Abstract
Ventricular tachyarrhythmia (VTA) are frequent arrhythmias in patients with hypertrophic cardiomyopathy (HCM). Representing a major risk factor for sudden cardiac death, Holter ECG at first clinical presentation appears insufficient. This study aims to investigate the ability of routinely obtained parameters associated with myocardial remodeling in stratifying for VTA in HCM. In this monocentric analysis, patients with HCM underwent 12-channel electrocardiography and echocardiography, including tissue doppler imaging. The study’s primary endpoint was the documentation of non-sustained and sustained ventricular tachycardia—summarized as ventricular tachyarrhythmias (VTA) on Holter ECG or active devices. The occurrence of VTA was exploratory. Based on our collective, we developed a risk model regarding VTA. Of 140 HCM patients, 38 (27.1%) had an episode of VTA. Patients with VTA were likelier to have a history of atrial fibrillation (p < 0.001), a thicker interventricular septum (p < 0.001) and lower peak systolic mitral annular velocity (p < 0.001). The parameters were independently associated with endpoint in univariate and multivariate logistic regression. We created a logistic equation and calculated a cut-off value. The resulting ROC curve revealed a discriminative ability with AUC of 0.80 (sensitivity, 63%; specificity, 88%). Our risk model including these widely available parameters is able to distinguish low and high-risk of VTA in patients with HCM.
Similar content being viewed by others
Introduction
Hypertrophic cardiomyopathy (HCM) is the most frequent inherited cardiovascular disorder in adults with a reported prevalence of up to 1 in 200 in the general population1. It is a genetic disease with a complex phenotype caused by mutations in more than a dozen genes mostly encoding sarcomere proteins, which lead to hypertrophy, fibrosis, and myocyte disarray2. These histological changes are substrates for the increased risk to develop ventricular arrhythmia, such as sustained or non-sustained ventricular tachycardia—summarized as ventricular tachyarrhythmias (VTA)2. Sudden cardiac death (SCD) is a feared complication, especially in young adults3. To assess the risk of SCD a risk calculator is recommended by the European Society of Cardiology (ESC)4. Age, history of syncope, various echocardiographic parameters (maximum left ventricular wall thickness, left atrial size, and maximum gradient of the left ventricular outflow tract), and ventricular tachycardia play an essential role in risk prediction4. The 48-h Holter ECG is the current standard for detecting VTA at initial clinical admission5. The ESC guidelines recommend a 48-h Holter ECG scan at the patient's initial presentation. However, ECG monitoring beyond 48 h may be required since ventricular tachycardia are often asymptomatic6. In our experience, patients who do not use specialty outpatient clinics or have limited proximity to urban centers with sound medical facilities tend to have infrequent access to Holter ECG or undergo such diagnostic procedures at inadequate intervals. We aim to identify HCM patients at high-risk for VTA, which may benefit from intensified monitoring via Holter ECG (even beyond 48 h) or event recorder. Previous studies have described that individual parameters in ECG7,8 or echocardiography9,10 may be associated with bad outcome in HCM, but no combination of parameters has ever been investigated regarding the occurrence of VTA.
Methods
Study design and participants
The study recruitment occurred between July 2011 and December 2022 at the special outpatient clinic for HCM of the Department of Cardiology and Angiology at Hannover Medical School (MHH). During their regular outpatient appointment, 140 patients were included in the study. The diagnosis of HCM was based on the recent ESC guidelines for the diagnosis and management of hypertrophic cardiomyopathies, which included patients with familial or genetically diagnosed HCM with wall thickness ≥ 13 mm in one or more left ventricular (LV) myocardial segments or patients with wall thickness ≥ 15 mm in the absence of any other cause for hypertrophy5. The following exclusion criteria were defined in advance: age < 18, moderate to severe aortic stenosis, amyloidosis, Fabry disease, and patients unable to provide informed consent. 12 patients were excluded because of incomplete acquisition of study data.
Each patient gave written informed consent. The study was approved by the ethics committee at Hannover Medical School (Ethics vote no.5632) and was conducted according to the ethical principles of the Declaration of Helsinki.
Baseline characteristics were collected by clinical records. At the presentation, each patient underwent a clinical examination, a 12-lead ECG, and a comprehensive echocardiographic examination. Following this, Holter ECG monitoring or interrogation of an implantable device was used to assess the primary endpoint. The primary endpoint was ventricular tachyarrhythmia (VTA) consisting of both sustained (sVT) and non-sustained (nsVT) forms. The nsVT was defined as ≥ 3 consecutive ventricular beats at a rate of ≥ 120 beats per minute and a duration of < 30 s according to the ESC guidelines for the diagnosis and management of hypertrophic cardiomyopathy5. In contrast, sVT lasted for 30 s or more5. In addition, for each patient, the ESC risk score, estimating the 5-year risk of SCD, was calculated.4.
Echocardiography
Echocardiographic images were acquired using Phillips ultrasound systems. Single-blinded investigators unaware of VTA status performed echocardiographic analyses.
Using 2D images, the thickness of the interventricular septum during diastole (IVSd), left ventricular end-diastolic diameter (LVEDD) and LV posterior wall during diastole (LVPWd) were measured. For quantification of the left atrial size, two different measurements were utilized: the left atrial diameter in maximum expansion in the parasternal long axis (LA PLAX) and the left atrial volume index (LAVI) measured from the apical four- and two-chamber views and then indexed to body surface area11. The echocardiography was carried out according to the recommendations of the American Society of Echocardiography12.
Different diastolic indices were evaluated using pulsed-wave Doppler: peak velocity of mitral E wave, peak velocity of the mitral A wave, ratio of mitral peak early to mitral peak late filling velocity (E/A) and deceleration time of the mitral E wave.
Using Tissue Doppler imaging, peak myocardial early (e′) and late (a′) diastolic velocity and peak systolic (s′) velocity were obtained in the apical four-chamber view at the medial and lateral mitral annulus at the highest possible frame rate. e′, a′ and s′ average were calculated by averaging medial and lateral values13. In 13 patients a′ average could not be measured because they were not in sinus rhythm during the echocardiogram. Transmitral early diastolic velocity ratio (E/e′ lateral, medial and average) was calculated for each patient.
Statistical analysis
Categorical variables are presented as numbers and percentages, and continuous variables either as mean ± standard deviation (SD) for normally distributed variables or as median and interquartile ranges (IQR) for non-normally distributed variables. The distribution of continuous data of our entire HCM cohort was tested for normality using Kolmogorov–Smirnov and Shapiro–Wilk tests. Group differences of continuous data were analyzed using the two-sided Student's t-test for normally distributed data or the Mann–Whitney U-test for ordinal or non-normally distributed data. The distribution of categorical variables was analyzed using chi-square or Fisher's exact test. A comparison between patients with and without VTA was performed for the complete dataset.
Risk stratification and score development
Binary logistic regression analysis was used to identify variables associated with the occurrence of VTA. Before multivariate analysis, multicollinearity was excluded. After checking for significant results in univariate analyses, multivariate logistic regression was performed. Backward elimination technique was used to find independently associated parameters with the development of VTA. Only variables with p < 0.05 were included in the final model. Goodness-of-fit was assessed by the Hosmer–Lemeshow test. Significant correlations between the predictors and other echocardiographic measurements were examined using Pearson correlation and t-test. Results from our regression analysis are presented as odds ratios (ORs) with 95% confidence intervals. Odds ratios and the beta coefficients used in the logistic equation can be converted into each other: OR = eβ. To avoid overfitting, the number of potential determinants was limited to a maximum of three14.
In the end, three parameters (AF, IVSd, s′ average), independently associated with our endpoint, were included in the risk stratification model. The area under the curve (AUC) was calculated to evaluate the discriminatory ability of the risk stratification score.
All statistical analyses were performed with Statistical Package for the Social Science, version 28.0 (IBM SPSS, Armonk, NY, USA), and statistical significance was defined by a p-value < 0.05. Figures were created using GraphPad Prism 9.0.1 (GraphPad Inc., La Jolla, CA, USA) and Biorender.com.
Statements and declarations
Each patient gave written informed consent. The study was approved by the ethics committee at Hannover Medical School (Ethics vote no.5632) and was conducted according to the ethical principles of the Declaration of Helsinki.
Results
Characteristics of the study cohort
Baseline characteristics, including a comparison of demographic, echocardiographic and TDI data, are summarized in Table 1. 140 HCM patients with a median age of 57 (IQR: 45–66) years were included in the study. 59% of the study cohort were male. 38 patients (27.1%) reached the clinical endpoint (Fig. 1), of whom 36 patients (25.7%) developed nsVT, while only two patients (1.4%) developed sVT. Patients with VTA did not differ significantly from those without VTA regarding age, gender, BMI or LV outflow tract obstruction. Neither the diastolic nor the systolic blood pressure differed significantly between both groups. Furthermore, no substantial differences were detected regarding interventions such as myectomy or Transcoronary ablation of septal hypertrophy (TASH) (Table 1). Patients with VTA were more likely to suffer from palpitations, and to have an implanted cardioverter-defibrillator (ICD), although these values did not reach statistical significance. Likewise, no significant differences in family history of HCM or SCD, syncope and dyspnea, assessed with NYHA classification, could be observed. The groups did not significantly differ concerning cardiac medication at admission. For the mineralocorticoid receptor antagonist, there was a formally statistically significant difference (p = 0.043).
ECG characteristics
HCM patients with VTA were more likely to have diagnosed atrial fibrillation (45% vs. 11% p < 0.001). The two groups did not significantly differ regarding ECG intervals and durations (PR, QRS or QTc).
Echocardiographic characteristics
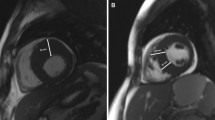
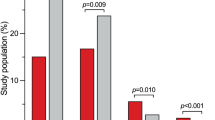
2D echocardiography revealed a significantly thicker interventricular septum (22 [18–27] vs. 18 [15–21] mm; p < 0.001) in HCM patients with proven VTA (Figs. 2a, 3a). The risk of VTA increased with increasing IVSd. In contrast, the thickness of the posterior wall of the left ventricle (LVPWd) or the left ventricular end-diastolic diameter (LVEDD) did not differ significantly between the two groups (13 [10–13] vs. 12 [10–14] mm; p = 0.759 and 43 [40–49] vs. 42 [39–47] mm; p = 0.934, respectively). LAVI, which represents the size of the left atrium in relation to body surface area, was significantly increased in patients with VTA compared to those without (43 [37–72] vs. 39 [32–54] ml/m2; p = 0.015). On the other hand, LA PLAX did not differ between the two groups (p = 0.381), nor did mitral E wave, mitral A wave, E/A and deceleration time of the mitral E wave.
In tissue doppler imaging late diastolic velocity, a′ average (7.1 [5.8–9.1] vs. 8.9 [7.4–10.3] cm/s; p < 0.001, Table 1) and peak systolic velocity, s′ average (5.9 [4.9–6.9] vs. 6.9 [6.1–8.0] cm/s; p < 0.001, Table 1), were significantly reduced in patients with VTA when compared with patients without clinical endpoints (Figs. 2b, 3b). The risk of VTA increased with decreasing tissue doppler velocities. We could not make this observation for e′ average: the early diastolic velocity (5.9 [4.7–7.9] vs. 6.2 [4.8–7.7] cm/s; p = 0.758).
Analysis for risk stratification
Based on the evaluated parameters, s′ average was the parameter most strongly associated with the occurrence of VTA. Its discriminative ability was assessed by ROC analysis revealing an AUC of 0.71 (95% CI 0.61–0.81) (Fig. 4c). The ability of IVSd to discriminate for VTA occurrence was similar with an AUC of 0.69 (95% CI 0.59–0.80) (Fig. 4b). In addition, this parameter strongly correlated with the left ventricular mass index (r = 0.74; p < 0.001). Concerning AF, calculation of the ROC curve revealed an AUC of 0.67 (95% CI 0.56–0.78) (Fig. 4a). For s′ average, the logistic regression yields a negative coefficient implying that a higher value of s′ average decreases the probability of VTA occurrence. The corresponding ROC curve was inverted to facilitate comparison with the other parameters (Fig. 4c).
Table 2 shows the results of univariate and multivariate logistic regression analysis. History of atrial fibrillation (OR = 6.7; 2.7–16.4; p < 0.001), IVSd (OR = 1.12; 1.05–1.20; p < 0.001) and s′ average (OR = 0.54; 0.39–0.75; p < 0.001) were independently associated with VTA occurrence in univariate logistic regression. All three parameters remained significant in multivariate regression (OR = 5.5; 2.1–14.4; p < 0.001; OR = 1.09; 1.02–1.17, p = 0.014; OR = 0.58; 0.4–0.85; p = 0.005, respectively), even after adjustment for age and gender (OR = 6.1; 2.2–16.9, p < 0.001; OR = 1.09; 1.01–1.17 p = 0.023; OR = 0.59; 0.40–0.86 p = 0.006). The beta coefficients in the logistic equation correspond to the natural logarithm of the Odds ratio (ln(OR) = β; OR = eβ).
Score development
A risk score for the occurrence of VTA was created using logistic regression based on data collected in routine clinical practice. Atrial fibrillation, the IVSd and s´ average were included in the final model since these parameters remained significant in multivariate logistic regression, even after adjustment for age and gender. The AUC was calculated to evaluate the discriminatory ability of the risk stratification score. The model demonstrated discrimination ability evidenced by an AUC of 0.80 (Fig. 4d). At a cutoff value of > 0.39; the model exhibited a sensitivity of 63% and a specificity of 88% in distinguishing HCM patients regarding the occurrence of VTA. Our risk stratification analysis revealed a positive predictive value of 67% and a negative predictive value of 87%.
The history of atrial fibrillation must be indicated in the score equation by assigning a value of 1 (apparent) or 0 (in-apparent), respectively. Additionally, the values of s′ average (in cm/s) and IVSd (in mm) must be inserted. The result of the logistic equation below is the probability (p) of the occurrence of VTA. If the score is > 0.39, this risk score permits the identification of patients with HCM at an increased risk for VTA and following intensified rhythm monitoring.
Probability (p) of the occurrence of VTA:
Discussion
In this study, we developed a risk stratification model based on routine clinical parameters to identify HCM patients at increased risk for VTA. The proposed model is straightforward to implement and requires only a few components, such as ECG, two-dimensional echocardiography, and TDI, routinely acquired during patient visits. This model or score should facilitate the identification of patients who will require intensified rhythm monitoring in the future. During the course of the disease, numerous remodeling processes such as fibrosis, myocyte disarray and hypertrophy occur in the myocardium, affecting both the atrium and the ventricle2,15. Several studies have demonstrated that these remodeling processes increase the risk of cardiac arrhythmia in HCM patients16,17. Consequently, parameters indicating these remodeling processes in both the atrium and the ventricle were included in our analysis.
There is a correlation between atrial fibrillation and structural remodeling processes in the atrium such as fibrosis.18. A systematic review including more than 7000 HCM patients revealed an overall AF prevalence of 22.45%19, similar to ours (20%). Kubo et al. have shown that patients suffering from HCM and AF simultaneously developed more adverse cardiovascular events, including sustained ventricular tachycardia, as compared to AF-free HCM patients, assuming AF to be a trigger of adverse events20. Different studies came to a similar conclusion revealing AF as an independent predictor of ventricular tachyarrhythmia in HCM patients who underwent ICD implantation for primary prevention and recurrence of ventricular tachyarrhythmia in ICD recipients21,22. Patients suffering from atrial fibrillation usually show an enlarged left atrium18. For this reason, we also inspected the left atrial size in our study using two measurement methods. When analyzing LA PLAX, the diameter of the left atrium in the parasternal long axis, there were no variances regarding VTA. However, we were able to determine significant differences when examining LAVI, a value normalized to body surface area. In a study by Debonnaire et al. LAVI could even predict appropriate ICD therapy in patients with HCM23. Significant differences in our study cohort were observed for atrial fibrillation and LA size. However, an enlarged left atrium predisposes to atrial fibrillation, so the two parameters would be interdependent values24,25. Since predictors in risk stratification models should be independent of each other, we decided to use atrial fibrillation because of its stronger p-value. With the addition of this parameter in the risk score, the atrial remodeling processes should be properly represented.
Another parameter associated with the remodeling processes in the HCM myocardium is the IVSd. Our echocardiographic examination showed a strong correlation with the left ventricular mass index (r = 0.74), indicating the close association between IVSd and the extent of ventricular hypertrophy. Consequently, IVSd is an excellent parameter for estimating the degree of hypertrophy. In addition, this parameter has been identified as a measure of the severity of HCM and an important prognostic factor26,27. Several other studies have revealed that the presence of VTA is associated with the magnitude of left ventricular hypertrophy10,27. It was even identified as a predictor of SCD in HCM patients9 and was therefore included in the ESC risk score estimating the 5-year risk of SCD4. With the addition of this parameter to the risk model, the extent of hypertrophy is particularly taken into account.
A parameter reflecting long-axis systolic function and remodeling processes in the ventricle is the averaged peak systolic longitudinal mitral annular velocity (s′) measured with TDI. It was recently demonstrated that s′ average and diastolic mitral annular velocities could predict poor outcomes in patients suffering from various heart diseases28,29,30. The decrease in s′ can be explained by progressive fibrosis and remodeling in the myocardium: longitudinal myofibrils predominate in the subendocardium resulting in the contraction being dominant in the longitudinal direction, while the subepicardial contraction generates mainly circumferential shortening and twist31. Due to extreme fluctuations in pressure and compression and large distances to the epicardial coronary flow, which are even longer in hypertrophic hearts, the subendocardium is uniquely vulnerable to injury31. Therefore, the subendocardium is usually the first myocardial layer to show structural changes such as fibrosis and ischemia31. These fibrotic remodeling processes, which predominantly affect longitudinal myofibrils, can result in a reduced systolic function obtained with s′ while the LVEF remains unaffected. In general, the LVEF is not a suitable method to assess the systolic function in HCM patients since it is usually normal or even increased, although the systolic function is impaired32,33. By adding s′ average to the risk score, attention is paid to the left ventricle, its remodeling and general systolic function.
Our results were in line with different TDI studies. Barakat et al. reported the association between a reduced s′ with the occurrence of VTA or ventricular fibrillation irrespective of age, gender and LVEF in cardiac device recipients34. Bayrak et al. identified a lower lateral s′ associated with a higher risk of cardiovascular death and hospitalization due to worsening of heart failure symptoms in patients with HCM35. Similar to other studies30,36, we also detected a significant reduction in late mitral annular diastolic velocity (a′), which reflects the ventricles passive motion (Table 1). However, this value cannot be measured if the patient is currently in atrial fibrillation during the echocardiographic examination. Our goal was to include parameters in the prediction model that can be measured not only in sinus rhythm, especially since many HCM patients suffer from atrial fibrillation.19. Moreover, a′ is a value that initially increases in the course of progressing LV dysfunction, while s′ decreases directly35. Therefore, we decided to utilize s′ average as a parameter for the risk model.
In some studies, a significantly prolonged QTc interval in patients with VTA could be observed7,8. Similar to the mentioned studies, we also observed a tendency towards a longer QTc interval albeit without statistical significance.
Our study selected parameters that reflect both atrial and ventricular remodeling processes for our risk stratification model: atrial fibrillation, thickness of interventricular septum and s′ average. These parameters appear capable of predicting the occurrence of VTA in such patients due to their association with remodeling processes of the atrium and ventricle. The developed risk stratification model resulted in an accuracy of 81.4% in correctly diagnosing VTA in our study cohort. The sensitivity of the model was 63%, while the specificity was 88%. The positive predictive value was 67%, while the negative predictive value was 87%. Thus, a negative result from our risk stratification model (below the cut-off value) can be used to rule out VTA. The negative predictive value is of particular importance in clinical applications. Patients scoring above the cut-off value may benefit from extended rhythm monitoring to detect VTA.
Our study has some limitations. One limitation is the inclusion of data obtained from Holter ECG and ICD interrogation, resulting in differences in patient observation periods. This discrepancy results from the consideration of the last interrogation period for ICD interrogations. In addition, it should be noted that VTA detection criteria vary for ICD because of different programming approaches. Acceptance of this selection bias in our study was guided by our primary focus on exploratory assessment of VTA occurrence to elicit these same events through our model to identify patients at increased risk for VTA in the future. In addition, the cohort included three patients who had received treatment with antiarrhythmic drugs at one time in the patient history. Specifically, two individuals were administered flecainide and belonging to the non-VTA group, whereas one patient received amiodarone and belonging to the VTA group. The main limitation is the rather small number of patients, especially those who reached the endpoint. However, the proportion of patients with VTA is similar to that from other studies37,38. Although larger studies are needed to confirm our results in a validation cohort, we were able to make corroborated statements with our case number. Furthermore, the occurrence of VTA was retrospectively evaluated using an exploratory data set derived from a single center.
Summary/conclusions
In summary, we have developed a risk stratification model using parameters that are easy to collect to detect HCM patients with an increased risk of ventricular tachyarrhythmia who consecutively require close rhythm monitoring. Independent prospective cohort studies should further validate our risk stratification model.
Data availability
The data sets generated in this study are available from the corresponding author upon reasonable request.
Abbreviations
- ACEi:
-
Angiotensin converting enzyme inhibitor
- AF:
-
Atrial fibrillation
- ARB:
-
Angiotensin receptor blocker
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- CCB DHP:
-
Dihydropyridine calcium channel blocker
- CCB non-DHP:
-
Non-dihydropyridine calcium channel blocker
- CI:
-
Confidence interval
- CMR:
-
Cardiovascular magnetic resonance
- ECG:
-
Electrocardiogram
- ESC:
-
European Society of Cardiology
- HCM:
-
Hypertrophic cardiomyopathy
- ICD:
-
Implantable cardioverter defibrillator
- IQR:
-
Interquartile range
- IVSd:
-
Interventricular septum in diastole
- LA PLAX:
-
Left atrium in parasternal long axis
- LAVI:
-
Left atrial volume index
- LGE:
-
Late gadolinium enhancement
- LVEDD:
-
Left ventricular end diastolic diameter
- LVEF:
-
Left ventricular ejection fraction
- LVPWd:
-
Left ventricular posterior wall in diastole
- MRA:
-
Mineralocorticoid receptor antagonist
- nsVT:
-
Non-sustained ventricular tachycardia
- NYHA:
-
New York Heart Association
- OR:
-
Odds ratio
- ROC:
-
Receiver operating characteristic
- SCD:
-
Sudden cardiac death
- SD:
-
Standard deviation
- sVT:
-
Sustained ventricular tachycardia
- TASH:
-
Transcoronary ablation of septal hypertrophy
- TDI:
-
Tissue Doppler imaging
- VTA:
-
Ventricular tachyarrhythmia
References
Semsarian, C., Ingles, J., Maron, M. S. & Maron, B. J. New perspectives on the prevalence of hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 65, 1249–1254 (2015).
Marian, A. J. & Braunwald, E. Hypertrophic cardiomyopathy: Genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ. Res. 121, 749–770 (2017).
Monserrat, L. et al. Non-sustained ventricular tachycardia in hypertrophic cardiomyopathy: An independent marker of sudden death risk in young patients. J. Am. Coll. Cardiol. 42, 873–879 (2003).
O’Mahony, C. et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur. Heart J. 35, 2010–2020 (2014).
Authors/Task Force members et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 35, 2733–2779 (2014).
Mitra, R. L. & Buxton, A. E. The clinical significance of nonsustained ventricular tachycardia. J. Cardiovasc. Electrophysiol. 4, 490–496 (1993).
Gray, B., Ingles, J., Medi, C. & Semsarian, C. Prolongation of the QTc interval predicts appropriate implantable cardioverter-defibrillator therapies in hypertrophic cardiomyopathy. JACC Heart Fail. 1, 149–155 (2013).
Debonnaire, P. et al. QRS fragmentation and QTc duration relate to malignant ventricular tachyarrhythmias and sudden cardiac death in patients with hypertrophic cardiomyopathy. J. Cardiovasc. Electrophysiol. 26, 547–555 (2015).
Olivotto, I. et al. Maximum left ventricular thickness and risk of sudden death in patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 41, 315–321 (2003).
Piva e Mattos, B., Torres, M. A. R., Freitas, V. C. D., Scolari, F. L. & Loreto, M. S. D. Ventricular arrhythmias and left ventricular hypertrophy in hypertrophic cardiomyopathy. Arq. Bras. Cardiol. 100, 452–459 (2013).
Lang, R. M. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 16, 233–270 (2015).
Cheitlin, M. D. et al. ACC/AHA/ASE 2003 Guideline Update for the Clinical Application of Echocardiography: Summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). J. Am. Soc. Echocardiogr. 16, 1091 (2003).
Nagueh, S. F. et al. Tissue Doppler imaging consistently detects myocardial abnormalities in patients with hypertrophic cardiomyopathy and provides a novel means for an early diagnosis before and independently of hypertrophy. Circulation 104, 128–130 (2001).
Peduzzi, P., Concato, J., Kemper, E., Holford, T. R. & Feinstein, A. R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 49, 1373–1379 (1996).
Derda, A. A. et al. Blood-based microRNA signatures differentiate various forms of cardiac hypertrophy. Int. J. Cardiol. 196, 115–122 (2015).
Hurtado-de-Mendoza, D. et al. Diffuse interstitial fibrosis assessed by cardiac magnetic resonance is associated with dispersion of ventricular repolarization in patients with hypertrophic cardiomyopathy. J. Arrhythm 33, 201–207 (2017).
McLELLAN, A. J. A. et al. Diffuse ventricular fibrosis on cardiac magnetic resonance imaging associates with ventricular tachycardia in patients with hypertrophic cardiomyopathy. J. Cardiovasc. Electrophysiol. 27, 571–580 (2016).
Sagris, M. et al. Atrial fibrillation: Pathogenesis, predisposing factors, and genetics. Int. J. Mol. Sci. 23, 6. https://doi.org/10.3390/ijms23010006 (2021).
Guttmann, O. P., Rahman, M. S., O’Mahony, C., Anastasakis, A. & Elliott, P. M. Atrial fibrillation and thromboembolism in patients with hypertrophic cardiomyopathy: Systematic review. Heart 100, 465–472 (2014).
Kubo, T. et al. Clinical significance of new-onset atrial fibrillation in patients with hypertrophic cardiomyopathy. ESC. Heart Fail. 8, 5022–5030 (2021).
Rusnak, J. et al. Atrial fibrillation increases the risk of recurrent ventricular tachyarrhythmias in implantable cardioverter defibrillator recipients. Arch. Cardiovasc. Dis. 114, 443–454 (2021).
Weissler-Snir, A., Dorian, P., Rakowski, H., Care, M. & Spears, D. Primary prevention implantable cardioverter-defibrillators in hypertrophic cardiomyopathy-Are there predictors of appropriate therapy?. Heart Rhythm 18, 63–70 (2021).
Debonnaire, P. et al. Global longitudinal strain and left atrial volume index improve prediction of appropriate implantable cardioverter defibrillator therapy in hypertrophic cardiomyopathy patients. Int. J. Cardiovasc. Imaging 30, 549–558 (2014).
Olivotto, I. et al. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation 104, 2517–2524 (2001).
Lee, S. et al. Impact of atrial fibrillation on the clinical course of apical hypertrophic cardiomyopathy. Heart 103, 1496–1501 (2017).
Elliott, P. M., Gimeno Blanes, J. R., Mahon, N. G., Poloniecki, J. D. & McKenna, W. J. Relation between severity of left-ventricular hypertrophy and prognosis in patients with hypertrophic cardiomyopathy. Lancet 357, 420–424 (2001).
Spirito, P. et al. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N. Engl. J. Med. 342, 1778–1785 (2000).
Hosseini, S. et al. Tissue Doppler echocardiography and outcome in arrhythmogenic right ventricular cardiomyopathy. Int. J. Cardiol. 368, 86–93 (2022).
Cianciulli, T. F. et al. Clinical significance of tissue Doppler imaging in chronic Chagas disease. Echocardiography 37, 1205–1212 (2020).
Biering-Sorensen, T. et al. Doppler tissue imaging is an independent predictor of outcome in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J. Am. Soc. Echocardiogr. 27, 258–267 (2014).
Stanton, T. & Marwick, T. H. Assessment of subendocardial structure and function. JACC Cardiovasc. Imaging 3, 867–875 (2010).
Maciver, D. H. A new method for quantification of left ventricular systolic function using a corrected ejection fraction. Eur. J. Echocardiogr. 12, 228–234 (2011).
Haland, T. F. et al. The systolic paradox in hypertrophic cardiomyopathy. Open Heart 4, e000571-000571 (2017) (eCollection 2017).
Barakat, M. F. et al. Tissue doppler-derived left ventricular systolic velocity is associated with lethal arrhythmias in cardiac device recipients irrespective of left ventricular ejection fraction. J. Am. Soc. Echocardiogr. 33, 1509–1516 (2020).
Biering-Sorensen, T. et al. Prognostic value of tissue Doppler imaging for predicting ventricular arrhythmias and cardiovascular mortality in ischaemic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 17, 722–731 (2016).
Yamamoto, T. et al. Prognostic value of the atrial systolic mitral annular motion velocity in patients with left ventricular systolic dysfunction. J. Am. Soc. Echocardiogr. 16, 333–339 (2003).
Di Salvo, G. et al. Non sustained ventricular tachycardia in hypertrophic cardiomyopathy and new ultrasonic derived parameters. J. Am. Soc. Echocardiogr. 23, 581–590 (2010).
Gimeno, J. R. et al. Exercise-induced ventricular arrhythmias and risk of sudden cardiac death in patients with hypertrophic cardiomyopathy. Eur. Heart J. 30, 2599–2605 (2009).
Acknowledgements
We thank all patients participating in this study.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research was conducted in the KlinStrucMed program of Hannover Biomedical Research School (HBRS) at Hannover Medical School and funded by the “Familie Felling Stiftung”. This work was supported by PRACTIS–Clinician Scientist Program of Hannover Medical School, funded by the German Research Foundation (DFG, ME 3696/3–1) to A.D.
Author information
Authors and Affiliations
Contributions
A.A.D., M.A. and K.S. collected data. A.A.D. and M.A. analyzed data; A.A.D., M.A., K.S., J.T.S., J.L., D.D., U.B., D.B., J. B. and T.T. interpreted results; A.A.D. and M.A. prepared figures; A.A.D., M.A., J.T.S and T.T. drafted manuscript; A.A.D., U.B., D.B., J.B. and T.T. revealed conception and design of research; U.B., A.A.D., K.S., J.T.S., F.J.G.W., N.W., N.Z., C.B., D.D., U.B., D.B., J.B. and T.T. edited and revised manuscript; A.A.D., M.A., K.S., J.T.S., F.J.G.W., N.W., N.Z., C.B., D.D., U.B., D.B., J. B., and T.T. approved final version of manuscript. All authors acquired and analyzed data and agreed to be accountable for all aspects of the work ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Derda, A.A., Abelmann, M., Sieweke, JT. et al. A pilot study for risk stratification of ventricular tachyarrhythmia in hypertrophic cardiomyopathy with routine echocardiography parameters. Sci Rep 14, 3799 (2024). https://doi.org/10.1038/s41598-024-54153-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-54153-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.