Abstract
Aiming at oil extraction from a tight reservoir, the Jilin oil field was selected as the research object of this study. Based on the molecular structures of conventional long-chain alkyl anionic surfactants, a new temperature-resistant anionic/nonionic surfactant (C8P10E5C) was prepared by introducing polyoxyethylene and polyoxypropylene units into double-chain alcohols. The resulting structures were characterized by Fourier transform infrared spectroscopy (FT-IR), nuclear magnetic resonance spectroscopy (1H-NMR), and electrospray ionization mass spectrometry (ESI–MS). Then, based on surface tension, interfacial tension, adsorption resistance, wettability, and emulsification performance tests, the performance of C8P10E5C was evaluated. The FT-IR, ESI–MS, and NMR spectra confirmed that C8P10E5C was successfully prepared. The critical micelle concentration (CMC) of C8P10E5C in water was 2.9510 × 10−4 mol/L (the corresponding mass concentration is 0.26%), and the surface tension of the aqueous C8P10E5C solution at this concentration was 30.5728 mN/m. At 0.3% concentration, the contact angle of the C8P10E5C solution was 31.4°, which is 60.75% lower than the initial contact angle. Under high-temperature conditions, C8P10E5C can still reduce the oil–water interfacial tension to 10−2 mN/m, exhibiting good temperature resistance. At 110 °C, upon adsorption to oil sand, the C8P10E5C solution could reduce the oil–water interfacial tension to 0.0276 mN/m, and the interfacial tension can still reach the order of 10−2 mN/m, indicating that C8P10E5C has strong anti-adsorption capability. Additionally, it has good emulsifying performance; upon forming an emulsion with crude oil, the highest drainage rate was only 50%. The forced imbibition oil recovery of C8P10E5C is 65.8%, which is 38.54, 24.22, and 27.25% higher than those of sodium dodecyl benzene sulfonate, alkyl polyoxyethylene ether carboxylate, and alkyl ether carboxylate, respectively.
Similar content being viewed by others
Introduction
As global energy requirements continue to increase, unconventional oil resources, such as tight oil, are attracting widespread attention1,2,3. Large reserves of tight oil have been found worldwide, and they have great potential for development2,4. A large number of micro- and nano-scale pores develop in tight reservoirs, resulting in low porosity and low permeability, complex pore throat structures, and strong heterogeneity, and production wells usually have no natural productivity5,6,7,8. Therefore, additional treatments, such as fracking, or other enhanced oil recovery techniques are required to achieve higher recovery rates9,10. Horizontal-well hydraulic fracturing is an effective technology to develop tight oil reservoirs11,12,13. By adding an imbibition solution to the fracturing fluid, crude oil can be recovered via imbibition during the shut-in process to improve the recovery efficiency of tight reservoirs14. Therefore, imbibition is one of the main mechanisms of enhanced oil recovery from tight reservoirs. However, because of the presence of acidic substances in crude oil, most reservoirs tend to be lipophilic15. Therefore, the capillary pressure of the well exerts a resistance during the imbibition process, which significantly inhibits the efficiency of imbibition16. Therefore, the prerequisite for reservoir imbibition is the wettability of rocks, and the stronger the hydrophilicity of the reservoir rock is, the greater the imbibition-based oil-transfer efficiency is17,18. Surfactants can enhance the wetting of rocks by adsorbing to them, thereby increasing the imbibition efficiency19,20,21,22.
However, tight reservoirs are generally buried deeper, resulting in higher reservoir temperatures. Surfactants suitable for conventional reservoirs have poor temperature resistance and are therefore unsuitable for high-temperature tight reservoirs. Consequently, there is an urgent need to develop novel temperature-resistant surfactants to improve the imbibition displacement efficiency of tight reservoirs. In this context, we developed a novel temperature-resistant anionic/nonionic surfactant. Based on the molecular structure of conventional long-chain alkyl anionic surfactants, we introduced polyoxyethylene and polyoxypropylene units to impart them with both nonionic and anionic properties, as well as better ductility, permeability, and temperature resistance. Then, we evaluated the properties of the novel temperature-resistant anionic/nonionic surfactant based on surface tension, interfacial tension, wettability, emulsification, and adsorption resistance tests. Finally, a forced imbibition experiment was conducted to evaluate the imbibition displacement efficiency.
Experimental section
Materials and instruments
Analytical reagent-grade isooctyl alcohol, chloroacetic acid, ethylene oxide, propylene oxide, sodium hydroxide, absolute ethyl alcohol, n-decane, sodium dodecyl benzene sulfonate (SDBS), alkyl polyoxyethylene ether carboxylate (APEC), and alkyl ether carboxylate (AEC) were purchased from Aladdin.
Simulated formation water was used in the experiments. The total salinity of the simulated formation water was 23,155 mg/L, and its mineral composition is listed in Table 1. Simulated formation water was used in the preparation of chemical agents used in the experiments. The dehydrated and degassed crude oil in block Q 246 of Jilin Oilfield was used as the experimental oil with a viscosity of 1.68 mP s(110 °C).
The following equipment were used in the study: DF-101S thermostatic heating instrument (Henan Yuhua Instrument Co., LTD.), HBYQ-2 high-temperature and high-pressure core flow test device (Huabao Petroleum Instrument Co., LTD.), vacuum rotary evaporator (Shanghai Shensheng Technology Co., LTD.), circulating water multi-purpose vacuum pump (Zhengzhou Hengyan Instrument Co., LTD.), high-temperature and high-pressure stainless steel reactor (Henan Yuhua Instrument Co., LTD.), constant-temperature water bath (Henan Yuhua Instrument Co., LTD.), DCAT21 table interface tensiometer (Dataphysics, Germany), LTD.), DCAT21 table interface tensiometer (Dataphysics, Germany), TX500C(U.S.A.) interface tensiometer (Dataphysics, Germany), OCA contact angle system (Dataphysics, Germany).
Preparation of novel temperature-resistant anionic/nonionic surfactants
(1) Synthesis of novel temperature-resistant anionic/nonionic surfactant intermediates: double-chain alkyl alcohols (0.01 mol) and potassium hydroxide (0.015 mol) were added to a high-temperature, high-pressure reactor, and nitrogen was continuously injected into the reactor for 30 min to remove air. An explosion-proof heating box and a reactor were opened for heating. Once the temperature reached 150 ℃, a vacuum pump was connected to the reactor to reduce its pressure to − 0.1 MPa over 3 h. A metal-sealed vessel containing ethylene oxide was then heated to 40 °C in a thermostatic water bath and connected to the reactor. Thereafter, the connecting valve was opened, the reaction temperature was controlled below 170 °C until the pressure in the reactor decreased to zero, and then the reaction was terminated. Then, a metal vessel containing propylene oxide was heated to 50 °C in a thermostatic water bath and connected to the reactor. The connection valve was opened, the reaction temperature was controlled below 170 °C until the pressure in the reactor decreased to zero, and the reaction was finally terminated. When the temperature of the reactor decreased to ~ 60 °C, the reaction products were decanted to obtain double-chain alkyl polyoxyethylene/polyoxypropylene ether as a colorless liquid product. The corresponding reaction process is shown in Fig. 1.
(2) Synthesis of novel temperature-resistant anionic and nonionic surfactants: a three-necked flask was secured in an oil bath pan, and the double-branched alkyl polyoxyethylene/ polyoxypropylene ether intermediate (0.005 mol) was added to it. The temperature of the oil bath was adjusted to 70 °C, and the reactants were stirred at 700 r/min for 30 min. To ensure full contact between NaOH and the intermediate, 0.01 mol of sodium hydroxide was added to the three-necked flask in several batches, and the reaction mixture was mixed continuously at a constant temperature for 3 h. Then, 0.02 mol of chloroacetic acid was added to the three-necked flask in multiple batches, and the oil bath pan was heated to 80 °C (Note: If the temperature is extremely low, the reaction rate is slow, and if the temperature is extremely high, the probability of side reactions increases). The reaction was stopped after continuously stirring the mixture for 5 h, and a pale-yellow waxy crude product was recovered. The crude product was subsequently dried in a vacuum freeze dryer for 48 h, and anhydrous ethanol (100 mL) was added to desalinate it for improving its purity. Finally, the anionic/nonionic surfactant was obtained by removing ethanol on a rotary evaporator. The corresponding reaction process is shown in Fig. 2.
The novel temperature-resistant anionic/nonionic surfactant was synthesized according to the aforementioned experimental methods. The structural formula of the obtained surfactant (hereafter denoted as C8P10E5C) is shown in Fig. 3.
Performance evaluation of the novel temperature-resistant anionic/nonionic surfactant
Surface tension test
The surface tensions of a series of C8P10E5C solutions of different concentrations (ranging from 1 × 10−8 to 1 × 10−2 mol/L, concentration gradient of half order of magnitude) were measured using a DCAT21 interfacial tensiometer until the surface tension reached a constant value. The error in measurement was less than 0.2 mN/m.
Experimental steps: ① The quartz tank was washed thoroughly with a large quantity of water, dried with a hair dryer, soaked in a chromic acid solution for more than 5 h, and washed alternately with distilled water and ultra-pure water, and then dried. ② C8P10E5C solutions of different concentrations were prepared via serial dilution. ③ A platinum sheet was burned to fiery red with an alcohol lamp for 10 min for removing the surfactant and other impurities present on the surface. ④ The platinum sheet was placed vertically in the solution and then pulled out carefully, ensuring that the bottom edge of the platinum sheet was just in contact with the solution interface, and the surface tension was measured at this moment. ⑤ The surface tensions of the C8P10E5C solutions with different concentrations were measured successively from low concentration to high concentration.
Wettability test
In this experiment, the ability to change the core wettability of C8P10E5C solutions with different concentrations (concentration range: 1 × 10−8 to 1 × 10−2 mol/L; concentration gradient: half order of magnitude) was evaluated by the contact angle test method.
The experimental steps are as follows: ① The contact angle chamber and mineral flakes were cleaned, the contact angle chamber was vacuumed and filled with nitrogen. ② The contact angle chamber was filled with a vacuumed C8P10E5C solution, ensuring that the mineral flakes were completely immersed in water, and then the mineral flakes were allowed to soak at 110 ℃ for 72 h. ③ Oil droplets were injected into mineral flakes using a micro-syringe, and the shape changes of oil droplets were observed through the transparent glass of the contact angle chamber; the contact angle was measured with the help of an optical lens. ④ The concentration of the C8P10E5C solution was increased, and steps ①–③ were repeated until the end of the experiment.
Interfacial tension test
The interfacial tension between C8P10E5C and crude oil was measured at 90–120 ℃ (temperature gradient 20 °C) using a 0.3% C8P10E5C solution prepared using simulated formation water. According to the PetroChina SY/T 5370-2018 standard, the rotary drop method was used, and a rotary interface tensiometer was utilized for measurements. The oil:water volume ratio was approximately 1:200, the rotation speed was 5000 r/min, and the measurement was performed until the steady-state value of the dynamic interfacial tension was reached, that is, when the value of the interfacial tension changed by less than 1% during 30 min.
Adsorption resistance test
Treatment of oil sands: The oil sand was suspended in petroleum ether, stirred with a glass rod, and soaked for approximately 10 min. Then, the solvent (petroleum ether) was removed, and the process was repeated two to three times until no oil remained on the surface of the oil sand. After drying (2 h), a small amount of the oil sand was placed in hot water; the absence of an oil slick indicated a thorough cleaning.
Experimental steps: ① The interfacial tension of the C8P10E5C solution was measured before adsorption to oil sand. ② The oil sand and a C8P10E5C solution were taken in a ground conical bottle with a stopper at a 1:10 solid/liquid ratio. ③ The conical bottle was subjected to constant-temperature oscillation in a water bath at 110 °C for 48 h. ④ The solution in the conical flask was shaken uniformly and transferred to a centrifuge tube. After centrifugation for 30 min, the supernatant was collected and its interfacial tension was measured.
Evaluation of the emulsifying property
The bottle test method was used to determine the stability of the emulsion: a 0.3% C8P10E5C solution and crude oil were maintained at 110 °C for 30 min, and then 10 g of the solution was taken according to the ratio of oil to water (1:1 mass ratio). The mixture was poured into a 25 mL stoppered test tube in the order of water first and then oil, the test tube was plugged with the stopper, and the contents were shaken vigorously by hand 100 times to ensure that it was completely emulsified. Once the desired emulsion was obtained, it was kept warm in a thermotank, and record the amount of water produced at different times.
Forced imbibition experiment
The forced imbibition experiment was conducted to evaluate the imbibition displacement efficiency of C8P10E5C by simulating the imbibition process of the imbibition liquid under the formation pressure (21.4 MPa) and temperature (110 °C) in a piston imbibition vessel. The experimental device is shown in Fig. 4.
Experimental procedure: ① The core was placed in an oven at 110 °C for 4 h and then cooled to room temperature. Its weight was recorded as m1. Then, it was dried further at 110 °C for 30 min, cooled to room temperature, and the weight was recorded as m2. When m2–m1 was less than 0.0005 g, the core was considered to be sufficiently dry, and the dry weight of the core was calculated as m3 = (m1 + m2)/2. ② The experimental apparatus was first connected to part A, and the rock sample was vacuumed for 4 h and then saturated with the simulated formation water. The core was removed and weighed (m4); the saturated water mass of the core was calculated as W1 = m4 − m3. ③ The core was placed in a gripper and aged for 24 h. The oil was injected at a rate of 0.02 mL/min until the water content in the produced liquid was 0. The volume of water in the produced liquid, V1 (volume of saturated oil in the core), was recorded. In addition, the bound water mass was determined as W2 = m4 − m3 − V1. ④ The core was removed, placed in a beaker with experimental oil for 2 h, the surface oil slick was removed, and it was quickly weighed for three consecutive times at different positions of the balance, and the average mass was recorded as W3; the weight of saturated oil was calculated as W4 = W3 − m3 − W2. Then, the experimental device was connected to part B, and different imbibition solutions were added according to the experimental scheme in Table 2. The rock sample was placed in the piston imbibition vessel, pressurized to the formation pressure, and the valves at both ends of the intermediate vessel were closed. The intermediate container was then placed in the incubator, removed after 24 h, cooled to room temperature, the core was retrieved, the surface floating oil and water were removed, and it was quickly weighed at different positions of the balance three consecutive times; the average mass of three weights was recorded as W5. The volume of the oil produced by forced imbibition was calculated as V2 = (W5 − W3)/(1 − W4/V1), and the imbibition oil recovery is given by V2/V1.
Results and discussion
Structural characterization of C8P10E5C
The structure of the C8P10E5C surfactant was analyzed by Fourier transform infrared (FT-IR) spectroscopy, electrospray ionization mass spectrometry (ESI–MS), and nuclear magnetic resonance (1H-NMR). The results are shown in Figs. 5, 6, and 7. As shown in Fig. 5, the FT-IR absorption peak at 3447 cm−1 was attributed to the hydroxyl or amino group (OH or NH) of C8P10E5C, and the absorption peaks at 2930 and 2872 cm−1 were assigned to the stretching vibrations of its methyl or methylene (–CH3 or –CH2) groups. The absorption peak at 1610 cm−1 was attributed to the carbonyl group (COO−) and that at 1458 cm−1 was assigned to methylene (–CH2) deformational vibrations. Further, the absorption peak at 1374 cm−1 corresponds to the deformational vibrations of the methyl groups (–CH3) and those at 1314 and 1252 cm−1 are due to the asymmetric stretching vibrations of the carbonyl group (COO−). The absorption peak at 1111 cm−1 corresponds to the vibrations of the ether bond (–CH2OCH2-), and the absorption peaks at 927 and 836 cm−1 are due to the vibrations of the polyether backbone (–CH2CH2O). Thus, the molecular chains contained all the designed groups and met the expected results. Therefore, the results of FT-IR spectroscopy preliminarily indicate that the target synthetic product, C8P10E5C, was formed.
As shown in Fig. 6, the molecular ion peaks in the mass spectrum are 303, 361, 419, 477, 535, 593, 651, 745, 803, 827, 899, 929, 973, 1031, 1061, 1105, 1163, 1207, 1279, etc. The multiple sets of data with peak differences of 44 and 58 indicate the presence of compounds containing both polyoxyethylene and polyoxypropylene units in the sample. Thus, ESI–MS further confirmed that the targeted synthetic product, C8P10E5C, was successfully obtained.
As shown in Fig. 7, the chemical shift of 0.83 ppm corresponds to the protons of –CH3, the chemical shift of 1.07 ppm corresponds to the protons of CH3CHCH2O, the chemical shift of 1.27 ppm corresponds to the protons of –CH2 groups, the chemical shifts of 3.67, 3.50, 3.41 and 3.30 ppm correspond to the protons of CH3CHCH2O and CH2O moieties, and the chemical shift of 3.92 ppm corresponds to the protons of the CH2COO− unit. The proton peaks corresponding to each unit were observed in the 1H-NMR spectra of C8P10E5C. Combined with the results of FT-IR spectroscopy and ESI–MS, these results confirm that the product was the new anionic/nonionic surfactant, C8P10E5C.
Evaluation of the performance of C8P10E5C as a surfactant
Surface tension
The surface tension results for C8P10E5C solutions of different concentrations are shown in Fig. 8. Initially, as the concentration of the C8P10E5C solution increased, its surface tension gradually decreased, and then the surface tension started to increase. The concentration corresponding to the shift in the change trend is the critical micelle concentration (CMC) of the C8P10E5C surfactant. The inflexion point of surface tension was determined using tangential fitting. Thus, the CMC value of C8P10E5C was determined to be 2.951 × 10−4 mol/L (the corresponding mass concentration is 0.26%). The surface tension of the solution at this concentration was 30.5728 mN/m. Thus, the synthesized C8P10E5C surfactant exhibited satisfactory surface activity.
Wettability test
Figure 9 shows the variation in the contact angle of the core with a change in the concentration of the C8P10E5C solution. When the concentration of C8P10E5C was less than 1 × 10−5 mol/L, the contact angle was greater than 75°, and the core was slightly wetted with water. When the concentration exceeded this range, the contact angle decreased slightly. However, when the CMC was reached, the contact angle decreased significantly. When the concentration of the C8P10E5C solution was 0.3%, the contact angle was 31.4°, which is 60.75% lower than the initial contact angle of the core, and the core exhibited water wettability. In summary, when the concentration of the C8P10E5C solution was higher than its CMC, the wettability improvement effect was better. Therefore, a C8P10E5C solution with a concentration higher than the CMC was selected for subsequent experiments to ensure better surface activity and improved wettability. To minimize the development costs, a 0.3% C8P10E5C solution was selected as the imbibition displacement agent.
Interfacial tension test
The test results of the interfacial tension between the C8P10E5C surfactant solution with a concentration of 0.3% and crude oil at different temperatures are shown in Fig. 10. The interfacial tension first decreased sharply with time, then decreased slowly, and finally stabilized. This is because the composition of the crude oil is complex. The initial oil–water interfacial tension is high. After a certain duration, the surfactant molecules emulsify the oil surface and penetrate the oil body, organizing at the oil–water interface to reduce the interfacial tension between them. The interfacial tension between the C8P10E5C solution and crude oil stabilized to 0.0211, 0.0243, 0.0273, and 0.0321 mN/m at 90, 100, 110, and 120 °C, respectively. At high temperatures, C8P10E5C could still decrease the oil–water interfacial tension to 10−2 mN/m. Thus, the capability of C8P10E5C in reducing the oil–water interfacial tension was not affected by the temperature, which indicates its good temperature resistance.
Adsorption property test
The change in the interfacial tension between the C8P10E5C solution (0.3% concentration) and crude oil before and after adsorption onto oil sand is shown in Fig. 11. Both before and after adsorption on the oil sand, the interfacial tension of the C8P10E5C solution first decreased sharply with increasing time, then decreased slowly, and finally became stable. Before adsorption, the interfacial tension between the C8P10E5C solution and crude oil was 0.0273 mN/m. After adsorption, the surfactant concentration decreased, and its effect of reducing the interfacial tension was weakened. Therefore, after adsorption, the interfacial tension between the C8P10E5C solution and crude oil was 0.0348 mN/m, 27.56% higher than that before adsorption, but it could still reach the order of 10−2 mN/m. This result indicates that C8P10E5C has strong anti-adsorption capability.
Emulsifying property test
Emulsification is an important mechanism by which surfactants enhance oil recovery. After the surfactant comes into contact with the crude oil, emulsification breaks the large oil droplets into smaller ones, allowing the crude oil to pass through the smaller pores and thus improving the oil-washing efficiency. The stability of the emulsion was observed using the bottle test method, and the results are shown in Fig. 12. As shown, with the prolongation of the placement time, the oil − water two-phase separation became evident, indicating that the synthesized C8P10E5C had an excellent emulsifying capability.
The stability of the emulsion formed using the C8P10E5C solution and crude oil was evaluated based on the drainage rate. The C8P10E5C solution was emulsified with crude oil to form an emulsion, and the drainage of the emulsion at different time points was evaluated (see Fig. 13). As shown, with the increase in time, the drainage first increased sharply to a certain value, then slowly to the highest value, and then tended to be stable. The highest drainage rate was 50%. The maximum drainage rate is small, indicating that the emulsion formed by the emulsification of the C8P10E5C solution with crude oil was stable, and the emulsification performance of C8P10E5C was strong.
Assessment of the forced imbibition efficiency
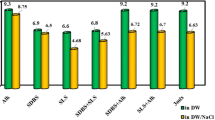
To evaluate the imbibition efficiency of C8P10E5C under high-temperature formation, its imbibition efficiency was compared with those of three other surfactants (SBDS, APEC, and AEC). The test results of the oil−water interfacial tension and contact angle of the four surfactants under the same conditions are shown in Fig. 14. Among the four surfactants, C8P10E5C exhibited the best effect in reducing the interfacial tension between oil and water and improving the wettability of the oil sand under the formation temperature conditions, indicating that it has strong high-temperature resistance.
A forced imbibition experiment was conducted to evaluate the imbibition oil recovery of different surfactants, and the imbibition oil recovery calculation results are shown in Table 3. As shown, the forced imbibition oil recovery values of C8P10E5C, SBDS, APEC, and AEC are 65.80, 27.26, 41.57, and 38.55%, respectively. According to these results, the forced imbibition oil recovery of C8P10E5C is 38.54, 24.22, and 27.25% higher than those of SBDS, APEC, and AEC, respectively. Thus, the efficiency of the new temperature-resistant anionic/nonionic surfactant in forced imbibition oil recovery from tight reservoir is significantly better than those of conventional anionic and nonionic surfactants. Therefore, compared with conventional anionic and nonionic surfactants, the new temperature-resistant anionic/nonionic surfactant is more suitable for enhanced oil recovery by imbibition from tight reservoirs.
Discussion
Based on the molecular structures of conventional long-chain alkyl anionic surfactants, a new temperature-resistant anionic/nonionic surfactant (C8P10E5C) was synthesized by introducing polyoxyethylene and polyoxypropylene units into double-chain alcohols. The FT-IR and mass spectra of C8P10E5C confirmed that the molecular chain of C8P10E5C contained all the designed groups and that the sample consisted of compounds containing both polyoxyethylene and polyoxypropylene units. In addition, the corresponding proton peaks of each unit were observed in the 1H-NMR spectrum of the synthetic product, C8P10E5C, consistent with the expected results. Thus, the final synthesized product was confirmed to be C8P10E5C.
The CMC of C8P10E5C was 2.951 × 10−4 mol/L, and the surface tension of the C8P10E5C solution at this concentration was 30.5728 mN/m. When the concentration of the C8P10E5C solution was higher than the CMC, the wettability improvement was better. When the concentration was 0.3%, the contact angle of the solution on the core was 31.4°, which is 60.75% lower than the initial contact angle of the core. Under high-temperature conditions, C8P10E5C could still reduce the oil–water interfacial tension to 10−2 mN/m. Thus, the capability of C8P10E5C in reducing the oil–water interfacial tension was not affected by the temperature, indicating its good temperature resistance. At 110 °C, the C8P10E5C solution adsorbed to oil sand could reduce the oil–water interfacial tension to 0.0276 mN/m, and it could still reach the order of 10−2 mN/m. This result indicates that the C8P10E5C has strong anti-adsorption ability. In addition, it has good emulsifying performance; when an emulsion was formed with crude oil, the highest drainage rate was only 50%.
The forced imbibition oil recovery of C8P10E5C was 38.54, 24.22, and 27.25% higher than those of SBDS, APEC, and AEC, respectively. Compared to conventional anionic and nonionic surfactants, the new temperature-resistant anionic/nonionic surfactant is more suitable for enhanced oil recovery by imbibition from tight reservoirs.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Sun, Y. P. et al. Experimental study on the mechanism of adsorption-improved imbibition in oil-wet tight sandstone by a nonionic surfactant for enhanced oil recovery. Pet. Sci. 18(4), 1115–1126 (2021).
Luo, Y. et al. Longitudinal reservoir evaluation technique for tight oil reservoirs. Adv. Mater. Sci. Eng. 2019(4), 1–8 (2019).
Zou, C. et al. Nano-hydrocarbon and the accumulation in coexisting source and reservoir. Pet. Explor. Dev. 39(1), 15–32 (2012).
Zhang, H. et al. Combined technology of PCP and nano-CT quantitative characterization of dense oil reservoir pore throat characteristics. Arab. J. Geosci. 12(16), 534 (2019).
Fu, L. et al. Study on the damage and control method of fracturing fluid to tight reservoir matrix. J. Nat. Gas Sci. Eng. 82, 103464 (2020).
Qun, L., Dingwei, W., Baoshan, G. et al. A novel approach of tight oil reservoirs stimulation based on fracture controlling optimization and design. Pet. Explor. Dev., (2020).
Wei, J. et al. Recovery efficiency of tight oil reservoirs with different injection fluids: An experimental investigation of oil-water distribution feature. J. Pet. Sci. Eng. 195, 10767 (2020).
Wu, B., Xie, R., Wang, X., et al. Characterization of pore structure of tight sandstone reservoirs based on fractal analysis of NMR echo data. J. Nat. Gas Sci. Eng. 103483 (2020).
Alvarez, J. O. & Schechter, D. S. Wettability alteration and spontaneous imbibition in unconventional liquid reservoirs by surfactant additives. SPE 20, 107–117 (2016).
Wang, F. et al. Mechanism of low chemical agent adsorption by high pressure for hydraulic fracturing-assisted oil displacement technology: A study of molecular dynamics combined with laboratory experiments. Langmuir 39(46), 16628–16636 (2023).
Jwa, B. et al. Recovery efficiency of tight oil reservoirs with different injection fluids: An experimental investigation of oil-water distribution feature. J. Pet. Sci. Eng. 195, 107678 (2020).
Li, H. et al. Quantitative analysis method of oil occurrences in tight reservoir. Energy Rep. 6, 1067–1072 (2020).
Zhang, Y., Zou, Y., Zhang, Y. Experimental study on characteristics and mechanisms of matrix pressure transmission near the fracture surface during post-fracturing shut-in in tight oil reservoirs. J. Pet. Sci. Eng., (2022).
Xu, J., Liu, R. & Liu, H. Optimization of shut- in time based on saturation rebalancing in volume- fractured tight oil reservoirs. Pet. Explor. Dev. 50(6), 1259–1267 (2023).
Xiao, L. et al. Imbibition mechanisms of high temperature resistant microemulsion system in ultra-low permeability and tight reservoirs. Pet. Explor. Dev. 49(6), 1206–1216 (2022).
Feng, C. et al. Nuclear magnetic resonance features of low-permeability reservoirs was conplex wettability. Pet. Explor. Dev. 44(2), 252–257 (2017).
Yu, F. et al. Features and imbibition mechanisms of Winsor I type surfactant solution in oil-wet porous media. Pet. Explor. Dev. 46(5), 950–958 (2019).
Osteboa, P. Early-and late-time analytical solutions for cocurrent spontaneous imbibition and generalized scaling. SPE J. 26(1), 220–240 (2021).
Xu, D. L. et al. A systematic research on spontaneous imbibition of surfactant solutions for low permeability sandstone reservoirs. J. Pet. Sci. Eng. 206, 109003 (2021).
Yang, K., Wang, F. T. & Zhao, J. Y. Experimental study of surfactant-enhanced spontaneous imbibition in fractured tight sandstone reservoirs: The effect of fracture distribution. Pet. Sci. 20, 370–381 (2023).
Wang, F. et al. Research on the adsorption law of HFAD agents on the surface of porous media during hydraulic fracturing-assisted oil displacement in low-permeability reservoirs. Langmuir 39(50), 18614–18620 (2023).
Yan, W. C. et al. Evaluation of wettabilities and pores in tight oil reservoirs by a new experimental design. Fuel 252, 272–280 (2019).
Acknowledgements
This research was supported by the Heilongjiang Provincial Natural Science Foundation of China (Grant No. LH2021E016).
Author information
Authors and Affiliations
Contributions
Conceptualization, Y.D. and C.A. ; methodology, Y.D. and B.C.; software, Y.D. and Y.L. ; validation, Y.D., C.A. and J.W.; writing—original draft preparation, Y.D. and B.C. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Duan, Y., Li, Y., Chen, B. et al. Preparation and performance evaluation of a novel temperature-resistant anionic/nonionic surfactant. Sci Rep 14, 5710 (2024). https://doi.org/10.1038/s41598-024-56342-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-56342-5
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.