Abstract
Mass mortality of the long line culture of the sea urchin Strongylocentrotus intermedius in summer, which is greatly associated with their disease, energy storage and resistant abilities, is the most serious problem for the development of the aquaculture. Here, a feeding experiment was conducted for ~ 9 weeks to investigate the survival, growth and gonadal development of small S. intermedius (~ 3 cm) fed either brown algae Sargassum horneri or Saccharina japonica. Subsequently, we assessed their resistant abilities via observing the behaviors of righting, tube feet extension and Aristotle's lantern reflex at both moderately elevated and acutely changed water temperatures. Sea urchins fed S. horneri showed significantly fewer diseased individuals and slower gonadal development than those fed S. japonica. Consistently, significantly greater Aristotle's lantern reflex occurred in sea urchins fed S. horneri at moderately elevated temperatures. These findings suggest that S. horneri has direct application potential as food for the long line culture of S. intermedius in summer because of the advantage in health, energy storage (avoid the energy loss caused by gonadal development at small body sizes) and resistance abilities. In comparison, sea urchins fed S. japonica outperformed those fed S. horneri for all experimental behaviors under the acutely changed water temperatures. These findings clearly suggest that S. intermedius fed S. japonica is more suitable for the areas with cold water mass in summer, because it can effectively avoid or reduce the negative impacts of acute changes of water temperature on sea urchins. The present study provides valuable information into the management of the long line culture of S. intermedius in summer.
Similar content being viewed by others
Introduction
The sea urchin Strongylocentrotus intermedius is a seafood delicacy with high commercial value in the world1,2. Interest in S. intermedius aquaculture has grown rapidly in the last decade3,4. The annual production of sea urchins, for example, was over 8,844 tons in China in 20185. Long line culture is the most important approach to meeting market demands for S. intermedius1. The brown alga Saccharina japonica is commonly used as food for S. intermedius aquaculture in Japan3 and northern China6. However, fresh S. japonica is largely unavailable in summer and thus dried S. japonica is used for food at that season1. Our previous study found that the brown alga Sargassum horneri is an alternative diet for S. japonica and consequently addressed the food shortage problem for the aquaculture of S. intermedius in summer7. However, mass mortality of the long line culture of S. intermedius in summer, which is greatly associated with their disease, energy storage, resistant abilities1, is the most serious unaddressed problem for the aquaculture of S. intermedius8.
Reproduction greatly consumes stored energy and is probably associated with various diseases even death in commercially important marine invertebrates such as the oyster Crassostrea gigas9. Cultured S. intermedius (~ 3 cm of test diameter) showed precocious gonads in July in northern China10, suggesting that the excessive allocation of energy to gametogenesis may be responsible for the mortality of sea urchins in summer. Strongylocentrotus intermedius fed S. horneri showed significantly lower gonad yield than those fed S. japonica in summer7. Low gonad production commonly results in less gametogenesis in sea urchin gonads1,11. Therefore, S. horneri is probably valuable to prevent precocious puberty of S. intermedius and subsequently to enhance the energy storage.
In addition, long line cultured S. intermedius are frequently exposed to local unfavorable water temperatures12. It has been well documented that S. intermedius are greatly impacted by elevated water temperatures in summer3,13. Besides, acute changes of water temperature also result in negative impacts on regional aquaculture14,15. For example, water temperature frequently decreases rapidly from 22 to 16 °C by the cold water mass at Haiyang island near Dalian (39° 03′ N, 123°09′ E) in summer16, causing mass mortality of cultured species such as the scallop Chlamys farreri17. Enhancing resistance abilities can improve the survival of sea urchins at adverse water temperatures. Food is one of the most important methods to facilitate biological resistance abilities18,19. Dietary intervention successfully reduced the mortality of aquacultural Australian greenlip abalone Haliotis laevigata in unfavorable conditions20. However, whether S. horneri and S. japonica have potential application for improving the resistance abilities of S. intermedius to adverse water temperatures is not known.
Sea urchins have fitness-related behaviors including righting, tube feet extension and Aristotle's lantern reflex. Righting behavior refers to an inverted sea urchin placed on its aboral surface to correct the posture with the aboral side up21,22 and has been commonly using as a stress indicator23,24,25. Tube feet extension, which refers to the ability of sea urchins to extend their tube feet26, is important for the fitness of sea urchins27. The Aristotle's lantern reflex is the process of opening and closing of the teeth, which affects the capacity of sea urchins to grasp food with their teeth28,29.
The main purposes of the present study are to investigate: (1) whether S. horneri decreases the mortality and morbidity of sea urchins, compared with S. japonica; (2) whether S. horneri slows the gonadal development of S. intermedius, compared with S. japonica; (3) whether S. horneri and S. japonica have potential application for improving the resistance of S. intermedius under adverse temperatures.
Results
Crude protein, fiber, fat and ash of S. horneri and S. japonica
The concentrations of crude protein (186.33 ± 2.66 g/kg) and crude fat (10.00 ± 4.36 g/kg) of dried S. horneri were not significantly different from those of dried S. japonica (210.50 ± 6.15 g/kg, t = 1.240, P = 0.283 for crude protein; 6.00 ± 2.83 g/kg, t = 1.315, P = 0.259 for crude fat). However, the concentrations of crude fiber (46.67 ± 6.80 g/kg) and ash (13.94 ± 4.02 g/kg) of dried S. horneri were significantly larger than those of dried S. japonica (31.50 ± 9.19 g/kg, t = 2.910, P = 0.044 for crude fiber; 1.95 ± 0.39 g/kg, t = 5.128, P = 0.007 for ash).
Experiment I
Number of survivors and diseased sea urchins
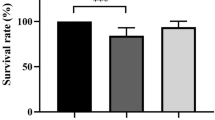
Diets did not significantly affect the number of survivors (χ2 = 0.116, P = 1.000, Table 1). However, S. intermedius fed S. horneri showed significantly fewer diseased individuals than those fed S. japonica (χ2 = 4.421, P = 0.036, Table 1).
Dried food consumption
The average dried food consumption of S. intermedius fed S. horneri (1.12 ± 0.29 g ind−1 day−1) was significantly more than those fed S. japonica (0.14 ± 0.08 g ind−1 day−1, P < 0.001, Fig. 1).
Test diameter and wet body weight
Compared with the initial conditions, test diameter significantly increased in both treatments (t = 4.272, P < 0.001 for S. intermedius fed S. horneri; t = 5.791, P < 0.001 for individuals fed S. japonica). However, there was no pronounced difference in wet body weight between groups (t = 1.784, P = 0.081 for S. intermedius fed S. horneri; t = 1.002, P = 0.321 for individuals fed S. japonica).
After ~ 9 weeks, test diameter and wet body weight of S. intermedius fed S. horneri (30.09 ± 2.93 mm for test diameter; 9.86 ± 2.34 g for wet body weight) were not significantly different from those fed S. japonica (30.19 ± 1.87 mm, t = 0.162, P = 0.872 for test diameter; 9.21 ± 1.48 g, t = 1.237, P = 0.223 for wet body weight).
Specific growth rate
There was no significant difference in specific growth rate (0.188 ± 0.189 for S. intermedius fed S. horneri; 0.080 ± 0.192 for individuals fed S. japonica) between both diet treatments (t = 0.891, P = 0.399).
Wet gut weight
No significant difference in wet gut weight was detected between treatments (0.19 ± 0.05 g for S. intermedius fed S. horneri; 0.13 ± 0.05 g for sea urchins fed S. japonica; t = 1.900, P = 0.087).
Aristotle's lantern length and wet Aristotle's lantern weight
Aristotle's lantern length (7.88 ± 0.74 mm) and wet Aristotle's lantern weight (0.46 ± 0.05 g) of S. intermedius fed S. horneri were not significantly different from those fed S. japonica (7.97 ± 0.34 mm, t = 0.275, P = 0.789 for Aristotle's lantern length; 0.40 ± 0.06 g, t = 2.036, P = 0.069 for wet Aristotle's lantern weight).
Gonadal yield
Compared with the initial conditions, no significant differences were found in wet gonad weight (t = 0.197, P = 0.846 for S. intermedius fed S. horneri; t = 0.491, P = 0.631 for individuals fed S. japonica) and gonad index (t = 0.281, P = 0.783 for S. intermedius fed S. horneri; t = 0.435, P = 0.670 for individuals fed S. japonica) in either treatment.
Significant differences were not detected in wet gonad weight (0.58 ± 0.45 g for S. intermedius fed S. horneri; 0.63 ± 0.35 g for individuals fed S. japonica; t = 0.195, P = 0.850) and gonad index (5.83 ± 1.90 for S. intermedius fed S. horneri; 7.17 ± 1.40 for individuals fed S. japonica; t = 0.564, P = 0.585) in either treatment.
Gonadal development
After ~ 9 weeks, all of the gonads of S. intermedius fed S. horneri were in the growing stage (stage II). However, 83.33% and 16.67% of individuals fed S. japonica were in the premature gonads (stage III) and growing stage (stage II), respectively.
In the gonads of S. intermedius fed S. horneri, the primary oocytes only attached to the follicular wall of ovaries (Fig. 2A) and sperms only occurred in the follicular wall of testes (Fig. 2B). Regarding the gonads of sea urchins fed S. japonica at the same period, the oocytes were detached from the wall and gradually replaced nutritive phagocytes in the follicular cavity (Fig. 2C). Consistently, the basophilic clusters of sperm with a length of ~ 2 μm occurred in both the follicular wall and follicular cavity of testes (Fig. 2D).
Histology of ovaries (A,C) and testes (B,D) of Strongylocentrotus intermedius (N = 6) fed different brown algae after ~ 9 weeks. Sargassum horneri and Saccharina japonica refer to the experimental and control groups, respectively. In the gonads of sea urchins in the experimental group, the primary oocytes only attached to the follicular wall of ovaries (A) and sperms only occurred in the follicular wall of testes (B). Regarding the gonads of sea urchins in the control group over the same period, the oocytes were detached from the wall and gradually replaced nutritive phagocytes in the follicular cavity (C). Consistently, the basophilic clusters of sperm with a length of ~ 2 μm occurred in both the follicular wall and follicular cavity of testes (D). NP means nutritive phagocytes.
Experiment II
Moderately elevated temperatures
Significant difference was not found between the treatments in either righting response time (89.3 ± 57.6 s for S. intermedius fed S. horneri, 74.9 ± 48.5 s for sea urchins fed S. japonica, Kruskal–Wallis H = 1.182, P = 0.277; Fig. 3A) or tube feet extension rating (3.32 ± 0.93 for S. intermedius fed S. horneri, 3.55 ± 0.90 for individuals fed S. japonica, Kruskal–Wallis H = 3.814, P = 0.051; Fig. 3B). However, Aristotle's lantern reflex of S. intermedius fed S. horneri (4.40 ± 0.95 times min−1) was significantly higher than those fed S. japonica (2.49 ± 1.39 times min−1, t = 3.343, P = 0.003, Fig. 3C).
Righting response time (mean ± SD, N = 15; A,D), tube feet extension rating (mean ± SD, N = 15; B,E) and Aristotle's lantern reflex (mean ± SD, N = 7 for experimental group and N = 10 for control group at moderately elevated temperatures; (C) mean ± SD, N = 10 for both groups; (F) in either treatment in respond to moderately elevated or acutely changed water temperatures. Sargassum horneri and Saccharina japonica refer to the experimental and control groups, respectively. Moderately elevated water temperatures refers to seawater temperature rose from 23.5 to 26.5 °C at a rate of 0.5 °C per day and maintained for 1 week. To simulate the changes of water temperature in Haiyang island near Dalian (39° 03′ N, 123° 09′ E) where water temperature frequently fluctuates from 22 to 16 °C instantly by the cold water mass, sea urchins were transferred quickly from 23.5 to 15 °C, maintaining at 15 °C for an hour and subsequently quickly returned to 23.5 °C for another hour to finish one cycle of acutely changed temperatures. After four cycles, these behaviors were observed. The asterisks *,** and *** mean P < 0.05 and P < 0.01 mean P < 0.001.
Acute changes in water temperatures
The righting response time of S. intermedius fed S. horneri (78.9 ± 49.9 s) was significantly higher than those fed S. japonica (38.6 ± 11.6 s, Kruskal–Wallis H = 17.149, P < 0.001, Fig. 3D). Consistently, tube feet extension rating (3.96 ± 1.05) and Aristotle's lantern reflex (4.04 ± 1.87 times min−1) of individuals fed S. japonica were significantly greater than those fed S. horneri (2.94 ± 1.27, Kruskal–Wallis H = 33.872, P < 0.001 for tube feet extension rating, Fig. 3E; 2.44 ± 1.94 times min−1, Kruskal–Wallis H = 6.281, P = 0.012 for Aristotle's lantern reflex, Fig. 3F).
Discussion
The increased incidence of bacteria causes black-mouth and spotting diseases greatly decreased the production of S. intermedius aquaculture1,4,8. We found that S. intermedius fed S. horneri showed significantly fewer morbidity than those fed S. japonica. A reasonable explanation is that polysaccharides enriched in S. horneri30 stimulate the innate immune system of sea urchins because cell walls of pathogenic bacteria possess polysaccharides that are identified as characteristic antigen molecules by the innate immune system31,32,33. This indicates that S. japonica could be an effective approach to the disease prevention in S. intermedius.
Strongylocentrotus intermedius necessarily requires at least one summer to develop from fertilized eggs to adults of commercial size (> 5 cm of test diameter) in long line culture in China4,7. It is not essential for small sea urchins to develop gametes, but more appropriate for somatic growth in aquaculture34. Gonadal precocity greatly consumes the stored energy and consequently leads to poor somatic growth35,36 and probable mortality of sea urchins. Dietary protein is the basis of gonadal developments of sea urchins35,37. The present study showed that S. intermedius fed S. horneri exhibited slower gonadal development than those fed S. japonica, even though no significant difference of crude protein concentration were found between the two brown algae. This indicates that gonadal development of sea urchins is probably due to other nutrient elements. Collectively, the present study indicates that S. horneri is an effective food to avoid the precocious puberty of S. intermedius and may subsequently contribute to their energy storage.
In addition, cultured S. intermedius requires higher resistance ability at adverse water temperatures in summer. Behaviors, which are realized by the coordination of neuromuscular systems38,39, display a strong correlation with the fitness of sea urchins40. Water temperature significantly affects neuromuscular activities. For example, the sea urchin Strongylocentrotus purpuratus showed a decreased adhesion when being exposed to the elevated water temperatures41. For the first time, the present study found that different species of brown algae have different effects on sea urchin behaviors under acutely changed and moderately elevated temperatures. Specifically, sea urchins fed S. horneri showed significantly greater Aristotle's lantern reflex than those fed S. japonica when being exposed to moderately elevated temperatures (an increase from 23.5 to 26.5 °C at a rate of 0.5 °C per day and maintained at 26.5 °C for 1 week). The Aristotle's lantern reflex represents the ability to operate sea urchin jaws to grasp a food around and is commonly used as an indicator for the food intake capacity29,42. Consistently, S. intermedius fed S. horneri exhibited significantly higher food consumption than those fed S. japonica. The study indicates that S. intermedius fed S. horneri displays a significantly greater capacity in thermal tolerance than those fed S. japonica. In comparison, sea urchins fed S. japonica outperformed those fed S. horneri in behaviors of righting, tube feet extension and Aristotle's lantern reflex under acutely changed water temperatures. These findings clearly suggest that S. intermedius fed S. japonica is more suitable for the areas with cold water mass in summer because it probably reduces the negative impacts of acute changes in water temperature on sea urchins. A possible explanation is that S. japonica contributes to the reduction of the free-radical level in organisms43, decreases tissue hypoxia and subsequently improves the neuromuscular activities of sea urchins.
In conclusion, S. horneri has direct application potential for the long line culture of S. intermedius in summer, because of the advantages in health, energy storage and resistance abilities. Further, S. japonica is appropriate for S. intermedius aquaculture in the areas with cold water mass where acute changes of water temperature exist. The present study provides valuable information into the management of the long line culture of small S. intermedius in summer.
Materials and methods
Sea urchins
Experimental sea urchins were produced in November 2018, fed Ulva pertusa ad libitum for ~ 2 months until the test diameter reached 0.3–0.4 cm diameter and subsequently fed S. japonica for culture1,4. Three hundred healthy S. intermedius (~ 3 cm of test diameter) were randomly selected from an aquaculture farm in Huangnichuan, Dalian (121° 45′ N, 38° 82′ E) and then were transported to the Key Laboratory of Mariculture and Stock Enhancement in North China’s Sea, Ministry of Agriculture at Dalian Ocean University (121° 56′ N, 38° 87′ E) on 9 July 2019. Sea urchins were maintained in a large fiberglass tank (length × width × height: 180 × 100 × 80 cm) of the recirculating system (Huixin Co., Dalian, China) to acclimatize to laboratory conditions and fed S. japonica ad libitum for 1 week with aeration. Water quality parameters were measured daily. Water temperature was 23.55 ± 0.07 °C, pH 7.72 ± 0.02 and salinity 33.76 ± 0.04. They were then fasted for another week until the experiment began.
Test diameter, wet body weight and wet gonad weight were evaluated for the initial conditions of sea urchins before the experiments started (N = 20 for test diameter and wet body weight; N = 10 for wet gonad weight).
Crude protein, fiber, fat and ash of S. horneri and S. japonica
Samples were taken from each dried brown alga to investigate their organic composition (crude protein, crude fiber and crude fat) and ash on 20 August 2019 (N = 3). Semi-micro Kjeldahl nitrogen was used to determine the crude protein concentration of the dried brown algae44. In order to measure the crude fiber concentration of brown algae, about 10 g of each sample of the dried brown algae was boiled with a mixed solution (1.25% dilute acid and dilute alkali) for 30 min and ashed at 550 °C to remove the minerals45. Five grams of each dried sample and 15 mL petroleum ether were added to the Soxhlet extractor and refluxed at constant temperature (45 ± 1 °C) for eight hours to assess the crude fat concentration of the brown algae46. To investigate the ash concentration of the brown algae, approximate two g dried samples were placed in a constant weight fritted glass and burned in a muffle furnace (M110, Thermo CO., U.S) at 550 °C for 48 h45.
Experiment I
Experimental design
Diet was the experimental factor, either S. horneri or S. japonica. Fresh S. horneri were collected from a farm in Huangnichuan Dalian (121° 45′ N, 38° 82′ E) and S. japonica from Dalian Bay (120° 37′ E 38° 56′ N) in July 2019. Individuals were fed dried S. horneri (experimental group) and dried S. japonica (control group) ad libitum for ~ 9 weeks during the experiment (from 23 July 2019 to 25 September 2019). One large fiberglass tank was used for each experimental treatment. One hundred sea urchins were haphazardly chosen and put into 100 individual cylindrical cages (length × width × height: 10 × 10 × 20 cm; 1.5 cm of mesh size) in each tank (length × width × height: 150 × 100 × 60 cm) of the recirculating system (Huixin Co., Dalian, China) with aeration, according to the experimental design. Diseased sea urchins were removed timely from the tanks to avoid the potential spread of infectious diseases in experimental treatments and were transported into new tanks (length × width × height: 75 × 45 × 35 cm) for individual culture and observation following with the previous management.
Water temperature was not controlled, ranging from 21.3 to 25.6 °C during the experiment. Water quality parameters were measured weekly as pH 7.59–7.85 and salinity 32.69–32.13. One-half of the seawater was renewed daily.
Number of survived and diseased sea urchins
Black-mouth disease refers to the perioral membrane turns black (Fig. 4A) with the decreased ability of attaching and feeding in sea urchins47. Sea urchin with spotting disease is indicated by the spotting lesions with red, purple or blackish color on the body wall followed by the detachment of local spines48 (Fig. 4B). The enlarging spotting lesions commonly cause ulceration on the body wall and finally result in death8. Sea urchin without disease performance is shown in Fig. 4C. The number of survived and diseased sea urchins (either black-mouth or spotting diseased) was recorded during the experiment.
Dried food consumption
The measurement of food consumption was conducted for six consecutive days (from 7 August 2019 to 12 August 2019). The total supplemented and remained diets were weighed (G & G Co., San Diego, USA) after removing the water on their surface. The samples of uneaten diets were collected, weighed and dried for 4 days at 80 °C and then reweighed (N = 5). To avoid the loss of uneaten food, a fine silk net (mesh size 260 μm) was set outside the cage to collect the fragments of uneaten brown algae7.
Dried food consumption was calculated as follows (according to Zhao et al.49 with some revisions):
F = dried food consumption (g), W1 = wet weight of total supplement diets (g), W2 = wet weight of total uneaten diets (g), Bs = wet weight of sample supplemented diets (g), Bu = dry weight of sample supplemented diets (g), Cs = wet weight of sample uneaten diets (g), Cu = dry weight of sample uneaten diets (g).
Growth
Test diameters, Aristotle’s lantern length were measured using a digital vernier caliper (Mahr Co., Ruhr, Germany). Body, Aristotle's lantern and gut were weighted wet using an electric balance (G & G Co., San Diego, USA) on 25 September 2019 (N = 27 for test diameter and wet body weight; N = 6 for Aristotle's lantern length, wet weight of Aristotle's lantern and gut).
Specific growth rate (SGR) was calculated according to the following formula:
SGR = specific growth rate, P2 = final wet body weight, P1 = initial wet body weight, D = experimental duration.
Gonad yield
Gonads were carefully collected from each treatment and weighed using an electric balance (G & G Co., San Diego, USA) on 25 September 2019 (N = 6). Gonad index was calculated according to the following formula:
GI = gonad index, GW = wet gonad weight, BW = wet body weight.
Gonadal development
One of five pieces of each gonad was preserved in the Bouin’s solution (saturated picric acid solution: formaldehyde: glacial acetic acid = 15: 5: 1) for 48 h between the treatments (N = 6). Standard histology technique, including embedment, infiltration, section and stain, was performed to make the gonad tissue slices50. Sections were classified according to the stage of development of germinal cells and nutritive phagocytes: stage I, recovering; stage II, growing; stage III, premature; stage IV, mature; stage V, partly spawned; stage VI, spent51,52,53.
Experiment II
Experimental design
Experiment II lasted for 4 weeks (from 25 September 2019 to 23 October 2019). Eighty healthy sea urchins were haphazardly selected from each treatment at the end of experiment I. They were then distributed into 80 cylindrical cages (5 × 10 × 10 cm) in each fiberglass tanks (length × width × height: 77.5 × 47.0 × 37.5 cm) of the temperature-controlled system (Huixin Co., Dalian, China) with aeration in both treatments. Sea urchins were maintained at 23.5 °C for 2 weeks (the average water temperature of experiment I) to eliminate the past thermal history, following the previous diet strategy of experiment I. Water quality was recorded daily as pH 7.83–7.85 and salinity 32.44–32.62. One-third of the seawater was renewed daily.
Subsequently, to investigate whether S. horneri and S. japonica contribute to the resistance abilities of small S. intermedius at moderately elevated temperatures, 40 individuals were haphazardly chosen from each treatment and placed into 40 cylindrical cages (5 × 10 × 10 cm) in each tank (length × width × height: 77.5 × 47.0 × 37.5 cm) of a temperature-controlled system (Huixin Co., Dalian, China) with aeration for both groups on 9 October 2019. They were subsequently exposed to the moderately elevated temperatures (rose from 23.5 to 26.5 °C at a rate of 0.5 °C per day and maintained at 26.5 °C for 1 week), according to the records of water temperature in Heishijiao sea area (~ 2 m water depth, 38° 51′ N, 121° 33′ E) in the summer of 2017 and 2018 (Fig. 5). Righting behavior, tube feet extension and Aristotle's lantern reflex were assessed on 23 October 2019.
Similarly, to explore the effects of S. horneri and S. japonica on the resistance abilities of small S. intermedius under acute changes of water temperature, another 40 individuals were randomly selected and placed into 40 cylindrical cages (5 × 10 × 10 cm) in each tank (length × width × height: 77.5 × 47.0 × 37.5 cm) of the temperature-controlled system (Huixin Co., Dalian, China) with aeration for both treatments on 9 October 2019. The water temperature was set at 23.5 °C. A tank of seawater was prepared at 15 °C. To simulate the changes of water temperature in Haiyang island near Dalian (39° 03′ N, 123° 09′ E) where water temperature frequently fluctuates from 22 to 16 °C instantly by the cold water mass17, sea urchins were transferred directly from 23.5 to 15 °C, maintained at 15 °C for an hour and subsequently quickly returned to 23.5 °C for another hour to finish one cycle of the acute change of water temperature. After four cycles, righting behavior, tube feet extension and Aristotle's lantern reflex of sea urchins were observed.
Righting behavior
Sea urchins were placed with the aboral side down on the bottom of an experimental tank (length × width × height: 60 × 40 × 16 cm, Fig. 4D). Righting response time is the time required for individuals in the inverted posture to right themselves with the aboral side up22. The righting response time in seconds was recorded during 10 min. If individuals did not right themselves within 10 min, the time was counted as 600 s (N = 15).
Tube feet extension
The method of assessing tube feet extension was established according to You et al.27, with some revisions. Sea urchins were maintained in a tank (length × width × height: 12 × 10 × 10 cm) with fresh seawater for ~ 5 min before the observation (N = 15). The subjective assessment of tube feet extension was evaluated by a well-trained team (5 persons) that was familiar with tube feet extension analysis of sea urchins. The ranking method was quantified based on the quantity and length of tube foot.
Tube feet extension (rating 1–5):
1 = extremely abnormal (not extending)
2 = severe abnormality (extremely low quantity and extremely short length)
3 = moderate anomaly (low quantity and short length)
4 = mild abnormality (slight decrease in quantity and length)
5 = normal (normal quantity and length)
Aristotle's lantern reflex
A simple device, which has two small compartments (length × width × height: 4.8 × 5.6 × 4.5 cm) with a food film on the bottom, was used to measure Aristotle's lantern reflex according to our previous study29. Food film was made by a mixture of ~ 2.5 g agar and 50 ml seawater in order to avoid the potential impacts of the food palatability on sea urchins. The number of Aristotle's lantern reflex were counted within 5 min using a digital camera (Canon Co., Shenzhen, China) under the device (N = 7 for sea urchins fed S. horneri and N = 10 for individuals fed S. japonica under moderately elevated temperatures; N = 10 for both groups under acutely changed temperatures; Fig. 4E).
Statistical analysis
Normal distribution and homogeneity of variance of the data were analyzed using the Kolmogorov–Smirnov test and Levene test, respectively. The number of survived and diseased S. intermedius were compared using the Fisher′s exact test. Food consumption was analyzed using one-way repeated measured ANOVA. Kruskal–Wallis test was performed to compare the difference of righting behavior and tube feet extension between the treatments and also used to analyze Aristotle's lantern reflex under acutely changed temperatures. Independent-samples t test was carried out to compare the difference between the final and initial conditions of sea urchins. Test diameter, wet body weight, SGR, Aristotle's lantern length, wet Aristotle's lantern weight, wet gut weight, gonad index and Aristotle's lantern reflex (under moderately elevated temperatures) were analyzed using the independent-samples t test. All data analyses were performed using SPSS 19.0 statistical software. A probability level of P < 0.05 was considered significant.
References
Chang, Y., Ding, J., Song, J. & Yang, W. Biology and Aquaculture of Sea Cucumbers and Sea Urchins (Ocean Press, Beijing, 2004).
Unuma, T. Introduction: Sea urchin fisheries in Japan. In Echinoderm Aquaculture (eds Brown, N. P. & Eddy, S. D.) 77–85 (Wiley, Hoboken, 2015).
Agatsuma, Y. Strongylocentrotus intermedius. In Developments in Aquaculture and Fisheries Science (ed. Lawrence, J. M.) (Elsevier, Amsterdam, 2013).
Lawrence, J. M., Zhao, C. & Chang, Y. Q. Large-scale production of sea urchin (Strongylocentrotus intermedius) seed in a hatchery in China. Aquacult. Int. 27, 1–7. https://doi.org/10.1007/s10499-018-0319-2 (2019).
Zhang, X. China Fishery Statistical Yearbook 2019 (China Agriculture Press, Beijing, 2019).
Chang, Y. Q. et al. Aquaculture of the sea urchins Strongylocentrotus intermedius in Fujian coastal areas. South China Fish. Sci. 16, 1–9. https://doi.org/10.12131/20190156 (2019).
Zhang, L. et al. Gulfweed Sargassum horneri is an alternative diet for aquaculture of juvenile sea urchins Strongylocentrotus intermedius in summer. Aquacult. Int. 25, 905–914. https://doi.org/10.1007/s10499-016-0088-8 (2017).
Zhang, W. et al. Transcriptome profiling reveals key roles of phagosome and NOD-like receptor pathway in spotting diseased Strongylocentrotus intermedius. Fish Shellfish Immunol. 84, 521–531. https://doi.org/10.1016/j.fsi.2018.10.042 (2019).
Lin, Q., Wu, J. S. & Zeng, Z. N. Comparative study on culture biology of triploid and diploid induced by Crassostrea gigas. Fish. Sci. Technol. Inf. 28, 265–267 (2001) (in Chinese).
Kong, Y. T., Cheng, Z. M., Wang, Q. & Zhong, W. Annual reproductive cycle of sea urchin, Stongylocentrotus intermedius, in raft cultivation. Fish. Sci. 21, 18–21 (2002) (in Chinese).
Joll, L. & Caputi, N. Geographic variation in the reproductive cycle of the saucer scallop, Amusium balloti, (Bernardi, 1861) (Mollusca: Pectinidae), along the Western Australian coast. Mar. Freshw. Res. 46, 779. https://doi.org/10.1071/MF9950779 (1995).
Apraiz, I., Mi, J. & Cristobal, S. Identification of proteomic signatures of exposure to marine pollutants in Mussels (Mytilus edulis). Mol. Cell. Proteomics 5, 1274–1285. https://doi.org/10.1074/mcp.m500333-mcp200 (2006).
Hokkaido Central Fisheries Experimental Station, Hiribeshihokubu Fisheries Extension Office & Hokkaido Institute of Mariculture. On the nature seeds collection, intermediate culture and release of the sea urchin, Strongylocentrotus intermedius. J. Hokkaido Fish. Exp. Station 41, 270–315 (1984) (in Japanese).
Du, B., Zhang, Y. J., Shang, Y. C. & Wang, H. The characteristic of cold water mass variation at the bottom of the north yellow sea and its hydrological effects on the mortality of shellfish cultured in the water of outer China-Shan islands. Mar. Sci. Bull. 14, 17–28 (1996) (in Chinese).
Guo, J., Ji, D., Hou, C., Guo, K. & Ji, L. Impact of tropical cyclone Matmo on mixed zone of the Yellow and Bohai Seas. J. Oceanol. Limnol. 36, 1484–1493. https://doi.org/10.1007/s00343-018-7085-x (2018).
Zhou, W. Water temperature characteristics of Oceanic islands which were affected by the yellow sea cold water mass. Acta Oceanol. Sin. 18, 113–118 (1996) (in Chinese).
Zhou, W. et al. The relation between the abnormal change of water temperature and the scallop (Chlamys farreri) death in Haiyang island sea area. Trans. Oceanol. Limnol. 4, 56–62 (1992) (in Chinese).
Sangha, J. S. et al. Bioactive components of the edible strain of red alga, Chondrus crispus, enhance oxidative stress tolerance in Caenorhabditis elegans. J. Funct. Foods 5, 1180–1190. https://doi.org/10.1016/j.jff.2013.04.001 (2013).
Yengkhom, O., Shalini, K. S., Subramani, P. A. & Michael, R. D. Non-specific immunity and disease resistance are enhanced by the polysaccharide fraction of a marine chlorophycean macroalga in Oreochromis niloticus (Linnaeus, 1758). J. Appl. Ichthyol. 34, 556–567. https://doi.org/10.1111/jai.13606 (2018).
Stone, D. A. J. et al. Dietary intervention improves the survival of cultured greenlip abalone (Haliotis laevigata Donovan) at high water temperature. Aquaculture 430, 230–240. https://doi.org/10.1016/j.aquaculture.2014.03.047 (2014).
Hyman, L. H. The Invertebrates: Echinodermata (McGraw-Hill, New York, 1955).
Chi, X. et al. Fitness benefits and costs of shelters to the sea urchin Glyptocidaris crenularis. PeerJ 8, e8886. https://doi.org/10.7717/peerj.8886 (2020).
Lawrence, J. M. The effect of temperature-salinity combinations on the functional well-being of adult Lytechinus variegatus (Lamarck) (Echlnodermata, Echinoldea). J. Exp. Mar. Biol. Ecol. 18, 271–275. https://doi.org/10.1016/0022-0981(75)90111-2 (1975).
Hagen, N. T. Is righting response a useful indicator of functional well-being in the green sea urchins Strongylocentrotus droebachiensis. In Echinoderms Through Time (eds David, G. & Reds, F.) 693–698 (Balkema Press, Rotterdam, 1994).
Böttger, S. A., McClintock, J. B. & Klinger, T. S. Effects of inorganic and organic phosphates on feeding, feeding absorption, nutrient allocation, growth and righting responses of the sea urchin Lytechinus variegatus. Mar. Biol. 138, 741–751. https://doi.org/10.1007/s002270000476 (2001).
Santos, R. & Flammang, P. Intra- and interspecific variation of attachment strength in sea urchins. Mar. Ecol. Prog. Ser. 332, 129–142. https://doi.org/10.3354/meps332129 (2007).
You, K., Zeng, X. Q., Liu, H., Zhang, X. M. & Liu, Q. Selectivity and tolerance of sea urchins (Hemicastrates pulcherrimus) to environmental change. Chin. J. Appl. Ecol. 14, 409–412 (2013) (in Chinese).
Brothers, C. J. & McClintock, J. B. The effects of climate-induced elevated seawater temperature on the covering behavior, righting response, and Aristotle’s lantern reflex of the sea urchin Lytechinus variegatus. J. Exp. Mar. Biol. Ecol. 467, 33–38. https://doi.org/10.1016/j.jembe.2015.02.019 (2015).
Ding, J. et al. Effects of water temperature on survival, behaviors and growth of the sea urchin Mesocentrotus nudus: New insights into the stock enhancement. Aquaculture 519, 734873. https://doi.org/10.1016/j.aquaculture.2019.734873 (2020).
Wen, Z. S. et al. Composition and anti-inflammatory effect of polysaccharides from Sargassum horneri in RAW264.7 macrophages. Int. J. Biol. Macromol. 88, 403–413. https://doi.org/10.1016/j.ijbiomac.2016.02.025 (2016).
Uribe, C., Folch, H., Enriquez, R. & Moran, G. Innate and adaptive immunity in teleost fish: A review. Vet. Med. 56, 486–503. https://doi.org/10.17221/3294-VETMED (2011).
Skjermo, J. et al. Immunostimulation of juvenile turbot (Scophthalmus maximus L.) using an alginate with high mannuronic acid content administered via the live food organism Artemia. Fish Shellfish Immunol. 5, 531–534. https://doi.org/10.1016/s1050-4648(95)80053-0 (1995).
Cheng, A., Tu, C., Chen, Y., Nan, F. & Chen, J. The immunostimulatory effects of sodium alginate and iota-carrageenan on orange-spotted grouper Epinephelus coicoides and its resistance against Vibrio alginolyticus. Fish Shellfish Immunol. 22, 197–205. https://doi.org/10.1016/j.fsi.2006.04.009 (2007).
Lawrence, J. M. Conflict Between Somatic and Gonadal Growth in Sea Urchins: A Review (2000). https://crdpm.umcs.ca/OURSIN/.
Hu, F. et al. Effects of macroalgae Gracilaria lemaneiformis and Saccharina japonica on growth and gonadal development of the sea urchin Strongylocentrotus intermedius: New insights into the aquaculture management in southern China. Aquacult. Rep. 17, 100399. https://doi.org/10.1016/j.aqrep.2020.100399 (2020).
Hammer, B. W. et al. The effects of dietary protein concentration on feeding and growth of small Lytechinus variegatus (Echinodermata: Echinoidea). Mar. Biol. 145, 1143–1157. https://doi.org/10.1007/s00227-004-1391-x (2004).
Kelly, M. S., Brodie, C. & McKenzie, J. D. Somatic and gonadal growth of the sea urchin, Psammechinus miliaris, maintained in polyculture with the Atlantic salmon. J. Shellfish Res. 17, 1557–1562 (1998).
De Ridder, C. & Lawrence, J. M. Food and feeding mechanisms: Echinoidea. In Echinoderm Nutrition (eds Jangoux, M. & Lawrence, M.) 57–115 (Balkema, Rotterdam, 1982).
Kleitman, N. The effect of temperature on the righting of Echinoderms. Biol. Bull. 80, 292–298. https://doi.org/10.2307/1537716 (1941).
Ling, S. D. & Johnson, C. R. Marine reserves reduce risk of climate-driven phase shift by reinstating size- and habitat- specific trophic interactions. Ecol. Appl. 22, 1232–1245. https://doi.org/10.1890/11-1587.1 (2012).
Percy, J. A. Thermal adaptation in the boreo-arctic echinoid, Strongylocentrotus droebachiensis (O. F. Muller, 1776). III. Seasonal acclimatization and metabolism of tissues in vitro. Physiol. Zool. 47, 59–67. https://doi.org/10.1086/physzool.47.1.30155622 (1974).
Agustina, Y. Effect of the covering behavior of the juvenile sea urchin Strongylocentrotus intermedius on predation by the spider crab Pugettia quadriceps. Fish. Sci. 67, 1181–1183. https://doi.org/10.1046/j.1444-2906.2001.00379.x (2001).
Sprygin, V. G., Kushnerova, N. F., Fomenko, S. E., Sizova, L. A. & Momot, T. V. The hepatoprotective properties of an extract from the brown alga Saccharina japonica. Russ. J. Mar. Biol. 39, 65–69. https://doi.org/10.1134/S1063074013010100 (2013).
Chen, Z. H., Shi, M., Wang, Q. X. & Zhang, X. H. Protein content measurement of food using the method of Kjeldahl determination. Xinjiang Anim. Husb. 5, 22–24 (2008) (in Chinese).
Cheng, J. Improvement of Determination Method of Feed Conventional Analysis (Northeast Agricultural University, Harbin, 2016).
Zhang, J., Yang, J. & Dong, W. Study on improved methods of determination of crude fat in foods. in Modern Agricultural Science Technology 333–334 (2012) (in Chinese).
Li, T. W., Xu, S. L., Wang, R. B., Xu, S. F. & Su, X. R. Preliminary studies on the black mouth disease of sea urchin, Strongylocentrotus intermedius. Mar. Sci. 24, 41–43 (2000) (in Chinese).
Wang, Y. N., Chang, Y. Q. & Lawrence, J. M. Sea Urchins: Biology and Ecology, Disease in Sea Urchins (Academic Press, Cambridge, 2013).
Zhao, C. et al. Effects of temperature and feeding regime on food consumption, growth, gonad production and quality of the sea urchin Strongylocentrotus intermedius. J. Mar. Biol. Assoc. U.K. 96, 185–195. https://doi.org/10.1017/S0025315415001617 (2016).
Johnstone, J., Nash, S., Hernandez, E. & Rahman, M. S. Effects of elevated temperature on gonadal functions, cellular apoptosis, and oxidative stress in Atlantic sea urchin Arbacia punculata. Mar. Environ.Res. 149, 40–49. https://doi.org/10.1016/j.marenvres.2019.05.017 (2019).
Byrne, M. Annual reproductive cycles of the commercial sea urchin Paracentrotus lividus from an exposed intertidal and a sheltered subtidal habitat on the west coast of Ireland. Mar. Biol. 104, 275–289. https://doi.org/10.1007/BF01313269 (1990).
Laegdsgaard, P., Byrne, M. & Anderson, D. T. Reproduction of sympatric populations of Heliocidaris erythrogramma and H. tuberculata (Echinoidea) in New South Wales. Mar. Biol. 110, 359–374. https://doi.org/10.1007/bf01344355 (1991).
King, C. K., Hoegh-Guldberg, O. & Byrne, M. Reproductive-cycle of Centrostephanus-rodgersii echinoidea, with recommendations for the establishment of a sea-urchin fishery in new-south-wales. Mar. Biol. 120, 95–106 (1994).
Acknowledgements
This work was supported by Chinese Outstanding Talents in Agricultural Sciences (for Yaqing Chang), Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao National Laboratory for Marine Science and Technology, China P. R. (2017-1B05), a grant for innovative talents in universities in Liaoning Province (for Chong Zhao). All applicable international, national, and/or institutional guidelines for the care and use of animals were followed by the authors. We thank Prof. John Lawrence for academic and editorial suggestions and Tianyang Zhang, Jiangnan Sun, Xiyuan Huang and Huiyan Wang for their assistance.
Author information
Authors and Affiliations
Contributions
C.Z., F.H. and Y.C. designed the experiments. F.H., M.Y., P.D., X.Z., Z.C. and J.L. carried out the experiments. F.H. and J.D. and X.C. did the data analysis. F.H. and C.Z. wrote the manuscript. All authors gave final approval for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hu, F., Yang, M., Ding, P. et al. Effects of the brown algae Sargassum horneri and Saccharina japonica on survival, growth and resistance of small sea urchins Strongylocentrotus intermedius. Sci Rep 10, 12495 (2020). https://doi.org/10.1038/s41598-020-69435-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-69435-8
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.