Abstract
The hemorrhagic progression of a contusion (HPC) after Traumatic brain injury (TBI) is one of the important causes of death in trauma patients. The purpose of this meta-analysis was to evaluate the predictive effect of imaging features of Computed tomography (CT) on HPC after TBI. A comprehensive systematic search was performed using PubMed, EMBASE, and WEB OF SCIENCE databases to identify all relevant literature. A total of 8 studies involving 2543 patients were included in this meta-analysis. Meta-analysis showed that subarachnoid hemorrhage (OR 3.28; 95% CI 2.57–4.20), subdural hemorrhage (OR 4.35; 95% CI 3.29–5.75), epidural hemorrhage (OR 1.47;95% CI 1.15–1.89), contrast extravasation (OR 11.81; 95% CI 4.86–28.71) had a predictive effect on the occurrence of HPC. Skull fracture (OR 1.64; 95% CI 0.84–3.19) showed no statistical significance, and midline displacement > 5 mm (OR 4.66; 95% CI 1.87–11.62) showed high heterogeneity. The results of this meta-analysis showed that some imaging features were effective predictors of HPC after TBI. Well-designed prospective studies are needed to more accurately assess the effective predictors of HPC after TBI.
Similar content being viewed by others
Introduction
Traumatic brain injury (TBI) is the important cause of death for trauma victims, mostly due to traffic accidents, followed by falls from a height. About 2.8 million cases of TBI occurred in the United States in 2013, while the incidence of TBI in Europe is 235 per 100,0001,2,3. Some studies have found that 35–65% of patients with TBI have hemorrhagic progression of contusion (HPC), which increases the risk of aggravation of clinical symptoms by 5 times. Therefore, this is an important cause of disability and death of trauma victims4,5,6,7,8. TBI has caused an increasing socio-economic burden in the world. The main reason is that HPC after TBI can not be predicted in time, and the patients cannot are managed hierarchically. If effective hierarchical management can be achieved, intervention measures can be put in place earlier to improve prognosis and allocate medical resource more rationally9,10.
In the past few decades, many studies have explored the predictive value of hematological and biochemical indicators for HPC, and relatively few studies have analyzed the predictive value of imaging features. Studies have found that platelet count, coagulation function test and plasma D-dimer have a predictive effect on HPC11,12,13,14,15,16. Hematological and biochemical parameters and imaging examinations are of great value in the diagnosis and prognosis of HPC. Computed tomography (CT) examination is the preferred imaging examination for the diagnosis of TBI, which can identify a variety of hematomas more quickly. Therefore, this study mainly discusses the imaging features of CT. Some studies have found that some imaging features is an independent predictor of HPC, such as subarachnoid hemorrhage (SAH), subdural hemorrhage (SDH), epidural hemorrhage (EDH) and contrast extravasation (CE)17,18,19,20,21,22,23,24. Therefore, the purpose of this study is to further systematically review and clarify the predictive value of some imaging features for HPC, and to provide a clinical basis for prediction of HPC after TBI.
Method
Systematic reviews and meta-analyses were performed according to the guidelines for systematic reviews and meta-analyses (PRISMA)25. This study was prospectively registered in the PROSPERO system evaluation database (CRD42022350212).
Literature search
A comprehensive literature search was performed in PubMed, EMBASE, and Web of Science databases to assess the association between imaging features and HPC after TBI. The search terms included ' cerebral contusion ', 'brain injury ', ' traumatic brain injury ', ' hemorrhagic progression of contusion ', ' extravasation of contrast agent ', ' leakage sign ', ' spot sign ' and their synonyms. The search time is from January 2007 to April 2022.
Inclusion and exclusion criteria
We selected articles according to the following inclusion criteria: (1) Head imaging examination with clear diagnosis of TBI and reexamination; (2) Outcome index was HPC; (3) The data of the selected articles can be used to calculate the OR value and 95% confidence interval; (4) The included population included mild, moderate and severe TBI patients (excluding only one or two groups). The exclusion criteria are as follows: (1) The same author is excluded; (2) Imaging factors are not the main object of study; (3) Reviews, conference reports and case reports are excluded. We reviewed the selected articles and summarized the relationship between imaging features and HPC in 34 studies, of which 8 studies met the inclusion criteria.
Data abstraction
The list of articles generated by the literature search was manually reviewed by two investigators (Peng and Luo). The following information is included: author, age, country, sample size, gender and hemorrhagic progression volume. Any differences will be resolved through consultation between the two authors.
Assessment of risk of bias and quality
Two authors (Peng and Luo) independently assessed the quality of the included article by the Newcastle–Ottawa Quality Assessment Scale (four for quality of selection, two for comparability, and three for quality of outcome and adequacy of follow-up). The quality of the study was classified as low (below 6), moderate (7), and high (8–9). Conflicts were resolved by consensus between the two authors.
Statistical analysis
All statistical analyses were performed using Review Manager Version 5.4 software. For dichotomous variables, we calculated the odds ratio (OR) with a 95% confidence interval (CI). Whenever I2 was less than 50%, the fixed-effects model results were used; otherwise, the random-effects model was preferred. For those with high heterogeneity, sensitivity analysis and subgroup analysis can be performed to evaluate the stability of the results.
Results
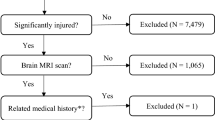
The process and results of retrieving the article are shown in Fig. 1. A total of 3356 articles were retrieved using PubMed, EMBASE, and Web of Science databases. After deleting duplicate articles, 3275 articles remained. After reading the title and abstract, a total of 3241 articles were excluded because they did not meet the topic. After reading the full text of the remaining 34 articles, 8 articles were retained (Fig. 1). Among them, 26 articles were excluded for the following reasons: 13 articles did not have enough data to calculate the OR value and 95% confidence interval; 2 articles did not analyze the imaging features; the outcome index of 4 articles was not HPC; 3 articles did not include patients with mild, moderate and severe TBI; 3 reviews were excluded; 1 article was repeated by the author.
Study characteristics
The characteristics of the included studies are shown in Table 1. All studies were published between 2007 and 2022. The diagnosis of TBI patients is confirmed by a clear history of TBI and imaging examination. Author, year, location, sample size, average age, gender, and hemorrhagic progression volume are shown in Table 1. Four studies were case–control studies and four studies were cohort studies.
Risk of bias assessment
The Newcastle–Ottawa quality assessment score included in the study was between 8 and 9 points. All the 8 studies were of high quality. Four case–control studies scored 8 (Table 2). In the cohort study, 2 articles scored 9 points and 2 articles scored 8 points (Table 3).
Data analysis
Eight articles were included in this meta-analysis, and 2543 patients were included. Six articles included SAH and SDH. Four articles were included in EDH. Four articles included skull fractures. Three articles were included in CE. Two articles included midline shift. The pooled results showed that SAH (OR 3.28; 95% CI 2.57–4.20; I2 = 24%; Fig. 2), SDH (OR 4.35; 95% CI 3.29–5.75; I2 = 0%; Fig. 3), EDH (OR 1.47 ; 95% CI 1.15–1.89 ; I2 = 46% ; Fig. 4), CE (OR 11.81; 95% CI 4.86–28.71; I2 = 0%; Fig. 5) all have a certain predictive effect on HPC. The above results were statistically significant (P < 0.05). The heterogeneity results were not high.
Four articles analyzed the predictive effect of skull fracture on HPC, and a total of 1476 patients were included. The pooled OR was 1.64 ([95% CI 0.84, 3.19]. I2 = 86.0%; Fig. 6), the results showed no statistical significance. Two studies have shown the predictive effect of midline shift > 5 mm on HPC. The results showed that midline shift > 5 mm was statistically significant (OR 4.66; 95% CI 1.87–11.62; I2 = 60%; Fig. 7). The reason for the high heterogeneity may be the insufficient number of included studies and the large difference between the two sample sizes.
Discussion
To our knowledge, there is no meta-analysis to systematically summarize and explore the predictive effect of imaging features on HPC after TBI. In TBI patients, HPC mostly occurs within 24 h before onset, and rarely occurs 3–4 days after onset4,26,27. Due to the high incidence of HPC after TBI, imaging features predict that HPC is crucial for the treatment of TBI patients. The purpose of this meta-analysis is to further clarify the predictive value of imaging features of CT. The results of this meta-analysis show that some imaging features are effective predictors of HPC. Among them, CE, SAH, SDH, and midline shift have obvious predictive effects. EDH has less predictive effect than the above imaging features. There is no statistical significance in skull fracture, and the heterogeneity of midline shift in forest map is high.
There is a lack of standardized definition of HPC in previous studies. When the amount of bleeding exceeds a threshold or the increase in the range of contusion is defined as HPC, but this threshold and the range of contusion increase are not uniform. This is the main reason for the difference in the incidence of contusion hemorrhage. Part of the reason is caused by the calculation method of assessing the volume of contusion. The previous traditional mechanism of HPC is coagulation dysfunction after TBI, but the occurrence of HPC in some studies does not seem to be related to coagulation dysfunction. In recent years, it has been found that ' traumatic penumbra ' may better explain the mechanism of HPC after TBI, but there are few studies on imaging features17,18,19,20,21,22,23.
Many studies have examined the predictive effect of hematological examinations such as coagulation function and platelets .TBI can cause small vessel injury and coagulation mechanism disorder, leading to HPC. Platelets can promote hemostasis, accelerate coagulation, and participate in the repair of vascular endothelium. Plasma D-dimer is an important indicator of coagulation function, reflecting disseminated intravascular coagulation.These indicators have a certain predictive effect on HPC11,12,13,14,15,16.
SAH causes extravasation of erythrocyte decomposition products and cerebral vasospasm, leading to ischemia and necrosis of the vascular wall. Extravasated red blood cells may trigger an inflammatory cascade5,19,28. SDH can reflect bridging vein rupture and venous sinus injury, with persistent bleeding, aggravating edema and compression effect5,17. After skull fracture, meningeal artery bleeding and veins can be damaged, resulting in persistent bleeding. Skull fracture may have middle meningeal artery injury, plate barrier bleeding and venous sinus rupture. The continuous bleeding of these arteries and veins leads to HPC19. The mechanism of HPC after EDH may be related to arteriovenous hemorrhage, secondary damage to the underlying cortex, and hematoma compression effect. Midline shift > 5 mm may also be an effective predictor of HPC. Midline shift > 5 mm shows a wide range of contusion, laceration, endothelial cell injury, swelling, vasospasm and microvascular injury4,14,28. CE shows that cerebrovascular endothelial cell damage after TBI patients, leading to active bleeding21,24. CE has been widely studied in spontaneous intracerebral hemorrhage (ICH) and is considered to be a predictor of cerebral hemorrhage expansion, adverse outcomes and mortality. Recent studies have shown that CE has predictive value for HPC after TBI, so this study included CE29,30,31.
In this penumbra, metabolic function is abnormal, and brain tissue is more vulnerable to secondary damage. Traumatic penumbra after TBI activates specific protein 1 and nuclear factor. Subsequently, the expression of sulfonylurea receptor 1 changed the expression of aquaporin 4, increased the permeability of the blood–brain barrier, formed vascular edema, endothelial cell death and capillary rupture, resulting in contrast agent exudation. Some studies have found that the loss of tight junction (TJ) protein and the increase of endocytosis of endothelial cells (EC) may also be related to the change of blood–brain barrier (BBB) permeability32,33,34,35. Dot signs and CE were more common in patients with spontaneous intracerebral hemorrhage, but CE was more common in TBI patients. Studies have shown that dot signs are vascular injury or perforation after spontaneous intracerebral hemorrhage. However, vascular injury or perforation directly caused by TBI is less likely. Secondary BBB rupture occurs within a few days after injury. In patients with TBI, secondary BBB damage, ischemic necrosis or perforation caused by ischemia or reperfusion injury takes time, which may explain the reason why there is no spot sign in the early stage of TBI21,24,36,37,38,39,40,41,42.
The traditional mechanism is that coagulation disorders lead to continuous or delayed bleeding of ruptured microvessels during primary injury, so there have been many studies on hematological and biochemical indicators5,36. However, the theory of ' traumatic penumbra suggests that vascular injury may be the main cause of HPC, and imaging features can show persistent bleeding after vascular injury, so some imaging features may be effective predictors. In recent years, studies proposed the multihematoma fuzzy sign after TBI. Multihematoma fuzzy sign usually refers to the contusion area with blood clots and fresh liquid blood. The presence of fresh liquid blood indicates that there may be ongoing active bleeding17.
This meta-analysis showed that skull fracture was not statistically significant, and the midline shift was > 5 mm shows high heterogeneity. There is no statistical significance of skull fracture may be due to the following reasons: (1) Rehman et al.11 only included patients with blunt brain injury, excluding penetrating brain injury. Because penetrating brain injury is more likely to have HPC than blunt brain injury, and this study has a small sample size and is likely to cause bias. (2) Skull fractures include linear fractures, depressed fractures, comminuted fractures and cavernous fractures. Yuan et al.20 only included linear fractures, but linear fractures are less likely to deteriorate than other fractures, and the risk of HPC is relatively small. Sensitivity analysis showed that the reason for the high heterogeneity of the midline shift > 5 mm may be related to the small number of included articles and the large difference in the sample size of the included studies18,20.
Although this meta-analysis shows that some imaging features are predictors of HPC, there are still some limitations: (1) Due to the small number of articles included, publication bias cannot be analyzed. Half of the included studies were case–control studies, and the quality of the literature was insufficient, which affected the accuracy of the results. (2) Hematoma volume after TBI, multiple hematoma blur sign, TBI impact location, the first CT examination time may be effective predictors11,17,19. Due to the lack of the number of studies, it is impossible to explore the relationship with HPC. (3) HPC has no clear definition, and the cut-off values in definition are different. Oertel described the definition of HPC, including EDH, SDH, SAH and intracerebral hematoma37. Alahmadi et al. proposed a rough definition of HPC and described the prognosis and risk factors of HPC43,44,45,46,47,48. Sheng J established a predictive model for acute traumatic intracerebral hematoma expansion17. (4) This study included people with mild, moderate and severe TBI, but severe TBI may be more prone to hemorrhagic injury. Because the included articles did not divide the population into mild, moderate and severe groups, we could not conduct a comparative study separately, which also led to some bias in the results of this study.
For the management of TBI patients, timely prediction of HPC is very important. Imaging examination and hematological and biochemical parameters have predictive value for the occurrence of HPC, and some imaging features can significantly predict the occurrence of HPC. The establishment of a risk assessment system based on imaging features may be more accurate in predicting the occurrence of HPC in TBI patients. The study of Randall Z. Allison et al. established a scoring system to predict the occurrence of HPC through imaging features. Studies have analyzed the relationship between imaging features and HPC in TBI patients, but there is no meta-analysis system to study the relationship. This study further demonstrated the predictive value of imaging features in predicting HPC in TBI, and provided further evidence for the establishment of imaging scoring system in the future.
Conclusion
The results of this meta-analysis show that some imaging features are effective predictors of HPC, and some mechanisms can explain these results, but more research is needed to explain the mechanism of HPC. High-quality cohort studies are needed to further demonstrate the predictive effect of imaging features on HPC.
Data availability
All data generated or analyzed during this study are included in these published articles.
References
Tagliaferri, F., Compagnone, C., Korsic, M., Servadei, F. & Kraus, J. A systematic review of brain injury epidemiology in Europe. Acta Neurochir. (Wien) 148(3), 255–268 (2006).
Taylor, C. A., Bell, J. M., Breiding, M. J. & Xu, L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Surveill. Summ. 66(9), 1–16 (2017).
Ma, D. et al. Establishment and preliminary application of craniocerebral trauma database platform. Chin. J. Neurosurg. 30(002), 159–161 (2014).
Sanus, G. Z. et al. Evolving traumatic brain lesions: Predictors and results of ninety-eight head-injured patients. Neurosurg. Q. 14(2), 97-104.0 (2004).
Kurland, D., Hong, C., Aarabi, B., Gerzanich, V. & Simard, J. M. Hemorrhagic progression of a contusion after traumatic brain injury: A review. J. Neurotrauma 29(1), 19–31 (2012).
Narayan, R. K. et al. Progression of traumatic intracerebral hemorrhage: A prospective observational study. J. Neurotrauma 25(6), 629–639 (2008).
Perel, P. et al. Intracranial bleeding in patients with traumatic brain injury: A prognostic study. BMC Emerg. Med. 3(9), 15 (2009).
Tong, W. S. et al. Prognosis analysis and risk factors related to progressive intracranial haemorrhage in patients with acute traumatic brain injury. Brain Inj. 26(9), 1136–1142 (2012).
Raj, R. et al. Costs, outcome and cost-effectiveness of neurocritical care: A multi-center observational study. Crit. Care 22(1), 225 (2018).
Alali, A. S. et al. Economic evaluations in the diagnosis and management of traumatic brain injury: A systematic review and analysis of quality. Value Health 18(5), 721–734 (2015).
Rehman, L. et al. Radiological parameters to predict hemorrhagic progression of traumatic contusional brain injury. J. Neurosci. Rural Pract. 10(2), 212–217 (2019).
Bakheet, M. F., Pearce, L. A. & Hart, R. G. Effect of addition of clopidogrel to aspirin on subdural hematoma: Meta-analysis of randomized clinical trials. Int. J. Stroke 10(4), 501–505 (2015).
Chauny, J. M. et al. Risk of delayed intracranial hemorrhage in anticoagulated patients with mild traumatic brain injury: Systematic review and meta-analysis. J. Emerg. Med. 51(5), 519–528 (2016).
Siracuse, J. J. et al. Antiplatelet agents, warfarin, and epidemic intracranial hemorrhage. Surgery 148(4), 724–729 (2010).
Mina, A. A. et al. Intracranial complications of preinjury anticoagulation in trauma patients with head injury. J. Trauma 53(4), 668–672 (2002).
Xu, D. X., Du, W. T., Li, X., Wu, Z. X. & Yu, G. F. D-dimer/fibrinogen ratio for the prediction of progressive hemorrhagic injury after traumatic brain injury. Clin. Chim. Acta 507, 143–148 (2020).
Sheng, J. et al. A clinical predictive nomogram for traumatic brain parenchyma hematoma progression. Neurol. Ther. 11(1), 185–203 (2022).
Tong, W. S. et al. Early CT signs of progressive hemorrhagic injury following acute traumatic brain injury. Neuroradiology 53(5), 305–309 (2011).
Wang, K. et al. Risk factors of progressive brain contusion and relationship with outcome. Zhejiang Da Xue Xue Bao Yi Xue Ban 44(4), 410–416 (2015).
Yuan, F. et al. Predicting progressive hemorrhagic injury after traumatic brain injury: Derivation and validation of a risk score based on admission characteristics. J. Neurotrauma 29(12), 2137–2142 (2012).
Huang, A. P. et al. Early parenchymal contrast extravasation predicts subsequent hemorrhage progression, clinical deterioration, and need for surgery in patients with traumatic cerebral contusion. J. Trauma 71(6), 1593–1599 (2011).
Letourneau-Guillon, L. et al. Traumatic intracranial hematomas: Prognostic value of contrast extravasation. AJNR Am. J. Neuroradiol. 34(4), 773–779 (2013).
Rosa, M. Jr. et al. Contusion contrast extravasation depicted on multidetector computed tomography angiography predicts growth and mortality in traumatic brain contusion. J. Neurotrauma 33(11), 1015–1022 (2016).
Orito, K. et al. Predictive value of leakage signs for pure brain contusional hematoma expansion. J. Neurotrauma 35(5), 760–766 (2018).
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 6(7), e1000097 (2009).
White, C. L., Griffith, S. & Caron, J. L. Early progression of traumatic cerebral contusions: Characterization and risk factors. J. Trauma 67(3), 508–514 (2009).
Yadav, Y. R., Basoor, A., Jain, G. & Nelson, A. Expanding traumatic intracerebral contusion/hematoma. Neurol. India 54(4), 377–381 (2006).
Vedantam, A., Yamal, J. M., Rubin, M. L., Robertson, C. S. & Gopinath, S. P. Progressive hemorrhagic injury after severe traumatic brain injury: Effect of hemoglobin transfusion thresholds. J. Neurosurg. 125(5), 1229–1234 (2016).
Simard, J. M. et al. Key role of sulfonylurea receptor 1 in progressive secondary hemorrhage after brain contusion. J. Neurotrauma 26(12), 2257–2267 (2009).
Martínez-Valverde, T. et al. Sulfonylurea receptor 1 in humans with post-traumatic brain contusions. J. Neurotrauma 32(19), 1478–1487 (2015).
Newcombe, V. F. et al. Microstructural basis of contusion expansion in traumatic brain injury: Insights from diffusion tensor imaging. J. Cereb. Blood Flow Metab. 33(6), 855–862 (2013).
Li, N. et al. Contrast extravasation on computed tomography angiography predicts clinical outcome in primary intracerebral hemorrhage: A prospective study of 139 cases. Stroke 42(12), 3441–3446 (2011).
Rosa Júnior, M., Rocha, A. J., Saade, N., Maia Júnior, A. C. & Gagliardi, R. J. Active extravasation of contrast within the hemorrhage (spot sign): A multidetector computed tomography finding that predicts growth and a worse prognosis in non-traumatic intracerebral hemorrhage. Arq. Neuropsiquiatr. 71(10), 791–797 (2013).
Peng, W. J., Reis, C., Reis, H., Zhang, J. & Yang, J. Predictive value of CTA spot sign on hematoma expansion in intracerebral hemorrhage patients. BioMed Res. Int. 2017, 4137210 (2017).
Adatia, K., Newcombe, V. F. J. & Menon, D. K. Contusion progression following traumatic brain injury: A review of clinical and radiological predictors, and influence on outcome. Neurocrit. Care 34(1), 312–324 (2021).
Mendelow, A. D. et al. Early surgery versus initial conservative treatment in patients with traumatic intracerebral hemorrhage (STITCH[Trauma]): The first randomized trial. J. Neurotrauma 32(17), 1312–1323 (2015).
Szczygielski, J., Kopańska, M., Wysocka, A. & Oertel, J. Cerebral microcirculation, perivascular unit, and glymphatic system: Role of aquaporin-4 as the gatekeeper for water homeostasis. Front. Neurol. 13(12), 767470 (2021).
Cash, A. & Theus, M. H. Mechanisms of blood-brain barrier dysfunction in traumatic brain injury. Int. J. Mol. Sci. 21(9), 3344 (2020).
Oertel, M. et al. Progressive hemorrhage after head trauma: Predictors and consequences of the evolving injury. J. Neurosurg. 96(1), 109–116 (2002).
Carnevale, J. A. et al. Blossoming contusions: Identifying factors contributing to the expansion of traumatic intracerebral hemorrhage. J. Neurosurg. 129(5), 1305–1316 (2018).
Alahmadi, H., Vachhrajani, S. & Cusimano, M. D. The natural history of brain contusion: An analysis of radiological and clinical progression. J. Neurosurg. 112(5), 1139–1145 (2010).
Sifri, Z. C. et al. A prospective evaluation of the value of repeat cranial computed tomography in patients with minimal head injury and an intracranial bleed. J. Trauma 61(4), 862–867 (2006).
Sifri, Z. C. et al. Value of repeat cranial computed axial tomography scanning in patients with minimal head injury. Am. J. Surg. 187(3), 338–342 (2004).
Allison, R. Z., Nakagawa, K., Hayashi, M., Donovan, D. J. & Koenig, M. A. Derivation of a predictive score for hemorrhagic progression of cerebral contusions in moderate and severe traumatic brain injury. Neurocrit. Care 26(1), 80–86 (2017).
Lakshmanan, R. et al. Metabolic crisis after traumatic brain injury is associated with a novel microdialysis proteome. Neurocrit. Care 12(3), 324–336 (2010).
Engel, D. C. et al. Changes of cerebral blood flow during the secondary expansion of a cortical contusion assessed by 14C-iodoantipyrine autoradiography in mice using a non-invasive protocol. J. Neurotrauma 25(7), 739–753 (2008).
Liao, Z. B. et al. Erythropoietin can promote survival of cerebral cells by downregulating Bax gene after traumatic brain injury in rats. Neurol. India 57(6), 722–728 (2009).
Hong, Y., Yan, W., Chen, S., Sun, C. R. & Zhang, J. M. The role of Nrf2 signaling in the regulation of antioxidants and detoxifying enzymes after traumatic brain injury in rats and mice. Acta Pharmacol. Sin. 31(11), 1421–1430 (2010).
Funding
This study was funded by the Kuanren Talent Program of the Second Affiliated Hospital of Chongqing Medical University, Chongqing Health Commission and Science and Technology Bureau (No.2024MSXM163), Chongqing Natural Science Foundation Project (cstc2021jcyj-msxmX0036) and Chongqing Science and Health Joint Traditional Chinese Medicine Research Project (2019ZY023458).
Author information
Authors and Affiliations
Contributions
J.P. and T.L.: conceptualization of the study; J.S., Q.H. and J.P.: design of the study; J.P. and T.L.: literature retrieval, study selection, statistical analyses, interpretation of the data and drafting of the initial manuscript; J.P. and T.L.: data extraction,quality evaluation; J.P., B.L., T.L., Y.C. and X.Y.L.: critical revision and comment for important intellectual content. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Peng, J., Luo, T., Li, X. et al. Imaging predictors of hemorrhagic progression of a contusion after traumatic brain injury: a systematic review and meta-analysis. Sci Rep 14, 5961 (2024). https://doi.org/10.1038/s41598-024-56232-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-56232-w
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.