Abstract
Birds play a crucial role in disseminating ticks that carry pathogens of high veterinary-medical importance. The aim of this study was to analyze data of a long-term tick collection from birds at a single stop-over site in Central Europe, Hungary. Over eight years (2015–2022) 5833 ticks (ten species) were collected from 2395 tick-infested birds. The most abundant species were Ixodes ricinus (n = 3971) and Haemaphysalis concinna (n = 1706). Ixodes ricinus nymphs and larvae were the most frequently occurring on resident and short-distance migratory birds with forest habitat but Ha. concinna was the most abundant species on reed-associated, long-distance migrants. Haemaphysalis concinna occurred mostly on birds feeding above the ground level, while I. ricinus predominated on ground feeding birds. Infestation with I. ricinus nymphs always peaked in the first half of the year, in contrast to larvae which were more abundant on avian hosts in the autumn. At the same time, Ha. concinna larvae and nymphs had their peak numbers in the summer. This is the first long-term study on the tick infestation of birds in Central Europe. The study shows that, migration distance, habitat type, and typical feeding level of birds, as well as characteristics of tick life cycle are all key factors in the role of birds as tick disseminators. It was revealed that Savi’s Warbler (Locustella luscinioides) is the most frequent hosts of Ha. concinna in Central Hungary.
Similar content being viewed by others
Introduction
Hard ticks (Acari: Ixodidae) are common carriers of pathogens that can affect both humans and animals, so it is not surprising that they are considered one of the world’s most important arthropod vectors1. Because of this, research on their ecology and distribution is of utmost importance2. This is especially true nowadays, as ecological systems are transforming rapidly due to climate change3.
The role of birds in the dissemination of ticks is unquestionable. Migratory birds can transport ticks between continents, for instance from Africa into Europe4. Thermophilic tick species, most notably from the genus Hyalomma, have long been considered adventitious, but in recent years these species have been reported in Europe with increasing numbers north of the Mediterranean Basin, and were even able to establish a resident population5,6,7,8. In this context, the importance of synanthropic, resident bird species (e.g. the Blackbird (Turdus merula)) is also crucial, regarding the local dissemination of ticks, as they can introduce ticks to urban areas9. The monitoring of bird-tick relationships is a long-standing and intensively researched field that has greatly contributed to what is known about ticks and the epidemiology of the pathogens they transmit10,11,12.
Research on this topic has been conducted in Hungary for decades, particularly at Ócsa Bird Ringing Station8,13,14,15,16,17. At this station more than 15 thousand birds are caught yearly. The area has several different habitat types (e.g. forest, arable field, reedbed)18 and is an important stop-over site for birds migrating along the Adriatic Flyway through Central Europe. Therefore, it is suitable for the examination of both migratory and resident birds of different habitats.
In order to evaluate factors that influence the association of ixodid ticks with avian hosts, ticks were collected from birds at Ócsa Bird Ringing Station between March 2015 and November 2022. The data collected over the course of eight years ensure a better understanding of ecological and ornithological factors that influence the epidemiological significance of birds as tick carriers.
Results
Identification of tick species and their occurrence on infested birds
During the study period 2395 tick-infested birds were captured, belonging to 51 different species, and a total of 5833 ticks were collected. The most frequent host species were the European Robin (Erithacus rubecula) (ninfested = 521, ncaptured = 14,809), followed by the Blackbird (T. merula) (ninfested = 359, ncaptured = 1525). The mean number of ticks found on infested birds (i.e., intensity of infestation) was 2.44, while the median intensity was 1.
Based on morphological characteristics, eight tick species were identified, namely Ixodes ricinus, Ixodes frontalis, Ixodes lividus, Ixodes festai, Ixodes arboricola, Haemaphysalis concinna, Haemaphysalis punctata and Dermacentor reticulatus (Table 1). Concerning molecular identification of Hyalomma species, one nymph showed 100% identity to sequences of Hyalomma rufipes reported in Africa and in Malta (cox1 gene: OQ540949 from Kenya; 12S rRNA gene: OL352890 from Malta, 16S rRNA gene: MK737649 from Egypt). Two further nymphs proved to be Hyalomma marginatum, one of them with 100% sequence identities to ticks reported from the eastern-middle-western Mediterranean region (cox1 gene: LC508365 from Portugal; 12S rRNA gene: OL352894 from Malta; 16S rRNA gene: KT391060 from Israel) and another with 99.8–100% sequence identities to specimens reported from the western Mediterranean region (cox1 gene: LC508365 from Portugal; 16S rRNA gene: LC508322 from Portugal).
Altogether, 1872 (78.16%) of the captured, tick-infested birds carried I. ricinus, while 592 (24.72%) of them carried Ha. concinna. Ixodes frontalis was present on 51 birds (2.13%). The remaining seven tick species were carried by a combined total of 13 (0.54%) birds. One hundred thirty-one birds carried two tick species, and on one Song Thrush (Turdus philomelos) three different tick species were present simultaneously (I. ricinus, I. frontalis and Ha. concinna).
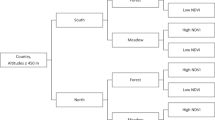
The 2395 tick-carrying birds captured during this study are summarized in Table 2, according to the characteristics that were examined. In the table, we also indicated how many bird species belonged to the given category. More detailed information can be found in Supplementary Tables 2 and 3.
Number and temporal occurrence of tick species, their host associations according to habitat and migration characteristics
All tick-host associations are listed in Supplementary Tables 1 and 2 and visualized in Fig. 1 and Supplementary Fig. 1 Statistical analyses on ticks and their hosts according to their habitat and migration characteristics were only calculated for the most abundant tick species (I. ricinus and Ha. concinna), due to the fact that the limited numbers of other tick species precluded robust statistical analyses.
Ixodes ricinus (ntotal = 3971, nlarvae = 1229, nnymphs = 2742): Ixodes ricinus subadults occurred most frequently on resident and/or short-distance migrating birds with forest or forest and meadow habitats (Tables 3, 4), This category includes the two species on which the most ticks were found: Blackbirds (nticks = 1073, nbirds = 326), and European Robins (nticks = 924, nbirds = 490) (Table 5). Ixodes ricinus ticks were present on birds year-round, with nymphs reaching their peak in the first half of the sampling periods (1st of March–30th of June), while larvae were the most abundant in the second half (1st of July–31 of October). This was consistent over the course of eight years. The difference between the numbers of nymphs and larvae regarding their half-yearly activity was significant (p < 0.0001) (Supplementary Table 1, Supplementary Fig. 2).
Ixodes frontalis (ntotal = 102, nlarvae = 52, nnymphs = 38, nfemale = 12): The European Robin was the most common host of this tick species (nticks = 52, nbirds = 21). The second most frequently identified host species was the Blackbird (nticks = 18, nbirds = 4). Ixodes frontalis occurred most frequently on resident and/or short-distance migrating birds with forest habitat (Tables 3, 4). All stages of I. frontalis were the most abundant in the second half of March every year, during the eight-year-long study period (Supplementary Fig. 3).
Ixodes lividus (ntotal = 13, nfemales = 12, nnymphs = 1): All 13 I. lividus ticks were collected from four Sand Martins (Riparia riparia).
Ixodes festai (ntotal = 8, nfemales = 6, nmales = 2): In total eight ticks of this species were collected. Five ticks were removed from two Blackbirds and three ticks from two Dunnocks (Prunella modularis). It is important to mention, that the two males collected were not feeding, but were in copulation with females.
Ixodes arboricola (ntotal = 1, nnymphs = 1): Only one nymph was collected. It was removed from a Great Tit (Parus major).
Haemaphysalis concinna (ntotal = 1706, nlarvae = 698, nnymphs = 1008): The majority (933 ticks, 54.7%) of individuals of this tick species were found on Savi’s Warbler (Locustella luscinioides) (nbirds = 238), making this bird the most common host of Ha. concinna. This tick species was also frequently collected from Eurasian Reed Warbler (Acrocephalus scirpaceus) (nticks = 157, nbirds = 82). The five most common hosts of Ha. concinna are listed in Table 5. Both Ha. concinna larvae and nymphs were active in the summer. However, the peaks of their abundance showed differences over the study period. In particular, the peak of infestation of birds with Ha. concinna larvae preceded that of its nymphs in 2015 and 2022, but the opposite trend was observed in 2017 and 2018. In other years, the peak larval and nymphal infestations occurred simultaneously (Supplementary Fig. 2).
It was found that the host habitat (reed, forest, meadow, meadow/forest) of I. ricinus and Ha. concinna was significantly different (p < 0.0001) (Table 3). We also compared the migratory habits (resident, short-distance migrants, middle-distance migrants, resident or short distance migrants, resident or middle-distance migrants, long-distance migrants) of the hosts of I. ricinus and Ha. concinna. The difference was strongly significant (p < 0.0001) (Table 4). According to our findings, Ha. concinna occurred most frequently on long-distance migrant and reed-associated birds, while I. ricinus was the most abundant on short-distance migrants, with forest habitat. (Tables 3, 4).
Haemaphysalis punctata (ntotal = 28, nlarvae = 28): A single Common Quail (Coturnix coturnix) was found with 28 feeding larvae.
Hyalomma marginatum (ntotal = 2, nnymphs = 2): One engorged nymph was collected from European Robin, and another from Song Trush (T. philomelos). Both were collected in the first half of April (in 2015, and 2016, respectively).
Hyalomma rufipes (ntotal = 1, nnymph = 1): Only one engorged nymph was found, which was feeding on a European Pied Flycatcher (Ficedula hypoleuca) in the second half of April 2015.
Dermacentor reticulatus (ntotal = 1, nfemale = 1): One female of this species was found on a Blackbird (Turdus merula), though it had not started to feed.
Host associations of ticks according to bird weight and feeding level characteristics
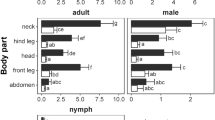
The mean intensity of infestation with I. ricinus nymphs was the highest among bird species with typical body weight above 100 g, whereas this was the lowest among bird species measuring below 10 g (Supplementary Fig. 4). The same was not true in the case of Ha. concinna nymphs, because the mean intensity of tick-infestation was the highest on birds weighing between 10.1 and 20 g (Supplementary Fig. 4).
When the numbers of I. ricinus, I. frontalis and Ha. concinna were compared according to the feeding level of their hosts (only ground level or above ground categories were tested), I. ricinus tended to predominate on ground-feeding birds, whereas Ha. concinna nymphs and larvae were more often found on birds looking for food items above the ground level (p < 0.0001). On the other hand, the difference was not significant between I. ricinus and I. frontalis (p = 0.2584) (Table 6).
Discussion
Although there are many similar previous reports from different countries (e.g.19,20,21,22,23,24), this study presents one of the longest continuous bird tick collections in Europe. Over an eight-year-long period, 5833 ticks of 10 species were collected from 2395 birds of 51 species. The dataset itself provides a valuable contribution to the field due to its size.
The two most abundant tick species collected were I. ricinus (ntotal = 3971) and Ha. concinna (ntotal = 1706). Only subadult stages were found in case of both species. Ixodes ricinus has long been known as the most common tick species that feeds on birds in the Palearctic region12 and is primarily a forests-dwelling tick species25. This fact was confirmed by data presented here, as I. ricinus occurred most frequently on birds with forest habitat. Interestingly, this was not true for Ha. concinna. According to the literature data, Ha. concinna can be found in a broad range of different habitats, mainly moist, wooded ecosystems, but in reedbeds as well25,26,27. However, according to our data, this species mainly parasitized reed-associated birds (1333 Ha. concinna ticks on birds with reed habitat). The difference between the host habitats of I. ricinus and Ha. concinna was strongly significant (p < 0.0001). Furthermore, 933 Ha. concinna ticks were collected from only one bird species, Savi’s Warbler. According to Csörgő et al.28 number of ringed Savi’s Warblers present in Hungary between 1951 and 2006 was 32 083 birds. Interestingly the same numbers of the second and third most common hosts from Ócsa (Eurasian Reed Warbler and Sedge Warbler (Acrocephalus schoenobaenus)) were over 220 000 for each species. Savi’s Warbler had the highest level of median infestation in the case of nymphs, and the second highest in the case of larvae. In our opinion however, larvae are not the greatest indicators in this regard, as a low presence of larvae can be overlooked easily due to the small size of the parasites.
Regarding the migration habit of the hosts of Ha. concinna, and I. ricinus, the results were in line with our previous observation8 on a strongly significant difference between the hosts of the two tick species: Ha. concinna most commonly occurred on long-distance migrants, whereas I. ricinus was most frequently collected from short-distance migrant or resident birds. Haemaphysalis concinna is a thermophilic tick species. Its larvae and nymphs have a similar period of peak activity (mainly the summer)27 and its typical avian hosts are reed-associated long-distance migrants28.
Among the latter, Savi’s Warbler was the most common host in Hungary. According to our hypothesis, the feeding habit of this bird species may explain this phenomenon. Savi’s Warbler shares a very similar ecological niche with Ha. concinna, because it mainly feeds on small invertebrates near the water surface in reedbeds. This behavior is consistent during the migration period28, and lakeshore vegetation was reported to be a preferred habitat type of Ha. concinna in Central Europe27.
Taken together, Ha. concinna subadults apparently share a much more similar ecological niche with Savi’s Warbler, than with other reed-associated songbirds. It is important to mention that this difference is only true among avian hosts and does not extend to mammals and reptiles, which are also favored hosts of Ha. concinna 27, especially roe deer29.
It was previously reported, that birds with larger body mass carry a higher number of I. ricinus nymphs30. In this study, the mean intensities of infestations with I. ricinus and Ha. concinna stages were categorized according to the average body mass of their avian hosts. In the case of I. ricinus, the mean intensity of nymphs showed a trend of increase with the average body mass of the hosts, unlike in the case of Ha. concinna nymphs. However, this could not be supported by the results of statistical analyses, because of the following reasons. First, the majority of Ha. concinna ticks were carried by Savi’s Warblers, which is a small size bird species. Second, I. ricinus occurred most frequently on ground feeding birds which in general have a larger body mass (e.g., Blackbirds). On the other hand, data on the body mass of bird individuals examined for tick-infestation in the present study were not available, and this parameter is known to change significantly even within the same bird species between different seasons.
The temporal distribution of I. ricinus and Ha. concinna developmental stages was also analyzed. Ixodes ricinus nymphs always reached their peak infestation on birds during the first half of the annual sampling periods throughout the study, whereas larvae reached their highest abundance from July to October. This is likely a consequence of the prolonged development of I. ricinus which takes several years in Central Europe31 implying that nymphs and larvae collected in the same year belonged to different generations. By contrast, the nymphal and larval peak activities of Ha. concinna were always close to each other, i.e., within 0.5–1.5 month over the summer period. This may reflect that a notable portion of these developmental stages belonged to the same generation, particularly when the larval peak preceded the nymphal peak (e.g., in 2015, 2022). This is in line with observations that under temperate climate this tick species can complete one generation in one year if hosts are available32 On the other hand, Ha. concinna larvae were also found on birds as early as March and April (e.g., in 2016 and 2017), suggesting overwintering in the larval stage and a generation time longer than one year27.
During the eight-year-long study period, 102 I. frontalis ticks were collected (nlarvae = 52, nnymphs = 38, nfemale = 12). According to the known literature data, I. frontalis reaches its peak in March and in November33,34. In this study, the vast majority of all developmental stages were collected in the second half of March. The absence of a peak in November is likely because the main sample collection period was finished at the end of October/beginning of November each year. From November tick collections, there are only a scarce and random amount of data. The fact that this tick is the most abundant at the beginning of spring and the end of autumn explains why I. frontalis showed association with resident and/or short-distance migrant birds according to our data (Table 4). Ixodes frontalis was almost exclusively found on birds with forest- or mixed meadow/forest habitats (98/102) (Table 3).
Altogether 8 specimens of I. festai, six females and two males were also collected during the study period. All hosts were Blackbirds and Dunnocks. The same host species were found to be infested in a previous study from another part of Central Europe, Switzerland 35, but literature data are available from multiple other host species as well 12.
Three Hyalomma nymphs were found in total. According to our molecular analyses, two were Hy. marginatum and one was Hy. rufipes. Each of these nymphs had 100% sequence identity with at least one specimen reported from the mid-Mediterranean region (Malta), situated along the Adriatic Flyway crossing Hungary toward the north. Thus, these data are highly relevant to their probable geographical origin. Hyalomma species are important vectors of sevedunral different pathogens, including the Chrimean-Congo haemorrhagic fever virus25. All Hyalomma ticks were collected in April (2015, 2016). Findings of Hyalomma subadults during the spring migration period are not uncommon in Central Europe36 and in Ócsa16. It is important to note, however, that the monitoring of Hyalomma-carrying birds is of great epidemiological importance.
Thirteen I. lividus were identified. This tick is the host-specific parasite of Sand Martin, that feeds extremely rarely on other birds12,25,37. It is not surprising that we have found all I. lividus ticks on Sand Martins. Ixodes lividus have been reported from Hungary long before our study38.
Twenty-eight Ha. punctata larvae were found feeding on a single Common quail. Haemaphysalis punctata has been already known in Hungary, as an uncommon parasite on birds 39.
Only one I. arboricola nymph was collected during this study. This low number is not surprising, since I. arboricola is a nidicolous tick that feeds on nestlings during the summer, and therefore adult birds are mostly infested during winter seasons, when roosting 40. For this study, sample collection period started in March and ended at the beginning of November each year so did not include the peak season for I. arboricola.
Lastly, one D. reticulatus female on a Blackbird was found in 2022. This tick was not (yet) feeding, and its occurrence is believed to be accidental, though D. reticulatus subadults have been found on Blackbirds (among other birds) before 41.
Conclusions
Based on this 8-year survey, the migration distance, the habitat type, and the feeding habit of birds, as well as the seasonal activity of ticks are all important factors determining the role of birds as tick disseminators. Haemaphysalis concinna was the most abundant on long-distance migrant, reed-associated, above-ground feeder birds, in contrast to I. ricinus, which predominated on resident or short-distance migrant, ground-feeder birds with forest or meadow habitat. In the study region, Savi’s Wabler (L. luscinioides) was by far the most common avian host of Ha. concinna larvae and nymphs.
Methods
Sample collection
Sample collection took place from March 2015 to November 2022 at Ócsa Bird Ringing Station (N47.2970, E19.2104), approximately 33 km south of Budapest. Ócsa is situated in a humid continental transitional climate zone. Summers are medium warm and dry, with relatively cold winters. The annual average temperature is 10.1 °C (minimum ca. − 15.6 °C, maximum ca. 34.1 °C). Annual precipitation is around 550–580 mm. Annual sunshine is ca. 2000 h. The mean elevation is 100 m above sea level18. There are a large variety of different habitats in the area of the station: it is surrounded by arable fields, but forests, shrubs, and garden yards, can also be found here. Due to the fact, that Ócsa is situated on the edge of a wetland, open water surfaces and reedbeds are also common18.
Birds were mist-netted for ringing by standard ornithological mist-nets (mesh size 16 mm). During the ringing procedure, birds were examined for the presence of ticks. Ticks were removed with the help of pointed tweezers and were placed and stored in 96% ethanol. Bird ringing and tick collection were constant from the beginning of March to the end of October each year. Only sporadic data were obtained during November and December. During 2020 and 2021, due to COVID-19 restrictions, bird ringing activity was reduced and tick collections were limited. Birds were handled, identified, and released by professional ringers throughout our study. Only data about tick-infested birds were recorded. Some data from 2022 were used for another study in a different context 8.
Morphological identification
Ticks were identified with a stereomicroscope (SMZ-2 T, Nikon Instruments, Japan, illuminated with model 5000-1, Intralux, Switzerland). To identify Ixodes ricinus, I. frontalis, I. lividus, I. arboricola, and Dermacentor reticulatus to the species level and Hyalomma species to the genus level, we used morphological keys provided by Estrada-Peña et al.25 Hyalomma ticks were identified to the species level with molecular methods8. Differentiation of subadults of Haemaphysalis concinna and Ha. punctata was based on the morphological keys by Filippova42. For the identification of I. festai, we used the manuscripts of Contini et al.43 and Hornok et al.14.
DNA extraction and molecular identification of Hyalomma species
These steps were carried out as in Keve et al.8. Purification and sequencing of the PCR products were done by Biomi Ltd. (Gödöllő, Hungary). Quality control and trimming of sequences were performed with the BioEdit program, then alignment with GenBank sequences by the nucleotide BLASTN program (https://blast.ncbi.nlm.nih.gov). New sequences were submitted to GenBank (cox1 gene: OR145129–OR145131, 16S rRNA gene: OR145132–OR145134, 12S rRNA gene: OR145138–OR145139).
Data curation and statistical analysis
All tick-host associations can be found in Supplementary Table 1. Birds were categorized according to their feeding place, minimum and maximum body mass, migration habits, and habitats according to ornithological data and previous reports14,15,28 (Supplementary Table 3). In order to categorize birds according to their feeding places, “Above ground” category was created. Birds belonging to this group are feeding on (e.g.) reed trunks, bushes, or branches that do not touch the ground directly but are not far from it either. For this categorization, the expertise of our co-authors (TCS and DK) were used, as well as the available literature data28.
Data curation and calculation of mean and median tick intensity was done in Microsoft Office Excel. Mean and median intensities were calculated for each tick and developmental stages according to Reiczigel et al.44. For comparing the half-yearly activity of I. ricinus larvae and nymphs, and the migration habits and the habitats of the hosts of tick species (I. ricinus, Ha. concinna), chi-squared tests were used. Chi-squared test was also used to compare feeding places of hosts of I. ricinus and Ha. concinna. The comparison of the feeding places of the hosts of I. ricinus and I. frontalis was done with Fisher’s exact test (R -program 4.3.0.). Results were considered significant if p < 0.05.
The average body mass of each bird species was calculated as the mean of the minimum and the maximum body mass registered and listed in Supplementary Table 3. \((\mathrm{Average\, body\, mass}\hspace{0.17em}=\hspace{0.17em}\frac{\mathrm{Minimum\, body\, mass}+\mathrm{Maximum\, body\, mass}}{2}\)).
The mean intensity of Ha. concinna and I. ricinus infestation was calculated for each group. The results are shown in Supplementary Fig. 4. Intensity was only calculated, if there were 10 or more infested birds in the respective category, to minimize the distortion caused by outliers. Data from one group (≤ 10g Average body mass for Ha. concinna) was therefore excluded (nbirds = 2).
In Supplementary Fig. 2, Relative, semimonthly numbers (RSN) were calculated as follows:
(SMN = Semimothly number of the tick species and stage; MON = number of the tick species and stage from the respective year, between March 01 - October 31).
English bird species names are capitalized following international recommendations (https://bou.org.uk/britishlist/bird-names/). In our figures and tables, HURING codes are used instead of the complete bird names. These abbreviations are clarified in Supplementary Table 3.
Ethical approval
The study was carried out according to the national animal welfare regulations (28/1998). License for bird ringing was provided by the National Inspectorate for Environment and Nature (No 14/3858-9/2012.). License for sample collection was provided by the Central Danube Valley Environmental Protection and Nature Conservation Inspectorate (KDV-KTF) (No KTF:27251-1/2014.). Where applicable, the ARRIVE guidelines were followed.
Data availability
The sequences generated during the current study are publicly available in GenBank (https://www.ncbi.nlm.nih.gov/genbank/). The following sequences were submitted: cox1 gene: OR145129–OR145131, 16S rRNA gene: OR145132–OR145134, 12S rRNA gene: OR145138–OR145139. All other relevant data are included in the manuscript and its supplementary files.
References
Sonenshine, D. E. The biology of tick vectors of human disease. in Tick-Borne Diseases of Humans 12–36 (Wiley, 2005).
Karpelles, L. Adalékok Magyarország atka-faunájáhozfaunájához [Contributions to the mite-fauna of Hungary]. Mathematikai és Természettudományi Közlemények 25, 439–441 (1893).
Grimm, N. B. et al. Climate-change impacts on ecological systems: Introduction to a US assessment. Front. Ecol. Environ. 11, 456–464 (2013).
Hoogstraal, H., Kaiser, M. N., Traylor, M. A., Gaber, S. & Guindy, E. Ticks (Ixodoidea) on birds migrating from Africa to Europe and Asia. Bull. World Health Organ. 24, 197 (1961).
Wallménius, K. et al. Spotted fever Rickettsia species in Hyalomma and Ixodes ticks infesting migratory birds in the European Mediterranean area. Parasit. Vectors 7, 1–20 (2014).
Hornok, S. & Horváth, G. First report of adult Hyalomma marginatum rufipes (vector of Crimean-Congo haemorrhagic fever virus) on cattle under a continental climate in Hungary. Parasit. Vectors 5, 170 (2012).
Földvári, G. et al. Emergence of Hyalomma marginatum and Hyalomma rufipes adults revealed by citizen science tick monitoring in Hungary. Transbound. Emerg. Dis. 69, e2240–e2248 (2022).
Keve, G. et al. Ornithological and molecular evidence of a reproducing Hyalomma rufipes population under continental climate in Europe. Front. Vet. Sci. 10, 272 (2023).
Gryczyńska, A. Urban and forest-living blackbirds Turdus merula as Hosts of Borreliella spp. Infect. Ticks. PJOE 66, 309–314 (2018).
Kaiser, M. N., Hoogstraal, H. & Watson, G. E. Ticks (Ixodoidea) on migrating birds in Cyprus, fall 1967 and spring 1968, and epidemiological considerations. Bull. Entomol. Res. 64, 97–110 (1974).
Hasle, G. Transport of ixodid ticks and tick-borne pathogens by migratory birds. Front. Cell. Infect. Microbiol. 3, 48 (2013).
Keve, G., Sandor, A. D. & Hornok, S. Hard ticks (Acari: Ixodidae) associated with birds in Europe: Review of literature data. Front. Vet. Sci. 9, 928756 (2022).
Flaisz, B. et al. Babesia genotypes in Haemaphysalis concinna collected from birds in Hungary reflect phylogeographic connections with Siberia and the Far East. Ticks Tick Borne Dis. 8, 666–670 (2017).
Hornok, S. et al. Bird ticks in Hungary reflect western, southern, eastern flyway connections and two genetic lineages of Ixodes frontalis and Haemaphysalis concinna. Parasites Vectors 9, 1–9 (2016).
Hornok, S. et al. Birds as potential reservoirs of tick-borne pathogens: First evidence of bacteraemia with Rickettsia helvetica. Parasit. Vectors 7, 128 (2014).
Hornok, S. et al. Synanthropic birds associated with high prevalence of tick-borne rickettsiae and with the first detection of Rickettsia aeschlimannii in Hungary. Vector Borne Zoonotic Dis. 13, 77–83 (2013).
Hornok, S., Karcza, Z. & Csörgő, T. Birds as disseminators of ixodid ticks and tick-borne pathogens: Note on the relevance to migratory routes. Ornis Hungarica 20, 86–89 (2012).
Csörgő, T., Harnos, A., Rózsa, L., Karcza, Z. & Fehérvári, P. Detailed description of the Ócsa Bird Ringing Station, Hungary. Ornis Hungarica 24, 91–108 (2016).
Norte, A. C. et al. Diversity and seasonal patterns of ticks parasitizing wild birds in western Portugal. Exp. Appl. Acarol. 58, 327–339 (2012).
Brinck, P., Svedmyr, A. & von Zeipel, G. Migrating birds at Ottenby Sweden as carriers of ticks and possible transmitters of tick-borne encephalitis virus. Oikos 88–99 (1965).
Klaus, C. et al. Tick infestation in birds and prevalence of pathogens in ticks collected from different places in Germany. Parasitol. Res. 115, 2729–2740 (2016).
Battisti, E. et al. Zoonotic pathogens in ticks from migratory birds, Italy. Emerg. Infect. Dis. 26, 2986–2988 (2020).
Hasle, G. et al. Transport of ticks by migratory passerine birds to Norway. J. Parasitol. 95, 1342–1351 (2009).
Ferianc, O. & Lichard, M. Birds in the Tribec and Hronský Inovec mountains as hosts of ticks. Bull. World Health Organ. 36(Suppl), 19–23 (1967).
Estrada-Peña, A., Mihalca, A. D. & Petney, T. N. Ticks of Europe and North Africa: A Guide to Species Identification. (Springer, 2017).
Rubel, F. et al. Geographical distribution, climate adaptation and vector competence of the Eurasian hard tick Haemaphysalis concinna. Ticks Tick-Borne Diseases 9, 1080–1089 (2018).
Nosek, J. The ecology, bionomics and behaviour of Haemaphysalis (Haemaphysalis) concinna Tick. Z. Parasitenk. 36, 233–241 (1971).
Csörgő, T. et al. Magyar madárvonulási atlasz [Atlas of bird migration in Hungary]. (Kossuth kiadó, 2009).
Hornok, S., Horváth, G., Jongejan, F. & Farkas, R. Ixodid ticks on ruminants, with on-host initiated moulting (apolysis) of Ixodes, Haemaphysalis and Dermacentor larvae. Vet. Parasitol. 187, 350–353 (2012).
Marsot, M. et al. Which forest bird species are the main hosts of the tick, Ixodes ricinus, the vector of Borrelia burgdorferi sensu lato, during the breeding season?. Int. J. Parasitol. 42, 781–788 (2012).
Daniel, M., Malỳ, M., Danielová, V., Kříž, B. & Nuttall, P. Abiotic predictors and annual seasonal dynamics of Ixodes ricinus, the major disease vector of Central Europe. Parasites Vectors 8, 1–12 (2015).
Meng, H. et al. The life cycle and occurrence of Haemaphysalis concinna (Acari: Ixodidae) under field conditions. Ticks Tick-Borne Diseases 5, 887–891 (2014).
Reynolds, C. et al. Shift in the seasonality of ixodid ticks after a warm winter in an urban habitat with notes on morphotypes of Ixodes ricinus and data in support of cryptic species within Ixodes frontalis. Exp. Appl. Acarol. 88, 127–138 (2022).
Plantard, O. et al. Where to find questing Ixodes frontalis ticks? Under bamboo bushes. Ticks Tick-Borne Diseases 12, 101625 (2021).
Papadopoulos, B., Humair, P. F., Aeschlimann, A., Vaucher, C. & Buttiker, W. Ticks on birds in Switzerland. Acarologia 42, 3–19 (2002).
Capek, M. et al. Ticks of the Hyalomma marginatum complex transported by migratory birds into Central Europe. Ticks Tick-Borne Diseases 5, 489–493 (2014).
Siuda, K., Majszyk, A. & Nowak, M. Ticks (Acari: Ixodida) parasitizing birds (Aves) in Poland. Biol. Lett. 43, 147–151 (2006).
Szép, T. & Møller, A. P. Cost of parasitism and host immune defence in the sand martin Riparia riparia: A role for parent-offspring conflict?. Oecologia 119, 9–15 (1999).
Hornok, S., Kováts, D., Horváth, G., Kontschán, J. & Farkas, R. Checklist of the hard tick (Acari: Ixodidae) fauna of Hungary with emphasis on host-associations and the emergence of Rhipicephalus sanguineus. Exp. Appl. Acarol. 80, 311–328 (2020).
Literak, I. et al. Winter infestation of wild birds by ticks and chiggers (Acari: Ixodidae, Trombiculidae) in the Czech Republic. Parasitol. Res. 101, 1709–1711 (2007).
Akimov, I. A. & Nebogatkin, I. V. Distribution of Ticks from the Genus Dermacentor (Acari, Ixodidae) in Ukraine. Becтник зooлoгии 45, 35–40 (2011).
Filippova, N. A. Fauna of Russia and Neighbouring Countries. Ixodid Ticks of Subfamily Amblyomminae. vol. 4 (Nauka Publishing House, 1997).
Contini, C., Palmas, C., Seu, V., Stancampiano, L. & Usai, F. Redescription of the male of Ixodes festai Rondelli, 1926 (Ixodida: Ixodidae) on specimens from Sardinia (Italy). Parasite 18, 235–240 (2011).
Reiczigel, J., Marozzi, M., Fábián, I. & Rózsa, L. Biostatistics for parasitologists—A primer to quantitative parasitology. Trends Parasitol. 35, 277–281 (2019).
Acknowledgements
The authors would like to express their deep gratitude to all of the contributing bird ringers from Ócsa, to Ms. Nóra Takács for performing PCRs, to Dr. Péter Fehérvári for his advices about statistical methods, and to Dr. Adrienn Gréta Tóth, Dr. Sándor Szekeres, and Dr. Gábor Keve for their help. The authors also highly appreciate that Ms. Ciara Reynolds (Virginia, USA) checked the text for English language.
Funding
Open access funding provided by University of Veterinary Medicine. Financial support was provided by the Office for Supported Research Groups, Hungarian Research Network (HUN-REN), Hungary (Project No. 1500107).
Author information
Authors and Affiliations
Contributions
G.K.: Tick identification, data curation, conceptualization, statistical analysis, study design manuscript writing, preparation figures. T.C.S.: sample collection, organization of sample collection, study design, and supervision. D.K.: sample collection, study design, ornithological categorization. S.H.: study design, conceptualization, DNA extraction, manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Keve, G., Csörgő, T., Kováts, D. et al. Long term evaluation of factors influencing the association of ixodid ticks with birds in Central Europe, Hungary. Sci Rep 14, 4958 (2024). https://doi.org/10.1038/s41598-024-55021-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-55021-9
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.