Abstract
This study aims to evaluate the safety of Alprazolam by analyzing the FAERS database, provide data analysis for monitoring adverse drug reactions. This research encompasses adverse event (AE) reports related to Alprazolam from the first quarter of 2004 to the second quarter of 2023. Four signal mining and analysis methods were utilized, including Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN), and Empirical Bayesian Geometric Mean (EBGM). Further exploration was conducted regarding patient characteristics and types of AEs. A total of 23,575 AE reports in which Alprazolam was the primary suspect drug were collected, identifying 347 Preferred Term (PT) signals and 27 System Organ Classes (SOCs). The number of AE reports increased annually, especially in 2015, 2018, 2019, and 2020. The main affected groups were females and the age range of 18 to 45. Psychiatric disorders, Nervous system disorders, and Gastrointestinal disorders were the most common the organ system in which the AEs occurred. There is a certain risk of drug abuse and suicide with Alprazolam. Most notably, several AEs not recorded in the Alprazolam leaflet appeared among the top 30 PTs in signal strength, including but not limited to Benzodiazepine drug level abnormal, Acquired amegakaryocytic thrombocytopenia, Cutaneous T-cell dyscrasia, and Coronary No-reflow Phenomenon. For the first time, AEs related to the cardiovascular system and platelet function were unveiled. The severe AE reports that resulted in "hospitalization" and "death" accounted for 30.96% and 21.86%. This study highlights the risks of suicide and misuse of Alprazolam. Other potential severe or fatal AEs, such as those related to the cardiovascular system, platelet function, and others, require further research to determine their precise mechanisms and risk factors.
Similar content being viewed by others
Introduction
Evaluating and regulating drug safety is pivotal to preserving public health. As pharmaceuticals are broadly consumed, it's crucial to report and monitor adverse events (AEs) to timely identify potential risks and safety concerns. A cornerstone of the drug monitoring ecosystem is the Adverse Event Reporting System (FAERS) of the U.S. Food and Drug Administration (FDA)—a nationwide database archiving AE information related to drug consumption1,2.
Alprazolam, as a commonly prescribed intermediate-acting benzodiazepine (BZD), primarily amplifies the inhibitory neurotransmitter Gamma-Aminobutyric Acid (GABA) system, thereby depressing the excitability of the central nervous system3. Recognized for its pronounced anti-anxiety effects, rapid onset, and efficacy against insomnia, Alprazolam has seen extensive use in medical practice. From 2015 to 2018, there were 71,481 dispensings of Alprazolam to 6772 people in Australia. Following a policy intervention in 2017, the overall dispensing of Alprazolam decreased by 51.2%, but the prescribing approvals increased by 17.5%4. Moreover, studies have indicated that short-term BZD usage can alleviate anxiety and insomnia symptoms in depression patients during the initial phase of antidepressant treatment, not only hastening relief from severe depression but also potentially enhancing the sustained efficacy of antidepressants5,6.
However, the broad clinical application of Alprazolam doesn't come without its slew of AEs and latent risks. At clinical doses, beyond its widely acknowledged sedative, hypnotic, anxiolytic, and muscle relaxant effects, Alprazolam may induce psychomotor disorders and cognitive degeneration. It's also possibly correlated with increased risks of Alzheimer's disease, strokes, and malignant brain tumors7,8. Additionally, due to its unique pharmacokinetics (high potency, short half-life, rapid absorption and withdrawal effects), Alprazolam manifests a higher propensity for abuse, potentially triggering withdrawal syndromes more severe than other BZDs, thereby negatively impacting health and quality of life9.
To better understand the safety profile and inherent risks of Alprazolam, and to deliver a more comprehensive insight to underpin prudent medicinal decisions, this study delves into AE data associated with Alprazolam in FAERS. It aims to unearth safety signals and risk factors through a meticulous analysis, thoroughly probing patterns, trends, and correlated factors of Alprazolam's AEs.
Materials and methods
Data source
The data for this study was sourced from the FAERS database. FAERS collects spontaneous safety reports and post-marketing clinical study reports related to drug use both within and outside the United States. This study selected data from the first quarter of 2004 to the second quarter of 2023. Analysis was performed using MySQL, and after deduplication, AE reports where Alprazolam was the primary suspected drug were obtained.
Data processing
Using "Alprazolam" as the keyword for retrieval from the database, we obtained details including personal information, drug information, AEs, and primary diseases. Following the FDA-recommended method for removing duplicate reports, we select the PRIMARYID (Primary Identification), CASEID (Case Identification), and FDA_DT fields from the DEMO table. We sort by CASEID, FDA_DT, and then PRIMARYID. For reports with the same CASEID, we retain the one with the largest FDA_DT value. FDA_DT refers to the FDA received date of the adverse event report. The rationale is that the report with the most recent received date likely contains the most up-to-date and complete information for that case. Secondly, for reports where both CASEID and FDA_DT are the same, we retain the one with the largest PRIMARYID value. PRIMARYID is a unique identifier assigned to each report. By keeping the report with the largest PRIMARYID, we aim to preserve the most complete data. Since the first quarter of 2019, each quarterly data package has included a list of deleted reports. After data deduplication, we remove reports based on the CASEID listed in the deleted reports list. The "Medical Dictionary for Regulatory Activities" (MedDRA) version 23.0 was used for AE terminologies. This included preferred SOC (System Organ Class) and PT (Preferred Term) for classifying and expressing AEs10.
Data analysis
Signal detection for AEs is essentially determining whether the reporting frequency of a specific AE for the target drug is higher than expected, thus establishing a statistical association between the drug and that specific AE. This study employed the Reporting Odds Ratio (ROR)11, Proportional Reporting Ratio (PRR) 12, Bayesian Confidence Propagation Neural Network (BCPNN)13,14, and the Empirical Bayesian Geometric Mean (EBGM)15 through SAS 9.4 software. The ROR helps mitigate biases in events with fewer reports. The PRR stands out for its greater specificity compared to ROR. BCPNN is adept at combining and cross-validating multi-source data. The MGPS is particularly effective in identifying signals from infrequent events. This study utilizes a blend of ROR, PRR, BCPNN, and MGPS to capitalize on their individual strengths, enhancing the scope of detection and validation from diverse angles. This integrated approach aids in more accurately identifying safety signals, reducing false positives through cross-validation and refining detection of rare adverse reactions by adjusting thresholds and variance. These methods are based on a 2 × 2 contingency table, as shown in Table 1. The formulas for each method and the conditions that satisfy signal generation are presented in Table 2.
Results
Constituent ratio of yearly data
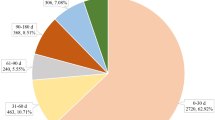
In this study, from January 1, 2004 to June 30, 2023, a total of 19,932,732 AE reports were obtained. Among them, 23,575 reports suspected Alprazolam as the primary drug. After analysis using the ROR, PRR, BCPNN, and EBGM methods, 347 signals on the PT level and 27 signals on the SOC level were detected. Female subjects dominated the AE reports for Alprazolam, accounting for 56.53%, while males accounted for 35.84%. Reports covered patients of all age groups, with the 18 to 45 age bracket having the highest proportion at 27.86%. Reports of AEs related to Alprazolam have shown an increasing trend year by year from 2004 to 2023. Notably, in 2015, 2018, 2019, and 2020, there was a significant increase in the number of reports, accounting for 8.97%, 10.52%, 13.86%, and 13.76% of the total reports, respectively. Consumers were the primary reporters, making up 35.37% of the total reports. The majority of reports came from the USA (54.43%), followed by France (18.57%) and Italy (9.96%). The outcomes of AEs showed that the most common were Hospitalization—Initial or Prolonged (30.96%) and Death (21.86%), suggesting that Alprazolam might be associated with some serious AEs (Table 3).
Risk signal analysis results
In Table 4, the SOCs with many signals included: Psychiatric disorders, General disorders and administration site conditions, Nervous system disorders, Injury, poisoning and procedural complications, Gastrointestinal disorders. Among them, General disorders and administration site conditions, Injury, poisoning and procedural complications, Respiratory, thoracic and mediastinal disorders, Musculoskeletal and connective tissue disorders, Vascular disorders had a large number of reports.
In Table 5, among the top 30 PTs by report count, most were common adverse reactions for psychiatric drugs. Drug abuse, Drug dependence, Overdose, and Withdrawal syndrome had high occurrence rates. The suicide risk has also been detected, including Completed suicide, Suicide attempt, and Suicidal ideation.
In Table 6, the top 30 PTs by signal strength included many adverse reactions not recorded in the Alprazolam leaflet. Among them, Benzodiazepine drug level abnormal, Acquired amegakaryocytic thrombocytopenia, Postnatal growth restriction, Prescription form tampering, Papillary muscle disorder ranked the top five, being new potential adverse reactions. Additionally, even though Cutaneous T-cell dyscrasia, Sarcomatoid carcinoma, Pseudophaeochromocytoma, and Coronary no-reflow phenomenon had fewer reports, their signal strengths were strong, necessitating further attention.
Discussion
Benzodiazepines (BZDs), derived from 1,4-benzodiazepine, play a pivotal role in the alleviation and treatment of emotional anxiety, hyperactive reactions, insomnia, and epilepsy. They are currently the most widely used and longest-standing drugs for insomnia treatment. The primary mechanism of these drugs is by acting on the reticular structure of the brainstem and the limbic system, enhancing the affinity between the inhibitory neurotransmitter GABA and its respective receptors, thus inducing a suppressive effect on the central nervous system. Alprazolam, introduced to the U.S. market in 1981, is a medium-acting benzodiazepine. It possesses various effects such as anti-anxiety, anti-convulsion, and anti-depression, making it one of the most extensively used BZDs. Past researches, via case reports, systematic reviews, prescription monitoring, and clinical trials, have primarily studied and evaluated the adverse reactions related to Alprazolam.
Previous case reports on Alprazolam's adverse reactions mainly include allergic reactions, addiction, withdrawal responses, short-term memory loss, sleepwalking, muscle weakness, mental disorders, frequent urination, bloody lactation, gum overgrowth, agitation, abnormal behaviors, lethal overdose, dose-dependent orgasmic disorders, and acute angle-closure glaucoma16. Additionally, studies indicated that women taking Alprazolam during the first three months of pregnancy may increase the risk of congenital anomalies17,18,19. Nursing mothers consuming Alprazolam might lead to withdrawal symptoms and mild drowsiness in infants. Consuming Alprazolam during pregnancy might be linked to inguinal hernia, fetal deformities, and neonatal withdrawal syndrome, while its use during breastfeeding could cause mild drowsiness and withdrawal symptoms in infants. This research deeply analyzes the AE report data of Alprazolam to gain a more comprehensive understanding of its safety and the overview of AEs, providing a foundation for further risk management and clinical practice.
Report trends and patient characteristics analysis
This study delved into the AE reports from 2004 to 2023, especially focusing on reports where Alprazolam was the primary suspected drug. The results unveiled a series of noteworthy phenomena and trends. Firstly, in terms of the number of AE reports, there's an annual increase in reports related to Alprazolam. Specifically, there were marked surges in 2015, 2018, 2019, and 2020. This trend hints at the rising societal and medical institutional concerns about the safety of Alprazolam. It might also suggest a broadening usage scope of this drug or its association with more complex and severe adverse reactions.
Secondly, the gender and age distribution in the reports is intriguing. Females dominate the AE reports for Alprazolam20. This could imply that females might be more susceptible to Alprazolam's adverse effects or are more inclined to report these events. The most common age group in these reports is between 18 to 45 years, typically considered the most socially and professionally active cohort. Hence, these adverse reactions could severely impact their work and social lives.
Existing adverse reactions
The AEs primarily associated with Alprazolam center around Psychiatric disorders, encompassing Drug abuse, Completed suicide, and Drug dependence. Issues of Drug abuse and Drug dependence hint at the potential addictiveness of Alprazolam, posing risks to patient safety. Of particular concern are suicide-related events such as Completed suicide and Suicidal ideation. This aligns with previous research, emphasizing the need for heightened vigilance regarding a patient's mental well-being while on Alprazolam, coupled with corresponding preventive measures.
Beyond Psychiatric disorders, Nervous system disorders also stake a claim in Alprazolam's list of adverse reactions. This includes Somnolence, Coma, and Confusional state. Such reactions could be life-threatening, especially when driving or operating machinery. Injury, poisoning, and procedural complications emerge as another area of concern, especially for long-term Alprazolam users. Such irregularities could lead to grave health issues, necessitating routine monitoring.
New adverse reactions and potential mechanisms
Risk signal analyses unveil various aspects warranting further research. While there's a plethora of signals regarding General disorders and administration site conditions, and Injury, poisoning and procedural complications, the medicine's documentation doesn't explicitly cite these adverse reactions. This suggests that both doctors and patients might lack adequate awareness and vigilance towards these latent risks.
Signal strength analyses further expose several potential adverse reactions not documented in Alprazolam's instructions. These newfound adverse reactions pertain to the cardiovascular system and platelet function, including Benzodiazepine drug level abnormal, Acquired amegakaryocytic thrombocytopenia, and Papillary muscle disorder. The mechanisms underlying these reactions remain elusive, but they might involve drug metabolism, interactions, and intricate physiological processes. For instance, Benzodiazepine drug level abnormal might stem from interactions between Alprazolam and other drugs, affecting its metabolism—a facet demanding further illumination21. Meanwhile, Acquired amegakaryocytic thrombocytopenia might be linked to an undiscovered correlation between Alprazolam and platelet functionality, necessitating in-depth experimentation and study22.
This research also identified rare but significant adverse reactions, namely Cutaneous T-cell dyscrasia, Sarcomatoid carcinoma, Pseudophaeochromocytoma, and the Coronary no-reflow phenomenon. For instance, Cutaneous T-cell dyscrasia might arise from Alprazolam's impact on the immune system23, and Sarcomatoid carcinoma could suggest that the drug indirectly affects biological pathways associated with cancer growth24. Pseudophaeochromocytoma might simulate some symptoms of pheochromocytoma by impacting the sympathetic nervous system. The Coronary No-reflow Phenomenon, a blood flow obstruction involving coronary arteries, may relate to Alprazolam's indirect effects on the cardiovascular system, particularly with high dosages or long-term use25,26.
Even though these adverse reactions are relatively less reported, given their severity and potential life-threatening nature, they warrant further research and attention. These findings signal the need for vigilance, regarding not only common side effects of Alprazolam but also these rare yet potentially severe reactions.
Limitations
There were several limitations regarding to the FAERS database27. The primary limitation of this study is that all data come from a voluntary AE reporting database. The spontaneous reporting system suffers from a significant underreporting issue, therefore the results do not reflect the full picture of the actual adverse reactions occurring. The study did not consider medication dosage data, making it impossible to interpret the results in the context of drug dosage, thus presenting certain limitations. Hence, these results should be viewed as a preliminary understanding of Alprazolam's safety concerns and should not replace more systematic and rigorous clinical studies. Besides, without specific data to directly correlate the rise in adverse event reports with increased usage, it's difficult to confirm this relationship definitively. Nonetheless, these initial findings undeniably set a direction and foundation for deeper future research and discussions.
Conclusion
In summary, this research provides vital safety information for the clinical use of Alprazolam. It unveils various concerns and risks that demand further scrutiny. The risks of suicide and abuse remain areas of significant concern. The cardiovascular system, platelet function, and other serious and potentially fatal issues require further studies to determine their precise mechanisms and risk factors. Given Alprazolam's widespread use in treating anxiety, insomnia, and other symptoms, it's imperative to deeply understand and address these issues. Future studies should examine Alprazolam's safety concerns more comprehensively and meticulously, aiming for more precise clinical guidance.
Data availability
The dataset generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
Roger, J. Y. et al. Emerging causes of drug-induced anaphylaxis: A review of anaphylaxis-associated reports in the FDA Adverse Event Reporting System (FAERS). J. Allergy Clin. Immunol. Pract. 9(2), 819-829.e2 (2021).
Fusaroli, M. et al. Post-marketing surveillance of CAR-T-cell therapies: Analysis of the FDA adverse event reporting system (FAERS) database. Drug Saf. 45(8), 891–908 (2022).
Rao, H. et al. Compritol-based alprazolam solid lipid nanoparticles for sustained release of alprazolam: Preparation by hot melt encapsulation. Molecules 27(24), 8894 (2022).
Schaffer, A. L. et al. Comparison of prescribing patterns before and after implementation of a national policy to reduce inappropriate alprazolam prescribing in Australia. JAMA Netw. Open 2(9), e1911590–e1911590 (2019).
Saberi, A. et al. Investigating the efficacy of fluoxetine vs. fluoxetine plus alprazolam (single therapy vs. combination therapy) in treatment of chronic tinnitus: A placebo-controlled study. Am. J. Otolaryngol. 42(3), 102898 (2021).
Lim, B. et al. Understanding the effects of chronic benzodiazepine use in depression: A focus on neuropharmacology. Int. Clin. Psychopharmacol. 35(5), 243–253 (2020).
French, J. A. et al. Inhaled alprazolam rapidly suppresses epileptic activity in photosensitive participants. Epilepsia 60(8), 1602–1609 (2019).
Mamtani, H. & Chaturvedi, S. K. Alprazolam: Good for some, not good for all!. J. Clin. Psychopharmacol. 43(3), 204–208 (2023).
Cardona-Acosta, A. M. et al. Alprazolam exposure during adolescence induces long-lasting dysregulation in reward sensitivity to morphine and second messenger signaling in the VTA-NAc pathway. Sci. Rep. 13(1), 10872 (2023).
Brown, E. G. Using MedDRA: Implications for risk management. Drug Saf. 27(8), 591–602 (2004).
Rothman, K. J., Lanes, S. & Sacks, S. T. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol. Drug Saf. 13(8), 519–523 (2004).
Evans, S. J. W., Waller, P. C. & Davis, S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol. Drug Saf. 10(6), 483–486 (2001).
Bate, A. et al. A Bayesian neural network method for adverse drug reaction signal generation. Eur. J. Clin.Pharmacol. 54, 315–321 (1998).
Almenoff, J. S. et al. Comparative performance of two quantitative safety signalling methods: Implications for use in a pharmacovigilance department. Drug Saf. 29, 875–887 (2006).
DuMouchel, W. Bayesian data mining in large frequency tables, with an application to the FDA spontaneous reporting system. Am. Stat. 53(3), 177–190 (1999).
O’sullivan, G. H. et al. Safety and side-effects of alprazolam controlled study in agoraphobia with panic disorder. Br. J. Psychiatry 165(1), 79–86 (1994).
Lee, H. et al. Pregnancy and neonatal outcomes after exposure to alprazolam in pregnancy. Front. Pharmacol. 13, 854562 (2022).
Kelty, E., Chitty, K. & Preen, D. B. 2023 safety of Alprazolam Use in pregnancy in Western Australia: A retrospective cohort study using linked health data. J. Psychoact. Drugs https://doi.org/10.1080/02791072.2023.2241465 (2023).
Lee, H. et al. Corrigendum: Pregnancy and neonatal outcomes after exposure to Alprazolam in pregnancy. Front. Pharmacol. 13, 934265 (2022).
Hockenhull, J. et al. Nonmedical use of alprazolam in the UK: Results from a nationally representative survey. Br. J. Clin. Pharmacol. 85(8), 1841–1845 (2019).
Corkery, J. M. et al. Alprazolam-related deaths in Scotland, 2004–2020. J. Psychopharmacol. 36(9), 1020–1035 (2022).
Burgos, C. F. et al. Anti-aggregation effect on platelets of Indiplon a hypnotic sedative non-benzodiazepine drug. Biomed. Pharmacother. 111, 378–385 (2019).
Lin, A. et al. Alprazolam prompts HIV-1 transcriptional reactivation and enhances CTL response through RUNX1 inhibition and STAT5 activation. Front. Neurol. 12, 663793 (2021).
Matsuda, Y. et al. Benzodiazepines for cancer dyspnoea: A nationwide survey of palliative care physicians. BMJ Support. Palliat. Care 10(2), 205–208 (2020).
Yeh, C. B. et al. Association of alprazolam with major cardiovascular events in patients with hypertension. J. Eval. Clin. Pract. 26(3), 983–991 (2020).
Costa, A. et al. Effects of oral administration of alprazolam and lorazepam as hypnotics on cardiovascular parameters in hypertensive patients. J. Clin. Psychopharmacol. 41(2), 191–195 (2021).
Noguchi, Y., Tachi, T. & Teramachi, H. Detection algorithms and attentive points of safety signal using spontaneous reporting systems as a clinical data source. Brief. Bioinform. 22(6), bbab347 (2021).
Acknowledgements
This study was performed using the FAERS source that was provided by the FDA. The information, results, or interpretation of the current study do not represent any opinion of the FDA.
Funding
The work is supported by the Medical Scientific Research Project of Jiangsu Provincial Health Commission (Z2022022), the Suzhou Health Youth Backbone Talent of National Mentor System (Qngg2021043), Suzhou Medical Key discipline construction project (SZXK202124).
Author information
Authors and Affiliations
Contributions
Feng Huang, Wenrong Xu conceived the study; Feng Huang, Xiao San, Qingqian Liu and Haohao Zhu collected the report; Feng Huang, Haohao Zhu and Wenrong Xu wrote the manuscript and edited the manuscript. All authors have approved publishment of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, F., San, X., Liu, Q. et al. Signal mining and risk analysis of Alprazolam adverse events based on the FAERS database. Sci Rep 14, 7489 (2024). https://doi.org/10.1038/s41598-024-57909-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-57909-y
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.