Abstract
Eustachian tube balloon dilatation (ETBD) has shown promising results in the treatment of ET dysfunction (ETD); however, recurrent symptoms after ETBD frequently occur in patients with refractory ETD. The excessive pressure of balloon catheter during ETBD may induce the tissue hyperplasia and fibrotic changes around the injured mucosa. Sirolimus (SRL), an antiproliferative agent, inhibits tissue proliferation. An SRL-coated balloon catheter was fabricated using an ultrasonic spray coating technique with a coating solution composed of SRL, purified shellac, and vitamin E. This study aimed to investigate effectiveness of ETBD with a SRL-coated balloon catheter to prevent tissue proliferation in the rat ET after ETBD. In 21 Sprague–Dawley rats, the left ET was randomly divided into the control (drug-free ETBD; n = 9) and the SRL (n = 9) groups. All rats were sacrificed for histological examination immediately after and at 1 and 4 weeks after ETBD. Three rats were used to represent the normal ET. The SRL-coated ETBD significantly suppressed tissue proliferation caused by mechanical injuries compared with the control group. ETBD with SRL-coated balloon catheter was effective and safe to maintain ET luminal patency without tissue proliferation at the site of mechanical injuries for 4 weeks in a rat ET model.
Similar content being viewed by others
Introduction
The Eustachian tube (ET) is a connector of the middle ear to the nasopharynx and has important functions in the middle ear such as ear ventilation, transport and secretion of pathogens, and protection from nasopharyngeal reflux1. The opening and closing of the ET are normally controlled by the movement of surrounding paratubal muscles including the tensor tympani, tensor and levator veli palatini, and salpingopharyngeus muscles; however, when the cartilaginous portion of the ET does not open properly, it causes obstructive ET dysfunction (ETD), which can lead to otitis media and cholesteatoma2,3. Although various pharmacologic agents, such as steroids, have been used in an attempt to relieve symptoms of ETD, they had limited effect3. Therefore, more direct therapeutic strategies, including laser Eustachian tuboplasty, ventilation tube insertion, and microdebrider tuboplasty, have been proposed for treating ETD4. However, these surgical procedures are associated with a risk of recurrence, and often result in adverse events such as ET obstruction, infection, and permanent perforation of the tympanic membrane5.
Eustachian tube balloon dilatation (ETBD) is emerging as a preferred alternative therapeutic option for obstructive ETD and has shown promising results6,7,8,9,10. Balloon dilatation in the cartilaginous portion of the ET can suppress adhesion and enlarge the mucous membranes; symptoms related with ETD are significantly improved in more than 70% of patients treated with ETBD11. However, resistant cases of ETD, such as patients who do not respond to primary ETBD, require repeated ETBD. These have a major negative impact on the quality of life of patients and are associated with recurrent symptoms12. Therapeutic mechanisms of ETBD have not yet been fully established; however, previous studies have indicated that ETBD leads to mild fractures within the ET cartilage and persistent dilation of the lumen13. A previous study of serial histological changes after ETBD in a rat model has reported that fibrosis of cartilage defects and increased thickness of the submucosa owing to tissue hyperplasia were observed. Although the lumen had been enlarged, luminal narrowing gradually developed over time owing to fibrotic tissue changes at the cartilage and submucosa injured by ETBD14.
In earlier studies, a drug-coated balloon catheter was used for homogenous delivery of anti-proliferative drugs at the target lesion through an inflated balloon to prolong patency and prevent restenosis15,16. Drug-coated ETBD may facilitate the compression of hypertrophic mucosal and submucosal injuries, potentially enabling healing with thinner, healthier layers and promoting rapid recovery after balloon dilation. A cobalt–chrome alloy stent eluting sirolimus (SRL), an anti-proliferative agent, effectively suppressed stent-induced tissue hyperplasia in a porcine ET model13,14,18. We hypothesized that SRL-coated balloon catheters would effectively prolong patency of the ET while inhibiting proliferation and/or fibrotic changes in submucosa resulting from mechanical injury caused by balloon dilation. This study aimed to investigate the effects of ETBD with SRL-coated balloon catheter to prevent tissue proliferation caused by fibrotic changes by ETBD in a rat ET model.
Results
Characterization of the sirolimus-coated balloon catheter
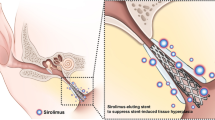
The SRL-coated balloon catheter was successfully fabricated using an ultrasonic spray coating process. The surface morphology of the SRL-coated balloon was observed to be smooth and did not have any trace of SRL crystallization (Fig. 1). The drug content and its uniformity in the SRL-coated balloon was verified using UV–Vis spectrophotometry over a period of 30 days. The coating layer was approximately 7.5-µm thick according to the optical microscopic images. (Fig. 1d). The payload of SLR was 1.27 µg/mm2 per balloon catheter. As shown in Fig. 1e, the SRL was slowly released from the balloon surface. The burst release at 7 days was approximately 39.5%, and approximately 85 ± 6% of the SRL was released at 30 days (Fig. 1b). SRL was securely attached to the surface of the balloon catheter.
Schematic of sirolimus (SRL)-coated balloon catheter. (a) Schematic of SRL coating onto the balloon surface. (b) Graph shows the in vitro release profiles of the SRL from the balloon catheter. (c) Representative SEM images show the morphological characterization of the drug-free and SRL-coated balloon surfaces. (d) Optical images of drug-free and SRL-coated balloon surfaces were analyzed using ImageJ (U.S. National Institutes of Health, Bethesda, MD) to measure the coating thickness.
Technical outcomes of ETBD
The ETBD was technically successful in all rats. Mild nosebleed was observed immediately after the procedure in two rats of the control group but resolved spontaneously within 30 min. The tympanic membrane was successfully punctured using a 24 G angiocatheter with a needle. During a contrast study of the rat ET, aspiration did not occur. A micro guidewire negotiated out of the nostrils. The balloon catheter was retrogradely advanced without any resistance. All rats survived until their respective follow-up periods without procedure-related complications (Fig. 2).
Study design and technical steps of Eustachian tube balloon dilatation (ETBD) in a rat model. (a) Flowchart shows the randomization of process and study follow-up. (b) The rat was placed in the prone position and a 24 G angiocatheter with a 0.014-inch micro guidewire was inserted from the tympanic membrane to the nasal cavity. (c) ETBD was performed under fluoroscopic guidance. (d) Contrast medium was injected through the angiocatheter to confirm the location of the rat ET (arrowheads). (e) Micro guidewire was inserted through the angiocatheter until it came out of the nostrils. (f) Balloon catheter was retrogradely advanced over the guidewire into the rat ET (arrowheads) and was inflated (arrows) to 9 atm for 1 min.
Luminal patency of the ET after balloon dilatation
The luminal patency of the ET after ETBD is shown in Fig. 3. The luminal patency of the ET in both groups was relatively expanded immediately after ETBD compared with that of normal ET. However, the epithelial layers were seriously damaged in both groups. At 1 week after ETBD, severe proliferative epithelial layer and submucosa were observed in the control group. The median percentage of ET luminal area in the control group was significantly lower than that in the SRL group (50.74%, interquartile range (IQR) 47.93–58.25% vs. 130.11%, IQR 119.50–140.38%, p < 0.01). However, no significant differences were observed between the two groups (106.83%, IQR 101.51–111.64% vs. 113.71%, IQR 109.57–131.06, p = 0.065) at 4-weeks follow-up, although it was relatively large in the SRL group.
Representative histological images of the ET treated with ETBD. (a) The changes in ET luminal area after ETBD with or without SRL-coated balloon over time in a rat ET model. The luminal area in the control group decreased owing to proliferative tissue caused by balloon dilation; however, the luminal area in the SRL group was adequately preserved. (b) Histological image of the normal ET and method of analysis of ET luminal area (c) The graph shows the percentage of ET luminal area in the study groups. ***p < 0.001.
Histological findings
The histological and immunohistochemical findings of serial changes in the ET tissue over time are summarized in Table 1 and shown in Figs. 4 and 5. Histological evaluations performed immediately after ETBD were not significantly different between the two groups (all variables; p > 0.05). The median thickness of epithelial layers was significantly higher in the control group than that in the SRL group at 1 week after ETBD (80.70 µm, IQR 72.45–94.35 µm vs. 15.90 µm, IQR 10.75–18.60 µm, p < 0.001). However, at 4 weeks after ETBD, the median thickness of the epithelial layers in the control group was significantly lower than that in the SRL group (27.10 µm, IQR 23.85–32.05 µm vs. 58.20 µm, IQR 48.00–62.40 µm, p < 0.001). The thickness of the submucosal fibrosis in the control group was significantly higher than that in the SRL group after 1 week and 4 weeks (both p < 0.001). The degree of inflammatory cell infiltration in the control group was also higher than that in the SRL group at both 1 week and 4 weeks (p < 0.05, respectively). However, no significant difference was observed in the collagen deposition between the two groups over time (1 week after ETBD: 2.00, IQR 1.00–3.50 in the control group vs. 2.00, IQR 1.00–2.25 in the SRL group, p = 0.632; 4 weeks after ETBD: 2.50, IQR 1.75–3.25 in the control group vs. 2.00, IQR 1.00–3.25 in the SRL group, p = 0.737). The degree of α-smooth muscle actin (α-SMA)-positive deposition was significantly higher in the control group than in the SRL group at 1 and 4 weeks (1 week after ETBD: 4.00, IQR 4.00–5.00 in the control group vs. 2.00, IQR 1.00–2.00 in the SRL group, p < 0.001; 4 weeks after ETBD: 3.00, IQR 2.00–3.00 in the control group vs. 2.00, IQR 1.00–2.00 in the SRL group, p < 0.05).
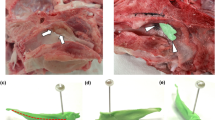
Fibrotic changes in the cartilaginous portion of the ET after drug-free or SRL-coated ETBD. (a) Histological images showing the ETBD-induced laceration (arrowheads) in the cartilaginous portion of the ET and the fibrotic tissue changes (arrows) that replaced the damaged areas of the cartilaginous portion. (b) Graphs showing the differences in histological findings between the two study groups. **p < 0.05, ***p < 0.001.
Cartilaginous fibrosis of the ET after balloon dilatation
The cartilage fractures of the cartilaginous portion of the ET were observed in 14 (77.7%) of 18 rat ETs (Fig. 5a). The cartilaginous defects were filled with fibrosis over time. The percentage of fibrotic area at the cartilaginous portion of the ET in the control group was significantly higher than that in the SRL group at 1-week follow-up (p < 0.001). However, no significant difference was observed between the two groups at 4-week follow-up, although the percentage in the control group was relatively high than that in the SRL group (20.50%, IQR 17.55–28.03% vs. 17.15%, IQR 11.03–20.35%, p = 0.102).
Discussion
The SRL-coated balloon catheter was successfully fabricated using an ultrasonic spray coating technique with a coating solution composed of SRL, purified shellac, and vitamin E. The shellac is a natural glue and clear coating material. The surface of the balloon can be rendered adhesive by the shellac and the purified shellac may increase attachment of the SRL to balloon surface and prevent SRL loss from the coating surface before the procedure. Vitamin E can reduce the inflammatory response immediately after ETBD17. Our in vivo study demonstrated that the degree of inflammatory cell infiltration in the SRL group was significantly lower than that in the control group. Using ultrasonic spray coating can decrease initial burst release and ensure uniform coating layers. Our in vitro results revealed that approximately 76% of the SRL was maintained on the balloon surface at 3 days.
The balloon dilatation of the cartilaginous portion of ET has been considered a safe and effective procedure for treating obstructive ETD18. The precise mechanism underlying tissue injuries owing to balloon dilation in the ET remains unclear and may involve a combination of factors, including direct pressure, hypoxia/hypoperfusion, and shearing trauma13,19,20. When BD is performed, the luminal area is significantly expanded while simultaneously causing crush injury to the epithelium and submucosa21. ETBD failure may be related to excessive tissue hyperplasia with fibrotic tissue formation caused by crush injury. Recurrence rates of 10–46% have been reported within the first month after ETBD11,22,23. Our histological findings revealed a significant increase in submucosal fibrosis over time in the rat ET treated with ETBD. However, the thickness of the submucosal fibrosis in the SRL group was significantly lower than that in the control group. The luminal patency of the rat ET treated with SRL-coated ETBD was maintained for 4 weeks.
SRL interrupts the mammalian target of rapamycin (mTOR) intracellular signaling pathway and inhibits phases of proliferation by blocking cell cycle progression at the G1/S transition24,25,26,27. Thus, SRL acts in the initial phase of the cell cycle and is considered to have lower toxicity compared with drugs that act in later stages of the cell cycle28. The severe proliferative epithelial layer was observed in the control group at 1 week after ETBD. In contrast, the SRL group had a significantly lower regenerated thickness of the epithelial layers. At 4 weeks after ETBD, the epithelial layers in the SRL group were increased compared with the control group. SRL inhibits the initial tissue hyperplasia owing to mechanical injuries and has low toxicity, suggesting that it does not affect the normal regeneration of epithelial layers after ETBD. The proliferative phase begins from days 4–14 of the wound healing process, with an excessive increase in fibroblasts and myofibroblasts29. Induction of fibrosis in the submucosa of ET increases the rigidity of the lumen. The strengthened ET lumen maintains patency30,31. However, excessive submucosal fibrosis not only hardens the ET lumen but may also affect ET movement and lead to patulous ETD. Richard et al. reported that the rate of patulous ETD symptoms was 7% after ETBD32. This rate is higher than the natural prevalence of patulous ETD33. α-SMA is commonly used as a marker of myofibroblasts, which mediate wound contraction but cause fibrosis when persist in the tissue25. The thickness of submucosal fibrosis and degree of α-SMA-positive deposition were significantly lower in the SRL group than in the control group both at 1 week and 4 weeks. SRL-coated balloons can be effective from a long-term perspective by suppressing fibrosis.
The ET cartilage is an elastic cartilage, which has the highest elasticity among cartilages4,34,35. Small full-thickness defects of elastic cartilage are primarily replaced by fibrocartilage, whereas partial defects have voids filled by the infiltration of fibrous scar tissue34. The cartilage fractures are closely related to the size and pressure of the balloon catheter. Many studies have emphasized the importance of the size and pressure of the balloon catheter used for ETBD18,36,37,38,39. In our study, ETBD was performed for 1 min at a pressure of 9 atm using a 1.25-mm diameter balloon catheter in the left ET of the rats, and partial cartilage fractures were observed in 77.7% of all ETs after ETBD. The fibrotic area in the cartilaginous portion increased at 1 week and decreased at 4 weeks after ETBD in the control group. In the SRL group, the fibrotic area gradually increased over time but was relatively low compared with that in the control group. This suggests that the infiltration of fibrous scar tissue into partial defects of the fractured cartilage is delayed owing to SRL but not replaced by elastic cartilage. The lack of response to repeat ETBD after the failure of primary ETBD40 may be owing to the increased fibrotic area of the fractured cartilage. Infiltration of fibrous scar tissue may interfere with cartilage expansion and contraction and result in additional defects41,42. Therefore, to prevent cartilage fractures, the size of the balloon catheter should be determined after diagnosing the patient's ET. Although SRL did not prevent fibrotic scar tissue infiltration in the cartilaginous portion, the histological results of this study provide the basis for early preclinical findings to address the limitations of ETBD.
The ETBD via trans-tympanic approach was technically successful in all rats without severe procedure-related complications. The rat ET model has several advantages compared with large animal models, including cost effectiveness, readily available, animal study on large sample size, and easy to use. Pig or sheep ET model has been commonly used, but these models are extremely expensive and is available only in a few laboratories. Although the anatomical structure is similar to that of human or large animal, the anatomy of the rat ET is relatively small, narrow and complicated43. We chose appropriate interventional equipment such as balloon catheter and guidewire and successfully performed ETBD in a rat ET model. Our ETBD rat model proved to be feasible for use in the rat ET and an efficient approach to stimulate balloon-induced mucosal injuries as a potential model for reproducing the mechanisms of mucosal injuries with healing process.
This study had several limitations. First, the number of animals was limited, and a robust statistical analysis could not be performed. Second, ETBD was performed in the normal rat ET and the wound healing process following ETBD may differ from that of obstructive ETD. Third, in vivo pharmacokinetic studies of the SRL-coated balloon were not conducted. Fourth, histological analysis was determined subjectively according to the distribution and density of the cells or antibody positive deposition. Finally, functional evaluation of the rat ET could not be performed. Nevertheless, although additional studies are required to identify the optimal SRL dose and verify its efficacy and safety in a large animal model, our study results support the basic concept of SRL-coated ETBD to prolong ET luminal patency in a rat model.
ETBD performed using an SRL-coated balloon catheter was effective and safe to maintain ET luminal patency without tissue proliferation at the site of mechanical injuries for 4 weeks in a rat ET model. In this study, we were able to successfully coat SRL with purified shellac and vitamin E using the ultrasonic spray coating. The SRL-coated ETBD significantly suppressed tissue proliferation compared with the control group in balloon-induced mechanical injuries. Although further studies are needed to investigate the efficacy and safety of SRL-coated ETBD for obstructive ETD, the SRL-coated ETBD should be a promising therapeutic option for patients with obstructive ETD or refractory ETD after ETBD.
Methods
Preparation of sirolimus-eluting balloon catheter
The balloon catheter (JLinker Inc., Jangseong, Korea) for the rat ET was 1.25 mm in diameter and 10 mm in length. SRL was coated onto the surface of the expanded balloon catheter using an ultrasonic coating machine (DEB coating machine, Noanix Inc., Korea). The coating solution consisted of vitamin E (5 mg, WonPoong Pharm. Co., Ltd, Seoul, Korea), purified shellac (5 mg, WonPoong Pharm. Co., Ltd), and SRL (5 mg, Biocon, Bangalore, India) dissolved in 5 mL of tetrahydrofuran. The balloon catheter was placed under an ultrasonic spray nozzle with a rotating shaft. The coating solution was sprayed over the balloon surface through the ultrasonic nozzle at room temperature (24 ± 1°C) with flow rate of 50 μL/min under a nitrogen atmosphere. The SRL-coated balloon catheter was vacuum-dried for 24 h. The prepared SRL-coated balloon catheter was crimped for the procedure using a crimping machine (J-Crimp; Blockwise Engineering, Tempe, Ariz).
Morphological analysis of sirolimus-coated balloon catheter
The surface morphology of the surface of the SRL-coated balloon was analyzed using optical and scanning electron microscopy (SEM; Sigma 300, Carl Zeiss, Oberkochen, Germany). The SRL-coated sample was fixed on an aluminum pin stub mount using carbon tape, and then the surface of the samples was analyzed to ensure distribution of the coating solution over the balloon surface.
Drug amount and release of sirolimus-coated balloon catheter
Amount of SRL on the balloon catheter was measured using a UV–Vis spectrophotometer at 278 nm. To determine the amount released of SRL from the balloon surface after inflating, a standard curve was prepared by measuring the concentration of SRL, which was dissolved in phosphate buffer saline (pH 7.4) and acetonitrile in the range of 0.5–32.5 µg/mL. The expanded balloon catheter was inserted within a silicone tube of 3 mm in diameter. The tubes were immersed in 5 mL of phosphate-buffered saline (PBS) within tinted vials and subjected to agitation at 100 rpm at 37°C. At predetermined intervals, the balloon sample was extracted from the tube, and the PBS medium was refreshed with a new solution. The concentration of released SRL was identified at immediately after coating, 1, 3, 7, 14, 21, and 30 days.
Animal study design
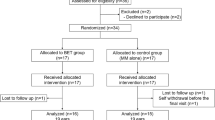
The protocol of animal study was approved by the Institutional Animal Care and Use Committee (No. 2023-40-112), and the animals used in this study were handled in accordance with the United States National Institutes of Health guidelines for the humane handling of laboratory animals. Overall, 21 male Sprague–Dawley rats, aged 12 weeks (weighing, 350–400 g; A BIO, Suwon, Korea) were used in this study. Of these, 18 rats were randomly assigned to the control and the SRL groups. The control group (n = 9) received a drug-free ETBD, whereas the SRL group (n = 9) received an SRL-coated ETBD. The rats in each group were euthanized immediately (n = 3), at 1 week and 4 weeks (both n = 3) after ETBD. The remaining three rats were euthanized to serve the normal ET as a reference without undergoing ETBD. All rats were housed under a 12-h control cycle at 55% ± 10% relative humidity and 24 ± 1°C environmental temperature. All rats were euthanized by administering inhalable pure carbon dioxide at the respective times.
Eustachian tube balloon dilatation
For all rats, anesthesia was performed using intramuscular injection of 50 mg/kg zolazepam and tiletamine (Zoletil 50; Virbac, Carros, France) and 10 mg/kg xylazine (Rompun; Bayer Healthcare, Leverkusen, Germany). The tympanic membrane was punctured through the external auditory canal using a 24 G angiocatheter with a needle (BD Angiocath Plus; Becton Dickinson, Franklin Lakes, NJ, USA). The angiocatheter was carefully inserted into the tympanic cavity and 1 mL of contrast medium was injected to visualize the tympanic orifice of the ET under a fluoroscopic guidance (MeteoR, NanoFocusRay Co., Iksan, Korea). A 0.014-inch micro guidewire (Transend; Boston Scientific, Marlborough, MA, USA) was inserted through the angiocatheter and negotiated consequently out of the nasal cavity. After the angiocatheter was removed, the balloon catheter was retrogradely advanced over the micro guidewire into the nasal cavity and located at the ET using fluoroscopic guidance. The balloon catheter was fully inflated to 9 atm as determined by the pressure gauge monitor of the balloon inflator (Genoss, Suwon, Korea) for 1 min14. The micro guidewire and balloon catheter were pulled out from the ET after ETBD.
Histological examination
Mandible and anatomical structures anterior to the soft palate were surgically resected to explore the nasopharyngeal opening of the ET after euthanasia14. Then, soft tissues around the ET orifice were removed. The dissected head samples were fixed in 10% neutral buffered formalin for 48 h and then decalcified for 2 weeks. The samples were embedded in a paraffin block and coronally sliced at intervals of 200 μm from the nasopharynx to the posterior wall of the tympanic cavity, which yielded three sections per sample. The slides containing the sections were stained with hematoxylin–eosin (H&E) and Masson’s Trichrome (MT). H&E-stained slices were evaluated for the percentage of ET luminal area, thickness of epithelial layers, thickness of submucosal fibrosis, degree of inflammatory cell infiltration, and percentage of fibrotic area at the cartilaginous portion of the ET13,14. The thickness of submucosal fibrosis was vertically measured from the tensor veli palatini muscle to the submucosal layer. The percentage of ET luminal area was calculated using the following equation: 100 × (balloon-treated ET lumen area/normal ET lumen area). The degree of inflammatory cell infiltration was classified according to the density and distribution of cells and graded as follows: 1, mild; 2, mild to moderate; 3, moderate; 4, moderate to severe; and 5, severe23,24,29. The percentage of fibrotic area at the cartilaginous portion of the ET was evaluated using the following formula: 100 × (fibrotic area at the cartilaginous portion/area of cartilaginous portion). On MT-stained slices, the degree of collagen deposition was subjectively classified into five grades as follows: grade 1, mild; grade 2, mild to moderate; grade 3, moderate; grade 4, moderate to severe; and grade 5, severe23,24,29. All stained samples were histologically visualized and measured via Caseviewer (3D HISTECH, Budapest, Hungary). This study was examined by three observers blinded to the experimental groups.
Immunohistochemistry
Immunohistochemistry (IHC) examination was performed on paraffin-embedded sections using α-smooth muscle actin (α-SMA; Abcam, Cambridge, England). The α-SMA-positive deposition was subjectively classified into five grades (1, mild; 2, mild to moderate; 3, moderate; 4, moderate to severe; and 5, severe)25.
Statistical analysis
Data were expressed as the median and interquartile range (IQR). Differences between groups were analyzed using a Bonferroni-corrected Mann–Whitney U test. Statistical analyses were performed using SPSS (version 27; IBM, Chicago, IL, USA). A p-value of < 0.05 was considered to indicate statistical significance.
Ethics declarations
All experiments were performed in accordance with relevant ARRIVE guidelines and regulations.
Approval for animal experiments
All experiments were approved by the Institutional Animal Care and Use Committee of the Asan Institute for Life Sciences (IACUC-2023-40-112).
Data availability
The authors confirm that the data supporting the findings of this study are available within the article. Raw data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Smith, M., Scoffings, D. & Tysome, J. Imaging of the Eustachian tube and its function: A systematic review. Neuroradiology 58, 543–556 (2016).
Schilder, A. et al. Eustachian tube dysfunction: Consensus statement on definition, types, clinical presentation and diagnosis. Clin. Otolaryngol. 40, 407 (2015).
van Heerbeek, N., Ingels, K., Rijkers, G. & Zielhuis, G. Therapeutic improvement of Eustachian tube function: A review. Clin. Otolaryngol. Allied Sci. 27, 50–56 (2002).
Adil, E. & Poe, D. What is the full range of medical and surgical treatments available for patients with Eustachian tube dysfunction?. Curr. Opin. Otolaryngol. Head Neck Surg. 22, 8–15 (2014).
Sudhoff, H. et al. Therapy of chronic obstructive Eustachian tube dysfunction: Evolution of applied therapies. Hno 61, 477–482 (2013).
Catalano, P. J., Jonnalagadda, S. & Yu, V. M. Balloon catheter dilatation of Eustachian tube: A preliminary study. Otol. Neurotol. 33, 1549–1552. https://doi.org/10.1097/MAO.0b013e31826a50c3 (2012).
Ockermann, T., Reineke, U., Upile, T., Ebmeyer, J. & Sudhoff, H. H. Balloon dilation eustachian tuboplasty: A feasibility study. Otol. Neurotol. 31, 1100–1103. https://doi.org/10.1097/MAO.0b013e3181e8cc6d (2010).
Poe, D. S., Silvola, J. & Pyykkö, I. Balloon dilation of the cartilaginous Eustachian tube. Otolaryngol. Head Neck Surg. 144, 563–569. https://doi.org/10.1177/0194599811399866 (2011).
Silvola, J., Kivekäs, I. & Poe, D. S. Balloon dilation of the cartilaginous portion of the eustachian tube. Otolaryngol. Head Neck Surg. 151, 125–130. https://doi.org/10.1177/0194599814529538 (2014).
Randrup, T. S. & Ovesen, T. Balloon eustachian tuboplasty: A systematic review. Otolaryngol. Head Neck Surg. 152, 383–392. https://doi.org/10.1177/0194599814567105 (2015).
Si, Y. et al. Effects of combination of balloon Eustachian tuboplasty with methylprednisolone irrigation on treatment of chronic otitis media with effusion in adults. Am. J. Otolaryngol. 39, 670–675. https://doi.org/10.1016/j.amjoto.2018.06.016 (2018).
Ockermann, T., Reineke, U., Upile, T., Ebmeyer, J. & Sudhoff, H. H. Balloon dilation eustachian tuboplasty: A feasibility study. Otol. Neurotol. 31, 1100–1103 (2010).
Kivekäs, I. et al. Histopathology of balloon-dilation Eustachian tuboplasty. Laryngoscope 125, 436–441 (2015).
Kim, Y. et al. Serial histological changes in the cartilaginous eustachian tube in the rat following balloon dilation. PLoS One 17, e0268763 (2022).
Clever, Y. P. et al. Novel sirolimus–coated balloon catheter: In vivo evaluation in a porcine coronary model. Circ. Cardiovasc. Interv. 9, e003543 (2016).
Ahmad, W. A. W. et al. Treatment of coronary de novo lesions by a sirolimus-or paclitaxel-coated balloon. Cardiovasc. Interv. 15, 770–779 (2022).
Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 72, 76–90 (2014).
Song, H. Y. et al. Fluoroscopic balloon dilation using a flexible guide wire to treat obstructive eustachian tube dysfunction. J. Vasc. Interv. Radiol. 30, 1562–1566. https://doi.org/10.1016/j.jvir.2019.04.041 (2019).
Ammit, A. J. The role of mRNA stability in airway remodelling. Pulmon. Pharmacol. Ther. 18, 405–415 (2005).
Stavrou, D. Neovascularisation in wound healing. J. Wound Care 17, 298–302 (2008).
Jurkiewicz, D., Bień, D., Szczygielski, K. & Kantor, I. Clinical evaluation of balloon dilation Eustachian tuboplasty in the Eustachian tube dysfunction. Eur. Arch. Oto-Rhino-Laryngol. 270, 1157–1160 (2013).
McCoul, E. D. & Anand, V. K. Eustachian tube balloon dilation surgery. Int. Forum Allergy Rhinol. 2, 191–198. https://doi.org/10.1002/alr.21007 (2012).
Ramakrishnan, N. & Kadambi, P. The use of balloon Eustachian tuboplasty in patients with eustachian tube dysfunction: A retrospective pilot usage experience. Indian J. Otolaryngol. Head Neck Surg. 72, 392–394. https://doi.org/10.1007/s12070-020-01822-z (2020).
Kang, J. M. et al. Sirolimus-eluting cobalt-chrome alloy stent suppresses stent-induced tissue hyperplasia in a porcine Eustachian tube model. Sci. Rep. 12, 3436. https://doi.org/10.1038/s41598-022-07471-2 (2022).
Kim, K. Y. et al. Sirolimus-eluting biodegradable poly-l-lactic acid stent to suppress granulation tissue formation in the rat urethra. Radiology 286, 140–148. https://doi.org/10.1148/radiol.2017170414 (2018).
Zhu, Y. et al. Biodegradable rapamycin-eluting nano-fiber membrane-covered metal stent placement to reduce fibroblast proliferation in experimental stricture in a canine model. Endoscopy 45, 458–468 (2013).
Klawitter, J., Nashan, B. & Christians, U. Everolimus and sirolimus in transplantation-related but different. Expert Opin. Drug Saf. 14, 1055–1070 (2015).
Parry, T. J. et al. Drug-eluting stents: Sirolimus and paclitaxel differentially affect cultured cells and injured arteries. Eur. J. Pharmacol. 524, 19–29 (2005).
Park, J. H. et al. Balloon-expandable biodegradable stents versus self-expandable metallic stents: A comparison study of stent-induced tissue hyperplasia in the rat urethra. Cardiovasc. Interv. Radiol. 42, 1343–1351. https://doi.org/10.1007/s00270-019-02239-0 (2019).
Smith, M. E. et al. The mechanism of balloon Eustachian tuboplasty: A biomechanical study. Med. Biol. Eng. Comput. 58, 689–699 (2020).
Goulioumis, A. K., Gkorpa, M., Athanasopoulos, M., Athanasopoulos, I. & Gyftopoulos, K. The eustachian tube dysfunction in children: Anatomical considerations and current trends in invasive therapeutic approaches. Cureus 14, e27193. https://doi.org/10.7759/cureus.27193 (2022).
Hubbell, R. D. et al. Patulous eustachian tube dysfunction symptoms following balloon dilation. Laryngoscope 133, 3152–3157. https://doi.org/10.1002/lary.30659 (2023).
Choi, S. W. et al. Prevalence and incidence of clinically significant patulous Eustachian tube: A population-based study using the Korean National Health Insurance Claims Database. Am. J. Otolaryngol. 39, 603–608. https://doi.org/10.1016/j.amjoto.2018.07.010 (2018).
Silver, F. H. & Glasgold, A. I. Cartilage wound healing: An overview. Otolaryngol. Clin. N. Am. 28, 847–864 (1995).
Takasaki, K., Sando, I., Balaban, C. D. & Ishijima, K. Postnatal development of eustachian tube cartilage. A study of normal and cleft palate cases. Int. J. Pediatr. Otorhinolaryngol. 52, 31–36. https://doi.org/10.1016/s0165-5876(99)00292-x (2000).
Kim, K. Y. et al. Fluoroscopy-guided balloon dilation in patients with Eustachian tube dysfunction. Eur. Radiol. 28, 910–919. https://doi.org/10.1007/s00330-017-5040-4 (2018).
Kim, K. Y. et al. Fluoroscopic subtraction Eustachian tubography: Initial feasibility test in a cadaver model. Eur. Radiol. 28, 3685–3691. https://doi.org/10.1007/s00330-018-5392-4 (2018).
Kang, B. C. et al. Fluoroscopic balloon diameter measurement at different pressures during Eustachian balloon dilation. Clin. Otolaryngol. 43, 1573–1577. https://doi.org/10.1111/coa.13218 (2018).
Kwak, M. Y. et al. Morphological analysis of the adult eustachian tube: A fresh-frozen human cadaver study. Otol. Neurotol. 42, e1583–e1591. https://doi.org/10.1097/mao.0000000000003294 (2021).
Keschner, D., Garg, R., Loch, R. & Luk, L. J. Repeat eustachian tube balloon dilation outcomes in adults with chronic eustachian tube dysfunction. Otolaryngol. Head Neck Surg. 166, 951–956. https://doi.org/10.1177/01945998211037975 (2022).
Harty, M., Neff, A. W., King, M. W. & Mescher, A. L. Regeneration or scarring: An immunologic perspective. Dev. Dyn. 226, 268–279 (2003).
Duynstee, M., De Krijger, R., Monnier, P., Verwoerd, C. & Verwoerd-Verhoef, H. Subglottic stenosis after endolaryngeal intubation in infants and children: Result of wound healing processes. Int. J. Pediatr. Otorhinolaryngol. 62, 1–9 (2002).
Wang, Z. et al. The rat eustachian tube: Anatomical, histological, and radiological features. J. Interv. Med. 6, 14–19. https://doi.org/10.1016/j.jimed.2022.12.002 (2023).
Acknowledgements
This research was supported by a grant from the Technology Innovation Development Project funded by the Korean Smal and Medium Business Administration (Project no. RS-2023-00224384). This work was supported by the Korea Evaluation Institute Of Industrial Technology (KEIT) grant funded by the Korea government (MSIT) (No. 2023OK9130-1).
Author information
Authors and Affiliations
Contributions
J.-K.P., H.J.P., and J.-H.P. conceptualized the project and designed the experiments. J.M.K., S.H.K., D.S.R., Y.P., D.-S.W., J.W.K., and J.-K.P. conducted the experimental work. J.M.K., S.H.K., D.S.R., Y.P., D.-S.W., J.W.K., and J.-H.P. collected and analyzed the data and prepared the manuscript. J.M.K., S.H.K., J.-K.P., H.J.P., and J.-H.P. wrote the manuscript. All the authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kang, J.M., Kim, S.H., Ryu, D.S. et al. Sirolimus-coated Eustachian tube balloon dilatation for treating Eustachian tube dysfunction in a rat model. Sci Rep 14, 8784 (2024). https://doi.org/10.1038/s41598-024-58869-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-58869-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.