lower genetic gains (− 0.1% to − 9.3%). Overall, based on the imagery analysis and visualization (Fig. 5), the body color of the DBS copepods appeared darker compared to the LBS copepods after the selective breeding program.
Reproductive performances
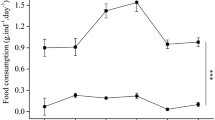
While nauplii production fluctuated across successive generations, no significant differences were found between the strains within each generation (Fig. 6). In terms of clutch interval, no noteworthy disparity was detected between the strains across generations, except for the G3 and G4 generations, where a significant difference surfaced (Fig. 6).
Astaxanthin content
In terms of astaxanthin content, a substantial contrast emerged between the DBS copepods and LBS copepod at 50-d PSB. Specifically, the 50-d PSB DBS copepods exhibited significantly higher astaxanthin concentration, measured at 10.19 μg/mg dry weight, in comparison to the LBS copepods with an astaxanthin concentration of 4.47 μg/mg dry weight (Fig. 7A). Further assessing astaxanthin content on a per individual basis (Fig. 7B), the 50-d PSB DBS copepods (female: 0.036 μg/ind; male: 0.022 μg/ind) showcased significantly higher astaxanthin compared to the LBS copepods (female: 0.013 μg/ind; male: 0.009 μg/ind). In the extended analysis, the DBS copepod (Fig. 7C) also showed significant higher astaxanthin concentration (0.04 μg/ind) than the LBS (0.01 μg/ind) and non-selective strain (0.02 μg/ind) 20-month PSB.
Astaxanthin contents of P. annandalei, (A) astaxanthin content per dry biomass of the LBS and DBS copepod at 50 days post selective breeding; (B) astaxanthin content per male and female individual of the LBS and DBS copepod at 50 days post selective breeding; (C) astaxanthin content per individual (non-sexual specific) at 20 months post selective breeding. Asterisk (*) and letters (a, b) indicate significant differences.
Discussion
In this study, the laboratory-cultured strain of copepod P. annandalei, originally isolated from brackish waters in Tungkang, southern Taiwan, underwent a domestication process over a decade. Intriguingly, a small subset of approximately 2% of individuals in the stock culture exhibited dark body coloration, denoted by an RGB value below 120. This novel observation of body color variation in P. annandalei, not documented in either wild or aquacultured populations before, suggests that the extended laboratory culture might have induced these distinct color traits, aligning with findings in other crustaceans, subjected to domestication or artificial selection49,50.
The trade-off between predation stress and copepod body colors has been noted in several studies51. Typically, highly pigmented copepods are more susceptible to predator detection in shallow waters52. If there are no predators in the scenario, as in the case of the mono-species copepod in this study, dark-colored individuals would not be captured and could survive and reproduce within the population. Therefore, a reduction in predators could potentially promote the prevalence of highly pigmented individuals within the long term copepod stock culture. Conversely, the astaxanthin content in copepod is positively correlated with light exposure. In their natural habitat, the semi-benthic P. annandalei tend to stay on the bottom of the water column46,53, where the light intensity is low. Conversely, in the laboratory conditions, characterized by clear water and transparent containers, the copepods could have experienced higher light intensity. This environmental difference might contribute to the emergence of dark individuals in our stock culture. While phenotypic plasticity is known to play an important role in regulating pigment content in zooplankton under strong seasonality31,54, the stable conditions of our P. annandalei stock culture suggest that the observed pigmentation enhancement in the dark-colored P. annandalei seems to be a result of genetic variation, rather than short-term and environmental-stimulated phenotypic plasticity.
Variations in body coloration among individuals are frequently observed in crustaceans. Weaver et al.55 identified body color variations in anchialine shrimp (Halocaridina rubra) from different locations, with individuals of higher astaxanthin content producing offspring exhibiting elevated astaxanthin levels, and the body color remained stable through a 14-year laboratory culture. Additionally, body color diversity in the ornamental shrimp Neocaridina denticulata has been linked to specific genes, hinting at potential biomarkers for the genetic selection of specific body colors or patterns50. These findings resonate with our observations during the selective breeding of P. annandalei, suggesting that traits such as body color and astaxanthin content can be genetically inherited in crustaceans. Future studies, such as cross breeding programs, could be conducted to investigate the genetics mechanisms involved in copepod astaxanthin metabolism.
Previous research has demonstrated that the efficiency of astaxanthin bioconversion in copepods is influenced by dietary astaxanthin precursors. For instance, dietary supplementation with zeaxanthin and canthaxanthin has been shown to elevate astaxanthin content in copepods14. Conversely, a decline of astaxanthin in copepod could occur when they are fed with carotenoid-deficient diets such as yeast14,56. In this study, microalga I. galbana, rich in carotenoids including fucoxanthin and β-carotene57, was used as a dietary source, providing astaxanthin precursors for both DBS and LBS populations. The strain-specific variations in astaxanthin content might stem from differential capacity in astaxanthin bioconversion and accumulation. The biosynthesis pathway of astaxanthin could involve many steps of enzymatic activities that produce a diverse groups of precursor metabolites (β-carotene, echinenone, and canthaxanthin), yet it is scarcely studied in copepod species51,58. In the future, the comparison of biosynthesize enzymes or relative gene expression levels between the two P. annandalei strains should be investigated using molecular biotechnology techniques, such as transcriptomic or metabolomic analysis, to uncover the molecular mechanisms that cause the variation of their astaxanthin concentration.
Studies have linked enhanced pigmentation to increased egg production rates in copepod52, and positive correlations between astaxanthin content and productivity in aquatic animals have been reported34,59. Interestingly, our findings differ, as DBS females displayed relatively longer clutch intervals across most generations. This discrepancy could be attributed to energy allocation shifts in copepods, possibly stemming from the allocation of more energy towards astaxanthin synthesis and accumulation, resulting in reduced reproductive frequency. However, there are many other life traits affecting the overall productivity of copepod production, including offspring survival rate, life span, developmental time, stress tolerance and cannibalism60,61. The various life traits of LBS and DBS copepods should be studied to further validate their productivity in the mass culture condition.
In this study, the high-astaxanthin DBS P. annandalei strain was established through selective breeding. This selective strain displays significantly rich astaxanthin content compared to levels reported in other copepod species (as shown in Table 3), indicating their great potential as live feed for marine or brackish larviculture. Furthermore, these selected strains could serve as experimental models for delving into various aspects of copepod physiological and ecological studies, including mechanisms of astaxanthin bioconversion, antioxidative capacities, and the role of body-color-based prey-predator interactions.
Data availability
The data that support the findings of this study are available on request from the corresponding author J.Y.P.
References
Atkinson, A., Ward, P., Hunt, B., Pakhomov, E. & Hosie, G. An overview of Southern Ocean zooplankton data: Abundance, biomass, feeding and functional relationships. Ccamlr Sci. 19, 171–218 (2012).
Mitsuzawa, A., Miyamoto, H. & Ueda, H. Feeding selectivity of early-stage fish larvae on the nauplii and eggs of different copepod species. Plankon Benthos Res. 12, 115–122 (2017).
Uriarte, A. et al. Evidence of density-dependent cannibalism in the diet of wild Atlantic bluefin tuna larvae (Thunnus thynnus) of the Balearic Sea (NW-Mediterranean). Fish. Res. 212, 63–71 (2019).
Dadzie, B. S., Abou-Seedo, F. & Al-Qattan, E. The food and feeding habits of the silver pomfret, Pampus argenteus (Euphrasen), Kuwait waters. J. Appl. Ichthyol. 16, 61–67 (2000).
Drillet, G. et al. Status and recommendations on marine copepod cultivation for use as live feed. Aquaculture 315, 155–166 (2011).
Burgess, A. I. & Callan, C. K. Effects of supplemental wild zooplankton on prey preference, mouth gape, osteological development and survival in first feeding cultured larval yellow tang (Zebrasoma flavescens). Aquaculture 495, 738–748 (2018).
Pouil, S., Tlusty, M. F., Rhyne, A. L. & Metian, M. Aquaculture of marine ornamental fish: Overview of the production trends and the role of academia in research progress. Rev. Aquac. 12, 1217–1230 (2020).
Shao, L. & Zeng, C. Survival, growth, ingestion rate and foraging behavior of larval green mandarin fish (Synchiropus splendidus) fed copepods only versus co-fed copepods with rotifers. Aquaculture 520, 734958 (2020).
Bruno, E., Højgaard, J. K., Hansen, B. W., Munk, P. & Støttrup, J. G. Influence of swimming behavior of copepod nauplii on feeding of larval turbot (Scophthalmus maximus). Aquac. Int. 26, 225–236 (2018).
Mahjoub, M.-S., Souissi, S., Michalec, F.-G., Schmitt, F. G. & Hwang, J.-S. Swimming kinematics of Eurytemora affinis (Copepoda, Calanoida) reproductive stages and differential vulnerability to predation of larval Dicentrarchus labrax (Teleostei, Perciformes). J. Plankton Res. 33, 1095–1103 (2011).
Moorhead, J. A. & Zeng, C. Development of captive breeding techniques for marine ornamental fish: A review. Rev. Fish. Sci. 18, 315–343 (2010).
Burgess, A. I., Callan, C. K., Touse, R. & Delos Santos, M. Increasing survival and growth in larval leopard coral grouper (Plectropomus leopardus) using intensively cultured Parvocalanus crassirostris nauplii. J. World Aquac. Soc. 51, 171–182 (2020).
van der Meeren, T., Olsen, R. E., Hamre, K. & Fyhn, H. J. Biochemical composition of copepods for evaluation of feed quality in production of juvenile marine fish. Aquaculture 274, 375–397 (2008).
Weaver, R. J., Cobine, P. A. & Hill, G. E. On the bioconversion of dietary carotenoids to astaxanthin in the marine copepod, Tigriopus californicus. J. Plankton Res. 40, 142–150 (2018).
Santhanam, P. et al. A novel technology towards the high-density and continuous production of the marine copepod, Pseudodiaptomus annandalei (Sewell, 1919). Biomass Conversion and Biorefinery, (2023).
Cahu, C., Infante, J. Z. & Takeuchi, T. Nutritional components affecting skeletal development in fish larvae. Aquaculture 227, 245–258 (2003).
Mejri, S. C., Tremblay, R., Audet, C., Wills, P. S. & Riche, M. Essential fatty acid requirements in tropical and cold-water marine fish larvae and juveniles. Front. Marine Sci., 557 (2021).
Rayner, T. A. et al. Biochemical composition of the promising live feed tropical calanoid copepod Pseudodiaptomus annandalei (Sewell 1919) cultured in Taiwanese outdoor aquaculture ponds. Aquaculture 441, 25–34 (2015).
Nielsen, B. L. H., Gréve, H. V. S. & Hansen, B. W. Cultivation success and fatty acid composition of the tropical copepods Apocyclops royi and Pseudodiaptomus annandalei fed on monospecific diets with varying PUFA profiles. Aquac. Res. 52, 1127–1138 (2021).
Pan, Y. J., Sadovskaya, I., Hwang, J. S. & Souissi, S. Assessment of the fecundity, population growth and fatty acid composition of Apocyclops royi (Cyclopoida, Copepoda) fed on different microalgal diets. Aquac. Nutrit. 24, 970–978 (2018).
Kabeya, N. et al. A complete enzymatic capacity for biosynthesis of docosahexaenoic acid (DHA, 22: 6n–3) exists in the marine Harpacticoida copepod Tigriopus californicus. Open Biol. 11, 200402 (2021).
Oester, R. et al. The influence of predator community composition on photoprotective traits of copepods. Ecol. Evol. 12, e8862 (2022).
Tume, R., Sikes, A., Tabrett, S. & Smith, D. Effect of background colour on the distribution of astaxanthin in black tiger prawn (Penaeus monodon): Effective method for improvement of cooked colour. Aquaculture 296, 129–135 (2009).
Zhang, C., Jin, Y., Yu, Y., Xiang, J. & Li, F. Effects of natural astaxanthin from microalgae and chemically synthetic astaxanthin supplementation on two different varieties of the ridgetail white prawn (Exopalaemon carinicauda). Algal Res. 57, 102347 (2021).
Díaz-Jiménez, L., Hernández-Vergara, M. P. & Pérez-Rostro, C. I. The effect of background colour and lighting of the aquarium on the body pigmentation of the peppered shrimp Lysmata wurdemanni. Aquac. Res. 49, 3508–3516 (2018).
Díaz-Jiménez, L., Hernández-Vergara, M. P., Pérez-Rostro, C. I. & Olvera-Novoa, M. Á. The effect of two carotenoid sources, background colour and light spectrum on the body pigmentation of the clownfish Amphiprion ocellaris. Aquac. Res. 52, 3052–3061 (2021).
Sommaruga, R. Preferential accumulation of carotenoids rather than of mycosporine-like amino acids in copepods from high altitude Himalayan lakes. Hydrobiologia 648, 143–156 (2010).
Caramujo, M.-J., De Carvalho, C. C., Silva, S. J. & Carman, K. R. Dietary carotenoids regulate astaxanthin content of copepods and modulate their susceptibility to UV light and copper toxicity. Marine Drugs 10, 998–1018 (2012).
Schneider, T., Grosbois, G., Vincent, W. F. & Rautio, M. Carotenoid accumulation in copepods is related to lipid metabolism and reproduction rather than to UV-protection. Limnol. Oceanogr. 61, 1201–1213 (2016).
Lim, K. C., Yusoff, F. M., Shariff, M. & Kamarudin, M. S. Astaxanthin as feed supplement in aquatic animals. Rev. Aquac. 10, 738–773 (2018).
Ahmadi, M., Bazyar, A., Safi, S., Ytrestøyl, T. & Bjerkeng, B. Effects of dietary astaxanthin supplementation on reproductive characteristics of rainbow trout (Oncorhynchus mykiss). J. Appl. Ichthyol. 22, 388–394 (2006).
Tran, D. V., Dang, T. T., Cao, T. T., Hua, N. T. & Pham, H. Q. Natural astaxanthin extracted from shrimp waste for pigment improvement in the Orange clownfish Amphiprion percula. Aquac. Res. 53, 4190–4198 (2022).
Zhao, W. et al. Effects of synthetic astaxanthin and Haematococcus pluvialis on growth, antioxidant capacity, immune response, and hepato-morphology of Oncorhynchus mykiss under cage culture with flowing freshwater. Aquaculture 562, 738860 (2023).
Hansen, Ø. J., Puvanendran, V. & Bangera, R. Broodstock diet with water and astaxanthin improve condition and egg output of brood fish and larval survival in Atlantic cod Gadus morhua L. Aquac. Res. 47, 819–829 (2016).
Wang, M. et al. Comparative transcriptome analysis provides new insights into the protective effect of astaxanthin on the liver of leopard coral grouper (Plectropomus leopardus). Aquaculture 565, 739118 (2023).
Dominguez, A., Ferreira, M., Coutinho, P., Fábregas, J. & Otero, A. Delivery of astaxanthin from Haematocuccus pluvialis to the aquaculture food chain. Aquaculture 250, 424–430 (2005).
Holeton, C., Lindell, K., Holmborn, T., Hogfors, H. & Gorokhova, E. Decreased astaxanthin at high feeding rates in the calanoid copepod Acartia bifilosa. J. Plankton Res. 31, 661–668 (2009).
Ambati, R. R., Siew Moi, P., Ravi, S. & Aswathanarayana, R. G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Marine Drugs 12, 128–152 (2014).
Beyrend-Dur, D., Souissi, S. & Hwang, J.-S. Population dynamics of calanoid copepods in the subtropical mesohaline Danshuei Estuary (Taiwan) and typhoon effects. Ecol. Res. 28, 771–780 (2013).
Blanda, E. et al. Trophic interactions and productivity of copepods as live feed from tropical Taiwanese outdoor aquaculture ponds. Aquaculture 445, 11–21 (2015).
Nguyen, T. T. et al. Salinity and temperature effects on productivity of a tropical calanoid copepod Pseudodiaptomus incisus. Aquac. Res. 51, 3768–3779 (2020).
Blanda, E. et al. An analysis of how to improve production of copepods as live feed from tropical Taiwanese outdoor aquaculture ponds. Aquaculture 479, 432–441 (2017).
Doan, N. X. et al. Temperature-and sex-specific grazing rate of a tropical copepod Pseudodiaptomus annandalei to food availability: Implications for live feed in aquaculture. Aquac. Res. 49, 3864–3873 (2018).
GrØnning, J., Doan, N. X., Dinh, N. T., Dinh, K. V. & Nielsen, T. G. Ecology of Pseudodiaptomus annandalei in tropical aquaculture ponds with emphasis on the limitation of production. J. Plankton Res. 41, 741–758 (2019).
Liao, I. C., Su, H. M. & Chang, E. Y. Techniques in finfish larviculture in Taiwan. Aquaculture 200, 1–31 (2001).
Lee, C.-H. et al. Predation of Pseudodiaptomus annandalei (Copepoda: Calanoida) by the grouper fish fry Epinephelus coioides under different hydrodynamic conditions. J. Exp. Marine Biol. Ecol 393, 17–22 (2010).
Walne, P. R. Studies on the food value of nineteen genera of algae to juvenile bivalves of the genera Ostrea, Crassostrea Mercenaria and Mytilus. Fish. Investig. Ser. 2, 26 (1970).
Wright, S. W. et al. Improved HPLC method for the analysis of chlorophylls and carotenoids from marine phytoplankton. Marine Ecol. Progress Ser. 77, 183–196 (1991).
Bernal Rodríguez, C. E. et al. The color of marine shrimps and its role in the aquaculture. Int. J. Aquac. Fish. Sci. 3(3), 062–065 (2017).
Huang, C.-W., Chu, P.-Y., Wu, Y.-F., Chan, W.-R. & Wang, Y.-H. Identification of functional SSR markers in freshwater ornamental shrimps Neocaridina denticulata using transcriptome sequencing. Marine Biotechnol. 22, 772–785 (2020).
Vilgrain, L. et al. Copepods’ true colors: astaxanthin pigmentation as an indicator of fitness. Ecosphere 14(6), e4489 (2023).
Gorokhova, E., Lehtiniemi, M. & Motwani, N. H. J. P. O. Trade-offs between predation risk and growth benefits in the copepod Eurytemora affinis with contrasting pigmentation. PLoS One 8(8), e71385 (2013).
Shang, X., Wang, G. & Li, S. Resisting flow–laboratory study of rheotaxis of the estuarine copepod Pseudodiaptomus annandalei. Marine Freshw. Behav. Physiol. 41, 91–106 (2008).
Lee, M. et al. Low-latitude zooplankton pigmentation plasticity in response to multiple threats. R. Soc. Open Sci. 6(7), 190321 (2019).
Weaver, R. J., Gonzalez, B. K., Santos, S. R. & Havird, J. C. Red coloration in an anchialine shrimp: carotenoids, genetic variation, and candidate genes. Biol. Bull. 238, 119–130 (2020).
Powers, M. J., Hill, G. E. & Weaver, R. J. An experimental test of mate choice for red carotenoid coloration in the marine copepod Tigriopus californicus. Ethology 126, 344–352 (2020).
Di Lena, G., Casini, I., Lucarini, M. & Lombardi-Boccia, G. Carotenoid profiling of five microalgae species from large-scale production. Food Res. Int. 120, 810–818 (2019).
Prado-Cabrero, A., Saefurahman, G. & Nolan, J. M. Stereochemistry of astaxanthin biosynthesis in the marine harpacticoid copepod Tigriopus californicus. Marine Drugs 18 (2020).
Higuera-Ciapara, I., Félix-Valenzuela, L. & Goycoolea, F. M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutrit. 46, 185–196 (2006).
Camus, T. & Zeng, C. Reproductive performance, survival and development of nauplii and copepodites, sex ratio and adult life expectancy of the harpacticoid copepod, Euterpina acutifrons, fed different microalgal diets. Aquac. Res. 43, 1159–1169 (2012).
Drillet, G. & Lombard, F. A first step towards improving copepod cultivation using modelling: the effects of density, crowding, cannibalism, tank design and strain selection on copepod egg production yields. Aquac. Res. 46, 1638–1647 (2015).
Acknowledgements
This study is part of the Master thesis of Mr. Sen Chen supported by National Taiwan Ocean University. We thank all the lab members for their assistance during this study, and Dr. Guillaume Drillet for his suggestion to improve this article.
Funding
This study is found by the National Science and Technology Council of Taiwan (111–2636-M-019–001- and 112–2636-M-019–001-), Ministry of Agriculture, Executive Yuan (112AS-1.3.2-ST-aL and 113AS-1.3.2-AS-30). This work was also supported by the research grants from the Center of Excellence for the Oceans, NTOU, which is financially supported by the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the National Science and Technology Council and Ministry of Education of Taiwan.
Author information
Authors and Affiliations
Contributions
S.C. conducted the experiments and wrote the original manuscript, Y.J.P. designed the experiments, supervised the study, and edited, reviewed, and visualized the manuscript, A.L. and J.L.L. conducted the astaxanthin analysis, C.H.W., J.S.C. and G.D. edited, reviewed, and visualized the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chan, S., Pan, YJ., Lu, A. et al. Establishing an astaxanthin-rich live feed strain of Pseudodiaptomus annandalei. Sci Rep 14, 8671 (2024). https://doi.org/10.1038/s41598-024-59224-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-59224-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.