Abstract
The study aimed to assess the metabolomic profile of the synovial fluid (SF) of dogs affected by spontaneous osteoarthritis (OA) and compare any differences based on disease progression. Sixty client-owned dogs affected by spontaneous OA underwent clinical, radiographic, and cytologic evaluations to confirm the diagnosis. The affected joints were divided into four study groups based on the Kallgreen–Lawrence classification: OA1 (mild), OA2 (moderate), OA3 (severe), and OA4 (extremely severe/deforming). The osteoarthritic joint’s SF was subjected to cytologic examination and 1H-NMR analysis. The metabolomic profiles of the study groups’ SF samples were statistically compared using one-way ANOVA. Sixty osteoarthritic joints (45 stifles, 10 shoulders and 5 elbows) were included in the study. Fourteen, 28, and 18 joints were included in the OA1, OA2, and OA3 groups, respectively (0 joints in the OA4 group). Metabolomic analysis identified 48 metabolites, five of which were significantly different between study groups: Mannose and betaine were elevated in the OA1 group compared with the OA2 group, and the 2-hydroxyisobutyrate concentration decreased with OA progression; in contrast, isoleucine was less concentrated in mild vs. moderate OA, and lactate increased in severe OA. This study identified different 1H-NMR metabolomic profiles of canine SF in patients with progressive degrees of spontaneous OA, suggesting 1H-NMR metabolomic analysis as a potential alternative method for monitoring OA progression. In addition, the results suggest the therapeutic potentials of the metabolomic pathways that involve mannose, betaine, 2-hydroxyisobutyrate, isoleucine, and lactate.
Similar content being viewed by others
Introduction
Osteoarthritis (OA) is a common chronic disease that affects the entire joint1. It is characterized by inflammation, alteration of synovial fluid, progressive deterioration of articular cartilage, synovial membrane dysfunction, subchondral bone sclerosis, and formation of periarticular osteophytes2, with the depletion of matrix protein driven by proteases including multiple matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTSs)3. OA is responsible for significant pain, reduction of joint mobility until loss of joint function, and disability in humans and dogs4. OA is a multifactorial pathology, and age, sex, obesity, activity level, prior joint injury, and inherited susceptibility are recognized risk factors5. Also, immune system activity and metabolic disorders can influence the development and progression of OA6. The incidence of this pathology is high in canines and humans10, and, in particular, it was estimated that affects 20% of the canine population over 1 year of age5 excluding possible cases not reported in veterinary medicine.
Currently, OA can be detected in the later stages of the disease because it is diagnosed predominantly through clinical examination and diagnostic imaging12, and there is no definitive treatment that can stop the progression and cure the disease11. For this reason, the scientific literature has increased its interest in researching novel therapies13,14 and alternative detection methods for early diagnosis and monitoring of disease activity and progression15.
Recently, studies have focused on metabolomics to detect OA’s metabolic fingerprint and describe and identify related biomarkers. Metabolomics is the study of the most abundant small molecules (< 1500 Da) in a biological system to understand the metabolic changes occurring during physiological and pathological conditions12. Different studies have attempted to identify biochemical biomarkers in urine19, plasma, blood serum21, and cerebrospinal fluid23, but the most common biofluid used in OA research is synovial fluid (SF)12. SF is in contact with numerous tissues altered during joint pathology (synovial membrane, cartilage, and subchondral bone), and the degradation products, enzymes, and signal transduction molecules involved in OA are first released from the cartilage matrix into SF. SF should, therefore, yield the most detailed available profile of joint metabolism, and it holds significant potential in the discovery of biomarkers whose levels are altered in the early stages of disease progression24. In healthy and pathologic joints of humans and animals, the metabolomic SF profile was recently investigated by 1HH-nuclear magnetic resonance spectroscopy (H-NMR), an analytical technique widely used in metabolomics analysis thanks to its capacity to quantify rapidly and simultaneously, with the same sensitivity, a large number of metabolites, requiring minimal sample pretreatment24.
Studies on the metabolomics of the SF of domestic animals affected by spontaneous OA are poor, and their results are not always in agreement with each other25. They have suggested a significant change in the concentration of metabolites due to the OA condition, emphasizing the complex mechanism underlying OA and the potential diagnostic and prognostic capacity of the metabolomic analysis. For this purpose, no studies have investigated the metabolic changes in SF of domestic animals during the OA progression.
The current study aimed to assess the metabolomic profile of the SF of dogs affected by spontaneous OA and compare any differences in the four groups of progressive degrees of OA. The study’s results could provide new information about the metabolic shifts induced by the evolution of OA not only in the dogs, but also in the human species. Indeed, the client-owned dogs are excellent models of human disease, because they share the same living environments and food resources and because the diseases are similar in physiology, presentation, and therapy response18.
Results
Animals, joints enrolled and cytological analysis
Seventy-two client-owned dogs of different breeds showing unilateral lameness were referred by the Veterinary Teaching Hospital of the University of Camerino. Of these, five patients were excluded from the study because metabolic and neoplastic pathologies were detected during clinical evaluations, and seven patients were excluded because cytological examination of SF detected a suppurative or immunomediated arthropathy. Sixty dogs, 24 males and 36 females, respected the inclusion criteria (mean ± SD age, 8.25 ± 2.41 years, and mean ± SD weight, 21.33 ± 9.19 kg), and they were enrolled in the study. Sixty joints were analyzed in the study, specifically 45 stifles, 10 shoulders, and 5 elbows (Table 1).
The OA1 group included 14 joints (11 stifles, 3 shoulders), the OA2 group included 29 joints (21 stifles, 7 shoulders, 1 elbows), the OA3 included 17 joints (13 stifles and 4 elbows), and the OA4 group included 0 joints. Therefore, three study groups were considered (OA1, OA2, and OA3 groups). In the OA1 was included 7 males and 7 females, in the OA2 group 11 males and 18 females, and in the OA3 group 6 males and 11 females. In the OA1, OA2, and OA3 groups, the mean ± SD patients’ age was 5.36 ± 3.86, 6.24 ± 3.08, and 6.82 ± 4.14 years, respectively. Peso corporeo (BW) mean ± SD was 25.71 ± 8.20 kg, 24.31 ± 8.75 kg, and 26.82 ± 6.40 kg, respectively. There were no statistically significant differences in the ages and BW among the three groups.
Considering the number of stifle joints included in our study compared to other joints, a statistical comparison of the concentration of metabolites identified in the SF of the stifles, affected by rupture of cranial cruciate ligament, was performed. In particular, the OA1stifle (n = 11 stifle joints), OA2stifle (n = 21 stifle joints) and OA3stifle (n = 13 stifle joints) groups were compared. In the OA1stifle, OA2stifle, and OA3stifle groups, the mean ± SD patients’ age was 5.18 ± 4.17, 6.09 ± 2.94, and 6.76 ± 3.82 years, respectively. BW mean ± SD was 24.91 ± 6.55 kg, 23.14 ± 8.73 kg, and 27.85 ± 7.01 kg, respectively. There were no statistically significant differences in the ages and BW among the three groups.
At semi-quantitative cytological analysis, the mean ± SD of the number of inflammatory cells in OA1, OA2 and OA3 groups was of 8.8 ± 5.71, 12.23 ± 9.05 and 12.38 ± 8.79 respectively. The number of inflammatory cells increased significantly between the OA1 and OA3 groups (p < 0.05) (Fig. 1).
Metabolomic analysis
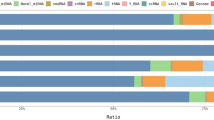
Forty-eight metabolites were identified and quantified in the SF sample during 1H-NMR metabolomic analysis (Fig. 2).
The concentrations of mannose, betaine, 2-hydroxyisobutyrate, isoleucine, and lactate were significantly different between the OA1, OA2, and OA3 groups (p < 0.05) (Table 2, Fig. 3). Between the OA1stifle, OA2stifle and OA3stifle groups, the concentration of mannose, betaine, 2-hydroxyisobutyrate, lactate, o-acetylcarnitine and pyruvate were significantly different (p < 0.05) (Table 3).
The concentration of mannose in canine SF was significantly elevated (p = 0.034) in the OA1 group (0.128 ± 0.239 mmol/L) compared with OA2 (0.034 ± 0.013 mmol/L). In the OA3 group, the mannose concentration was lower (0.034 ± 0.014 mmol/L), but not significantly, compared with the OA1 group (Fig. 3A). The same evolution could be observed for the concentration of mannose in the SF of stifle joints, because it was significantly higher (p = 0.052) in the OA1stifle group (0.151 ± 0.267 mmol/L) than in the OA2stifle group (0.036 ± 0.014 mmol/L) and in the OA3stifle group (0.038 ± 0.014 mmol/L).
As the mannose, the concentration of metabolite betaine showed a significantly higher concentration (p = 0.0196) in the OA1 group (0.157 ± 0.068 mmol/L) than in the OA2 group (0.112 ± 0.038 mmol/L), but there was no statistically significant difference between the OA1 and OA3 groups (0.123 ± 0.038 mmol/L) (Fig. 3B), unlike what was observed in the SF of the stifles, where the difference was also significant between OA1stifle (0.115 ± 0.077 mmol/L) and OA3stifle groups (0.115 ± 0.034 mmol/L).
The OA progression led to a significant decrease in the concentration of 2-hydroxyisobutyrate: a significantly higher concentration could be observed in the OA1 group (p = 0.011; 0.087 ± 0.129 mmol/L) than in the OA2 (0.002 ± 0.001 mmol/L) and the OA3 groups (0.004 ± 0.002 mmol/L) (Fig. 3C), and in the OA1stifle group (p = 0.046; 0.102 ± 0.169 mmol/L) than in the OA2stifle group (0.002 ± 0.001 mmol/L) and the OA3stifle group (0.004 ± 0.003 mmol/L).
An opposite evolution is observed for concentrations of isoleucine and lactate. Isoleucine significantly increased (p = 0.0313) with the OA progression. In particular, its concentration was significantly lower in the OA1 group (0.048 ± 0.017 mmol/L) compared to the OA2 group (0.05952 ± 0.011 mmol/L) and in the OA1 group compared to the OA3 grous (0.059 ± 0.013 mmol/L) (Fig. 3D). In the OA3 group, lactate showed a significantly higher concentration (2.008 ± 1.118 mmol/L) compared with the OA2 group (1.335 ± 0.525 mmol/L) (p = 0.0186) (Fig. 3E). Different was the significance observed in the concentration of these two metabolites in the SF of the only stifle joints. Although isoleucine tends to increase with the progress of OA in the stifles, this increase is not statistically significant (OA1stifle: 0.050 ± 0.019 mmol/L; OA2stifle: 0.058 ± 0.011 mmol/L; OA3stifle: 0.062 ± 0.014 mmol/L), while the concentration of lactate revealed a significant increment between all study groups (p = 0.009; OA1stifle: 1.038 ± 0.543 mmol/L; OA2stifle: 1.141 ± 0.422 mmol/L; OA3stifle: 1.675 ± 0.686 mmol/L).
From the comparison between the concentrations of the metabolite o-acetylcarnitine and pyruvate in the SF of the stifle joints, a significant initial decrease in these metabolites was observed (o-acetylcarnitine: OA1stifle 0.006 ± 0.003 mmol/L, OA2stifle 0.003 ± 0.001 mmol/L; pyruvate: OA1stifle 0.045 ± 0.033 mmol/L; OA2stifle 0.036 ± 0.017 mmol/L), and then a subsequent significant increase was detected with OA progression (o-acetylcarnitine: OA3stifle 0.004 ± 0.002 mmol/L; pyruvate: OA3stifle 0.060 ± 0.014 mmol/L).
Discussion
This study demonstrated biochemical differences in the 1H-NMR metabolomic profile of canine SF in patients with progressive degrees of spontaneous OA. To our knowledge, this is the first study in domestic animals that quantified and compared different metabolite concentrations in progressive OA stages, and, in particular, the first study that compared three stages of osteoarthritic joints. In humans, Kim et al.31 investigated changes in metabolite concentrations in SF in progressive stages of human knee OA using the Kallgren-Lawrence scale, identifying 28 metabolites, related to fatty acid metabolism, glycerolipid metabolism and tricarboxylic acid cycle, as important molecules for discriminating between the early and late OA stages. In our study, 48 metabolites were identified in SF samples, and five of them showed a significant shift during OA progression: mannose, betaine, 2-hydroxyisobutyrate, isoleucine, and lactate. Furthermore, pyruvate and o-acetylcarnitine exhibited significant shifts during OA progression only in the SF of the evaluated stifle joints.
Mannose is a simple sugar, five times more active than glucose in non-enzymatic glycation32, and is the major monosaccharide component of the N-glycans33. The high-mannose type N-glycans are more highly localized in the superficial chondrocytes34. The OA changes, which were characterized by a decrease in the extracellular matrix, chondrocyte apoptosis, and dedifferentiation, were accompanied by a decrease in high-mannose type N-glycans in chondrocytes throughout the entire cartilage layer35. Urita et al.35 observed that the high-mannose type N-glycan structure is altered during the processes of human articular cartilage degradation, and that the related N-glycogen (GlcNAc-TI), an enzyme that converts high-mannose type N-glycans to complex-type N-glycan, influences the regulation of MMP-13 and ADAMTS-5 (aggrecanase 2) expression in mouse chondrocytes in response to stimulation with IL-1α. These findings suggest that high-mannose type N-glycans are key molecules in the initiation and pathogenesis of OA35, and the sudden decrease of concentration of mannose observed in our study in advanced stages of OA could be explained by increased demand for mannose used for N-glycosylation in response to the alteration of N-glycans localized in superficial chondrocytes.
Furthermore, mannose is phosphorylated by hexokinase (HK) to produce mannose-6-phosphate (Man-6-P) directed into N-glycosylation via phosphomannomutase (PMM2), but it can also be catabolized by phosphomannose isomerase (MPI) within the cell33. Saito et al.36 profiled sugar metabolism in leukemia cells and found that mannose is an energy source for glycolysis thanks to leukemia cells’ high levels of expression of phosphomannose isomerase (PMI). With regard to these recent studies, the use of mannose as an energy source for glycolysis could be a possible explanation for its reduced concentration in SF, but to be able to affirm these hypotheses, further studies are needed to assess the mechanism of N-glycosylation and mannose consumption and to assess the activity of MPI in the intraarticular environment during OA.
Different metabolomic studies have also observed the increment of isoleucine with the OA progression. In particular, increased levels of branched-chain amino acids (BCAAs), such as isoleucine, leucine, and valine, in OA patients could be due to either blocked catabolism of BCAAs or increased release from the breakdown of proteins15. The BCAAs are essential amino acids making up one-third of skeletal muscle protein and are relevant for energy metabolism15. An animal model study of OA showed an enhancement of the resonance at 0.85 ppm of the 1H high-resolution magic angle spinning NMR spectra of the OA-affected cartilage sample, which could be attributable to the increase in leucine and isoleucine54, suggesting the association in the current study could be due to the release of amino acids from joint collagen breakdown. Studies of osteoarthritic canine SF also showed an increased concentration of isoleucine, supporting this hypothesis24. Recently, the ratio of BCAAs to the amino acid histidine was suggested as a biomarker for OA: the increase in serum BCAAs correlated with OA’s radiographic severity22. BCAAs have been shown to increase the production of cytokines, including interleukin 1 (IL-1) and 2 (IL-2), TNF-α, and IFN-γ40. It could be possible that an increased concentration of BCAAs leads to increased production of cytokines, which leads to an increased rate of joint collagen degradation associated with OA22. Our results showed a significant increment of isoleucine and a slight increment of valine and leucine, supporting a possible alteration of BCAA metabolism in the OA progression.
In previous studies, the concentration of ketone bodies increased in osteoarthritic SFs compared to healthy SFs, suggesting that fat metabolism plays an important role as a source of energy in OA joints7. In particular, hydroxybutyrate and other ketone bodies are a part of the regulatory mechanism affecting glucose and lipid metabolism. The ketone bodies decrease glucose utilization and pyruvate oxidation, maintaining normal glycolytic intermediates for biosynthetic purposes24. The results of our study demonstrated a significant decrease in the concentration of 2-hydroxyisobutyrate: a significantly higher concentration can be observed in the OA1 group compared with more advanced stages of pathology (OA2 and OA3 groups). 2-hydroxyisobutyrate is a molecule that mediates lysine 2-hydroxyisobutyrylation, one of the newly identified post-translational modifications (PTMs), which are important epigenetic modifications of the nucleosomal core histones44. The study of histone modification has emerged as a new field in the context of OA46. Some epigenetic changes are considered possible causes of the abnormal gene expression and subsequent alteration of the chondrocyte phenotype (hypertrophy, proliferation, senescence) and extracellular matrix homeostasis, as observed in osteoarthritic cartilage46. In the intraarticular environment, the PTMs, such as lysine 2-hydroxyisobutyrylation, activate fibroblastic proliferation and migration, reduce cartilage erosion by decreasing the production of interferon γ (IFN-γ) in natural SF killer cells, and reduce the formation of osteoclasts, which mediate bone erosion in rheumatoid arthritis models47. It could reasonably be assumed that 2-hydroxyisobutyrate has a protective role. In addition, in the mouse model of OA, hydroxyisobutyrylation also reduces the production of TNF-α in macrophages, increasing the synthesis of collagen (COL2A1 and COL10A1) and glycosaminoglycan in chondrogenic mesenchymal stem cells, protecting from cartilage erosion. Hydroxyisobutyrylation finally inhibits the expression of matrix metallopeptidases 9 and 13 (MMP-9, MMP-13) and a disintegrin and metalloproteinase with type 1 motif 5 (ADAMTS-5) in joint chondrocytes46. Therefore, a progressive reduction of 2-hydroxyisobutyrate during the OA progression, as suggested by our data, could promote the activation of all factors favoring inflammation and articular cartilaginous dystrophy, reducing the activation of the arthro-chondroprotection factors. Using 2-hydroxyisobutyrate for the lysine 2-hydroxyisobutyrylation could explain this decrease in its concentration.
Betaine, a stable and nontoxic natural substance widely distributed in animals, plants, and microorganisms, is an important methyl group donor in transmethylation, a process catalyzed by betaine-homocysteine methyltransferase (BHMT), and it is an essential osmoprotectant, primarily in the kidneys, liver, and brain. A large amount of betaine can accumulate in cells without disrupting cell function and protects cells under osmotic stress50. In particular, it can increase cells’ cytoplasmic volume and free water content to prevent shrinkage in hyperosmotic conditions51. Bush et al. observed that the volume of chondrocytes within the superficial and mid-zones increased with cartilage degeneration. Cell swelling was greater than that expected from the increased hydration in OA, suggesting that increased chondrocyte volume might arise from factors other than increased cartilage hydration, such as elevated cell osmolyte concentration53. Betaine could participate in this mechanism and accumulate within chondrocytes, justifying its decreased concentration in the SF of the OA2 group, but not its mild increment in the OA3 group. In addition, it could also be justified because betaine could be used for its anti-inflammatory effect. It inhibits the nuclear factor-κB (NF-κB) activity, which controls many genes involved in inflammation: the pro-inflammatory cytokines tumor necrosis factor-α (TNF-α), interleukin 1β (IL-1β), interleukin 23 (IL-23), and leucine-rich pyrin-containing 3 (NLRP3) inflammasome activation54. In contrast to our study, a recent metabolomic article reported a lack of significance between betaine concentration in healthy and osteoarthritic joints, but reported a lower concentration of choline in the OA joints27. The betaine content of body is heavily influenced by the amount of betaine consumed through diet54, but it can be also synthesized from choline in the mitochondria54, so its concentrations could influence betaine. Our results suggested a decrease of betaine concentration in the OA2 group with respect to the OA1 groups, and the same evolution can be observed for the concentration of the metabolite choline, but without significantly different concentrations between study groups.
In addition, the concentration trend of betaine and choline showed an important analogy with the evolution of concentration of methionine in the SF of dogs with OA, attributed to their interconnection within the methionine synthesis cycle. The methionine is involved in complex metabolic pathways56, including the production of the methyl donor S-adenosylmethionine (SAME), which is the source of the three methyl groups of choline by transmethylation57. Choline is a fundamental donor of methyl (CH3) groups after oxidation to betaine for the re-methylation of homocysteine to methionine. In practice, in the presence of choline or betaine, the body could methylate the sulfur of homocysteine to give methionine58. Many studies indirectly corroborate the decrease in choline found in our study because betaine is derived from choline, and this latter metabolite is also positively related to lipid metabolism and inflammation59. Other studies corroborate our observations also regarding the dramatic decrease of hydroxybutyrate metabolites and the decrease of choline (fundamental for phosphatidylcholine synthesis), as well as betaine and methionine61.
Our investigations focused on potential correlations between metabolite alterations and the degree of articular involvement in canine progressive OA, reflecting the severity of inflammation and immune cells within the inflamed joints. Normally, during OA1 to OA3 progression, different leukocytes are recruited to the site of inflammation, including macrophages, CD4+ and CD8+ T cells, B cells, and neutrophils63. Resident macrophages, the predominant immune cell type in chronic joint diseases, and mast cells, proliferate during chronic inflammation while neutrophils are still present, but their percentage is lower than that in the acute state due to the presence of other recruited cell types66. In our study, the chronic higher degree of canine joint inflammation enhances macrophage recruitment, as observed by cytology in fact an increasing number of inflammatory cells were in observed in OA2 and OA3 groups compared to OA1 group. In vitro studies have demonstrated that methionine sulfoxide treatment alters the extracellular nucleotide metabolism, promoting an increase in ATPase/ADPase activities in macrophages, promoting alterations in the redox state of macrophages, and increasing reactive oxygen and nitrogen species (RONS) levels62, and it is known that M1/classical macrophage activation is related to pro-inflammatory responses characterized by increased iNOS activity and TNF-α release64. Our data showed a not statistically significant increase of concentration of methione sulfoxide, but we could observe that different levels of chronic inflammation led to differences in the immune cell composition, illustrated by the changes in the concentration of metabolites involved in redox, immune, energy, cell growth, and nucleotide metabolism.
Currently, doubts are being raised about the role of lactate as a discriminating factor for an osteoarthritic joint. Lactate represents the end product of glycolysis, and anaerobic conditions favor its production. Different authors have documented increased concentrations of lactate in the SF of an osteoarthritic joint and a reduced concentration of glucose, suggesting an intraarticular environment more hypoxic and acidotic than that of a healthy joint24. In contrast, some authors have not found such differences in lactate concentration25. Considering the inconsistent results in the literature and our significant increase in lactate concentration, especially in the severe stage of of OA, it is likely that lactate is a discriminating factor for the most advanced stages of OA, rather than for mild pathological conditions. In fact, lactate accumulation has been observed during chronic inflammation69. This metabolite, associated with an accumulation of pyruvate in OA3 group, significantly noticeable in the stifle joints, indicates a progressive disruption of the tricarboxylic acid (TCA) cycle, reflecting a response to increased energy consumption and reduced fatty acid reserves. The high increase in lactate concentration in SF during the progression of OA could also be explained by conversion of pyruvate to lactate due to the inflammatory peak and the necessary stabilization of the acidic environment to recruit additional inflammatory cells24.
The OA is characterized by alterations in energy metabolism and cloud contribute to changes in o-acetylcarnitine levels. In the SF of the stifle joints, affected by rupture of the cranial cruciate ligament, o-acetyl-carnitine decreases during the OA progression, only to show a slight increase in subjects with severe OA. The o-acetylcarnitine (or acetyl-L-carnitine) is a specific form of acylcarnitine that is derived from the acylation of carnitine with acetic acid71. It is involved in cellular energy metabolism, in particular transports the acyl groups from the cytosol into the mitochondrial matrix for β-oxidation and it is a regulator of energy metabolism controlling the Acyl-CoA:CoA ratio37. Inflammatory and degenerative processes in the joint may require increased use or consumption of o-acetylcarnitine to respond to tissue damage and energy demand. As a result, o-acetylcarnitine levels in SFs may decrease. On the contrary, the slight increase that we observed in the more advanced stages of OA, can be explained by an alteration of mitochondrial proteins identified in advanced OA in a recent proteomic study of mitochondria from normal or osteoarthritic chondrocytes37. Further research is needed to better clarify the role of o-acetylcarnitine in pathogenesis and progression of joint disease.
Some studies suggest a potential beneficial effect of supplementation or restriction of some of these metabolites on OA47. Fontana et al.73 suggested that restriction of BCAA intake might provide novel nutraceutical approaches to OA management. Recently, Zhou et al.74 demonstrated that exogenous administration of D-mannose in a mouse model alleviates OA progression by suppressing the hypoxia-inducible factor 2α (HIF-2α) mediated chondrocyte sensitivity to ferroptosis. Ferroptosis is a form of oxidative cell death characterized by the iron-dependent accumulation of lipid hydroperoxides to lethal levels74, which contributes to the progression of OA75. The decrease in the SF concentration of mannose during the progression of OA observed in our data and the capacity of exogenous administration of D-mannose to alleviate OA progression, thanks to its therapeutic strategy for ferroptosis-related diseases, could make the mannose metabolic pathway an interesting therapeutic target to investigate74. Besides betaine’s anti-inflammatory, antifibrotic, and antiangiogenic properties, Yajun et al. demonstrated its capacity to suppress osteoclastogenesis in vitro by inhibiting reactive oxygen species (ROS) production and subsequent mitogen-activated protein kinase (MAPK) signaling, in addition to having anti-inflammatory, antifibrotic, and antiangiogenic properties76. These data demonstrated that betaine attenuated OA progression by inhibiting hyperactivated osteoclastogenesis and maintaining microarchitecture in the subchondral bone. This study on the exogenous administration of betaine in a mouse model with OA76 and the decreased betaine concentration in our results may further prompt us to consider this metabolite a potential therapeutic target. Future analysis of altered metabolic pathways during OA progression may highlight their potential therapeutic effect15.
Stifle joints, affected by rupture of cranial cruciate ligament, represented the majority of the joints included in our study. The cranial cruciate ligament disease is cause of pelvic limb lameness; it is associated with joint instability and it leads to secondary pathologic change such as OA80. The results of the statistical comparison of the concentration of metabolites identified in the SF of the stifles were similar to the results obtained from the comparison of all joints, however, the significant difference was found for the pyruvate and o-acetylcarnitine concentrations, where the significant difference was not detected from the first statistical comparison of all included joints. So, localization or pathology could affect the metabolome, but further studies are needed to assess how the localization or the rupture of the cruciate ligament may affect the metabolome, in particular studies that compare healthy stifles with affected stifles. The same analysis should be performed for other joints affected by other conditions.
Our study has some limitations. First, the metabolomic profiles of dogs of different breeds that were enrolled could be different, as previously demonstrated18; secondly, we have not analyzed the potential influence of body condition score on the metabolome of the SF18. In addition, the clinical nature of the study and the standards recommended by EU Directive 2010/63/EU for experiments on animals did not allow us to have a control group to include healthy dogs’ joints without OA. The execution of an arthrocentesis of a healthy joint, without clinical or radiographic indication, does not comply with animal welfare laws, and the difficulty of obtaining the SF of dogs subjected to euthanasia free of metabolic pathologies is evident. It is not yet clear the mechanism of action of these metabolites in relation to the evolution of OA, so some considerations are hypothetical and should therefore be contextualized.
In conclusion, this study emphasized the presence of different 1H-NMR metabolomic profiles of canine SF in patients with progressive degrees of spontaneous OA. The results suggested the existence of therapeutic potentials of the metabolic pathways that involve mannose, betaine, choline, 2-hydroxyisobutyrate, isoleucine, and lactate, and monitoring their concentrations can give information on the evolution of OA.
In both humans and domestic animals, this is the first study to quantify and compare different metabolite concentrations in three stages of osteoarthritic joints, encouraging the application of metabolomic analysis in clinician research for diagnosis, monitoring of progression, and treatment of OA. Further studies are needed to assess the actual therapeutic potential of the involved metabolic pathways.
Materials and methods
Ethics statement
This study was performed in accordance with relevant guidelines and regulation and it was approved by the Institutional Animal Care and Use Committee of the University of Camerino (protocol no. 11/2023) according to the standards recommended by EU Directive 2010/63/EU for experiments on animals.
Animals
Client-owned dogs showing lameness in one limb and affected by spontaneous OA were referred to the Veterinary Teaching Hospital of the University of Camerino and prospectively enrolled in the study. The inclusion criteria were dogs aged 1 to 15 years, with no weight and sex restriction, belonging to the ASA 1–2 anesthetic risk class82. The presence of comorbidity, pregnancy, or lactation; the presence of OA in other joints of the same limb; and joints affected by arthropathies not on a degenerative basis, such as septic arthritis, immunomediated arthritis, hemarthrosis, and neoplasia, represented exclusion criteria. Dogs that had received anti-inflammatory drugs or nutraceuticals in the 20 days before enrollment or intraarticular treatments in the 180 days before enrollment were excluded from the study.
Clinical, radiographic, and synovial cytologic examinations
Clinical, radiographic, and cytologic evaluations were performed to confirm the OA diagnosis.
Anamnestic and signaling data were collected. Subsequently, an expert orthopedic surgeon performed clinical and radiographic examinations under anesthesia (more than 20 years of experience).
Patients were sedated with 2 μg/kg of dexmedetomidine and 0.2 mg/kg of methadone IM and then anesthetized by 2–3 mg/kg of propofol IV until tracheal intubation was achieved. Anesthesia was maintained with 1.2% isoflurane in oxygen for the radiographic evaluation. The radiographic examination was performed, and orthogonal projections of the affected joint were obtained from each patient to blindly assess the OA according to the modified Kellgren–Lawrence scale (Table 4): score 0 = normal (grade 0); score 1–3 = mild OA (grade 1); score 4–6 = moderate OA (grade 2); score 7–9 = severe OA (grade 3); score > 10 = extremely severe/deforming OA (grade 4)13.
During anesthesia, the affected joint was trichotomized and SF samples were collected aseptically by arthrocentesis for qualitative and semiquantitative cytological examination and metabolomic analysis. An aliquot of SF was blindly assessed in the Veterinary Pathology Unit of the University of Camerino to characterize the type of disease affecting the joint (for diagnostic purposes), and subsequently, joints affected by arthropathies different from OA, such as septic or immunomediated arthropathy, hemarthrosis, and neoplasia, were excluded from the study. Only joints with cytologic findings of degenerative arthropathy characterized by an increased number of nucleated cells with a predominance of vacuolated macrophages (Fig. 4) were included in the study. For each sample the number of mononuclear cells (macrophages and lymphocytes) was counted in 5 representative high-power filed (40X) and reported as the mean ± SD. A second aliquot was collected into 0.4 mL Eppendorf tubes and stored at − 80 °C until metabolomics analysis via 1H-NMR was performed.
When the OA diagnosis was confirmed, the recruited affected joints were definitively divided into four study groups of OA grade (according to the modified Kellgren–Lawrence scale): OA1 group (mild OA), OA2 group (moderate OA), OA3 group (severe OA), and OA4 group (extremely severe/deforming OA) (Fig. 5).
Medio-lateral (ML) (A) and caudo-cranial (CaCr) (B) radiographic projections of a stifle joint with mild OA according to the modified Kellgren–Lawrence scale (OA1 group); ML (C) and CaCr (D) radiographic projections of a knee joint with moderate OA according to the modified Kellgren–Lawrence scale (OA2 group); ML (E) and CaCr (F) radiographic projections of a stifle joint with severe OA according to the modified Kellgren–Lawrence scale (OA3 group).
Synovial metabolomic analysis
For the observation of the metabolome of synovial fluids, an NMR analysis solution with 3-(trimethylsilyl)-propionic acid-2,2,3,3-d4 sodium salt (TSP) 10 mM in D2O was prepared and then set at pH 7 with a 1 M phosphate buffer. The solution contained 0.01 mL of 2 mM NaN3 to avoid microbial proliferation. SF samples were prepared for 1H-NMR analysis by centrifuging 0.4 SF with 0.4 mL of H2O (2540×g; 300 s; 4 °C). The obtained solution was centrifuged (18,630×g; 900 s; 4 °C), and 0.6 mL of supernatant was added to 0.1 mL of 1H-NMR analysis solution. As described above, each sample was centrifuged once again.84
1H-NMR spectra were registered (600.13 MHz; 298 K) with an AVANCE™ III spectrometer (Bruker, Milan, Italy) set at a frequency of 600.13 MHz and operated using Topspin v3.5 software84. A CPMG filter of 330 ms allowed the suppression of signals with a very short relaxation time due to large molecules. In addition, a presaturation allowed suppression of the residual water signal. The double suppression was achieved by the cpmgpr1d sequence, part of the standard pulse sequence library. Each spectrum was acquired by summing up 256 transients constituted by 32,000 data points over a 7184 Hz spectral window, and then phased84.
Assignment of signals to specific molecules was performed in Chenomx software (Chenomx Inc., Canada, ver. 8.3) by comparison with the internal database (ver. 10) and the HMDB database85 implemented in Chenomx (version 2), together with previous investigations on the same biofluid of horses27. The assignment strategy, utilizing multiple sources, enabled us to categorize the rigor of each assignment as either level 1 or 2, in accordance with the scale introduced by Sumner et al.86 After bringing the spectra into the R environment87 through custom scripts, quantification was performed in one sample with a median water dilution, assessed through probabilistic quotient normalization (PQN)88, by relying on the TSP area and known concentration. The area of each signal was measured by global spectra deconvolution, implemented in the MestReNova software (Ricerca Mestrelab S. L. Santiago De Compostela (Spain), ver. 14.2.0-26256). This was done after applying a line-broadening noise reduction of 0.3 and a baseline adjustment through the Whittaker smoother procedure.
Statistical analysis
Data about the presence and concentration of metabolites detected in SF samples were analyzed using the GraphPad Prism 9 software (GraphPad Software, Inc., La Jolla, CA, USA). All data are presented as mean ± standard deviation (SD) and were first checked for normality using the Kolmogorov–Smirnov test. An Ordinary one-way ANOVA followed by the Tukey multiple comparison test was used to compare differences in metabolite concentration among the groups with different degrees of OA (OA1, OA2 and OA3 groups) and among the groups with different degrees of OA considering only the stifle joints (OA1stifle, OA2 stifle and OA3 stifle groups). Conversely, a Kruskal–Wallis test followed by the Dunn’s multiple comparison test was used to compare differences in synovial mononuclear cells among the groups the three aforementioned groups. The level of significance was set at p < 0.05.
Institutional review board statement
This study was approved by the Institutional Animal Care and Use Committee (OPBA) of the University of Camerino (Protocol No. 11/2023) according to the standards recommended by EU Directive 2010/63/EU for experiments on animals. This study is reported in accordance with ARRIVE guidelines.
Data availability
The data present in this study are available within the article.
References
Smith, G. K. et al. Lifelong diet restriction and radiographic evidence of osteoarthritis of the hip joint in dogs. J. Am. Vet. Med. Assoc. 229, 690–693. https://doi.org/10.2460/javma.229.5.690 (2006).
Lam, M. R., Lee, H. B., Kim, M. S. & Kim, N. S. Surgical model of osteoarthritis secondary to medial patellar luxation in dogs. Vet. Med. 56, 123–130. https://doi.org/10.17221/3155-VETMED (2011).
Struglics, A. et al. Human osteoarthritis synovial fluid and joint cartilage contain both aggrecanase- and matrix metalloproteinase- generated aggrecan fragments. Osteoarthr. Cartil. 14(2), 101–113. https://doi.org/10.1016/j.joca.2005.07.018 (2006).
Abramoff, B. & Caldera, F. E. Osteoarthritis: Pathology, diagnosis, and treatment options. Med. Clin. N. Am. 104(2), 293–311. https://doi.org/10.1016/j.mcna.2019.10.007 (2020).
Anderson, K. L., Zulch, H., O’Neill, D. G., Meeson, R. L. & Collins, L. M. Risk factors for canine osteoarthritis and its predisposing arthropathies: A systematic review. Front. Vet. Sci. 7, 2020. https://doi.org/10.3389/fvets.2020.00220 (2020).
Kluzek, S., Newton, J. L. & Arden, N. K. Is osteoarthritis a metabolic disorder?. Br. Med. Bull. 115(1), 111–121. https://doi.org/10.1093/bmb/ldv028 (2015).
Zhai, G. Alteration of metabolic pathways in osteoarthritis. Metabolites 9, 1–11. https://doi.org/10.3390/metabo9010011 (2019).
Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 21(1), 16–21. https://doi.org/10.1016/j.joca.2012.11.012 (2013).
Scanzello, C. R., Plaas, A. & Crow, M. K. Innate immune system activation in osteoarthritis: Is osteoarthritis a chronic wound?. Curr. Opin. Rheumatol. 20(5), 565–572. https://doi.org/10.1097/BOR.0b013e32830aba34 (2008).
Murphy, L. & Helmick, C. G. The impact of osteoarthritis in the United States: A population-health perspective: A population-based review of the fourth most common cause of hospitalization in U.S. adults. Orthop. Nurs. 31, 85–91. https://doi.org/10.1097/NOR.0b013e31824fcd42 (2012).
Aragon, C. L., Hofmeister, E. H. & Budsberg, S. C. Systematic review of clinical trials of treatments for osteoarthritis in dogs. J. Am. Vet. Med. Assoc. 230(4), 514–521. https://doi.org/10.2460/javma.230.4.514 (2007).
Michiewicz, B. et al. Metabolic analysis of knee synovial fluid as a potential diagnostic approach of osteoarthritis. J. Orthop. Res. 33(11), 1631–1638. https://doi.org/10.1002/jor.22949 (2015).
Botto, R. et al. Effects of intra-articular autologous adipose micrograft for the treatment of osteoarthritis in dogs: A prospective, randomized, controlled study. Animals 12(14), 2022. https://doi.org/10.3390/ani12141844 (1844).
Lee, M. I. et al. A placebo-controlled study comparing the efficacy of intra-articular injections of hyaluronic acid and a novel hyaluronic acid-platelet-rich plasma conjugate in a canine model of osteoarthritis. J. Orthop. Surg. Res. 14(1), 314. https://doi.org/10.1186/s13018-019-1352-1 (2019).
Zhai, G., Randell, E. W. & Rahman, P. Metabolomics of osteoarthritis: Emerging novel markers and their potential clinical utility. Rheumatology 57(12), 2087–2095. https://doi.org/10.1093/rheumatology/kex497 (2018).
de Sousa, E. B., Dos Santos Junior, G. C., Duarte, M. E. L., Moura Neto, V. & Aguiar, D. P. Metabolomics as a promising tool for early osteoarthritis diagnosis. Braz. J. Med. Biol. Res. 50(11), e6485. https://doi.org/10.1590/1414-431X20176485 (2017).
Li, J. T., Zeng, N., Yan, Z. P., Liao, T. & Ni, G. X. A review of applications of metabolomics in osteoarthritis. Clin. Rheumatol. 40(7), 2569–2579. https://doi.org/10.1007/s10067-020-05511-8 (2021).
Carlos, G., dos Santos, F. P. & Fröehlich, P. E. Canine metabolomics advances. Metabolomics 16, 16. https://doi.org/10.1007/s11306-020-1638-7 (2020).
Lamers, R. J. et al. Identification of a urinary metabolite profile associated with osteoarthritis. Osteoarthr. Cartil. 13(9), 762–768. https://doi.org/10.1016/j.joca.2005.04.005 (2005).
Loeser, R. F. et al. Association of urinary metabolites with radiographic progression of knee osteoarthritis in overweight and obese adults: An exploratory study. Osteoarthr. Cartil. 24(8), 1479–1486. https://doi.org/10.1016/j.joca.2016.03.011 (2016).
Zhang, W. et al. Metabolomic analysis of human plasma reveals that arginine is depleted in knee osteoarthritis patients. Osteoarthr. Cartil. 24(5), 827–834. https://doi.org/10.1016/j.joca.2015.12.004 (2016).
Zhai, G. et al. Serum branched-chain amino acid to histidine ratio: A novel metabolomic biomarker of knee osteoarthritis. Ann. Rheum. Dis. 69(6), 1227–1231. https://doi.org/10.1136/ard.2009.120857 (2010).
Musteata, M., Nicolescu, A., Solean, G. & Deleanu, C. The 1H NMR profile of healthy dog cerebrospinal fluid. PLoS ONE 8(12), 12–14. https://doi.org/10.1371/journal.pone.0081192 (2013).
Damyanovich, A. Z., Staples, J. R., Chan, A. D. & Marshall, K. W. Comparative study of normal and osteoarthritic canine synovial fluid using 500 MHz 1H magnetic resonance spectroscopy. J. Orthop. Res. 17(2), 223–231. https://doi.org/10.1002/jor.1100170211 (1999).
Stabile, M. et al. 1H-NMR metabolomic profile of healthy and osteoarthritic canine synovial fluid before and after UC-II supplementation. Sci. Rep. 12(1), 19716. https://doi.org/10.1038/s41598-022-23977-1 (2022).
Lacitignola, L., Fanizzi, F. P., Francioso, E. & Crovace, A. 1H NMR investigation of normal and osteoarthritic synovial fluid in the horse. Vet. Comp. Orthop. Traumatol. 21(1), 85–88. https://doi.org/10.3415/VCOT-06-12-0101 (2008).
Laus, F. et al. Synovial fluid metabolome can differentiate between healthy joints and joints affected by osteoarthritis in horses. Metabolities 13, 913. https://doi.org/10.3390/metabo13080913 (2023).
de Sousa, E. B. et al. Normal and osteoarthritic synovial fluid present different metabolomic profile. Osteoarthr. Cartil. 25(1), S384. https://doi.org/10.1016/j.joca.2017.02.657 (2017).
Jaggard, M. K. J. et al. A systematic review of the small molecule studies of osteoarthritis using nuclear magnetic resonance and mass spectroscopy. Osteoarthr. Cartil. 27, 560–570. https://doi.org/10.1016/j.joca.2018.08.024 (2019).
Meeson, R. L., Todhunter, R. J., Blunn, G., Nuki, G. & Pitsillides, A. A. Spontaneous dog osteoarthritis—A one medicine vision. Nat. Rev. Rheumatol. 15, 273–287. https://doi.org/10.1038/s41584-019-0202-1 (2019).
Kim, S. et al. Metabolite profiles of synovial fluid change with the radiographic severity of knee osteoarthritis. Jt. Bone Spine 84(5), 605–610. https://doi.org/10.1016/j.jbspin.2016.05.018 (2016).
Bunn, H. F. & Higgins, P. J. Reaction of monosaccharides with proteins: possible evolutionary significance. Science 213(4504), 222–224. https://doi.org/10.1126/science.12192669 (1981).
Sharma, V., Ichikawa, M. & Freeze, H. H. Mannose metabolism: More than meets the eye. Biochem. Biophys. Res. Commun. 453(2), 220–228. https://doi.org/10.1016/j.bbrc.2014.06.021 (2014).
Matsuhashi, T. et al. Alteration of N-glycans related to articular cartilage deterioration after anterior cruciate ligament transection in rabbits. Osteoarthr. Cartil. 16(7), 772–778. https://doi.org/10.1016/j.joca.2007.11.004 (2008).
Urita, A. et al. Alterations of high-mannose type N-glycosylation in human and mouse osteoarthritis cartilage. Arthritis Rheum. 63(11), 3428–3438. https://doi.org/10.1002/art.30584 (2011).
Saito, Y. et al. Mannose and phosphomannose isomerase regulate energy metabolism under glucose starvation in leukemia. Cancer Sci. 112(12), 4944–4956. https://doi.org/10.1111/cas.15138 (2021).
Adams, S. B. et al. Global metabolic profiling of human osteoarthritic synovium. Osteoarthr. Cartil. 20, 64–67. https://doi.org/10.1016/j.joca.2011.10.010 (2012).
Borel, M. et al. Longitudinal profiling of articular cartilage degradation in osteoarthritis by high-resolution magic angle spinning 1H NMR spectroscopy: Experimental study in the meniscectomized guinea pig model. J. Proteome Res. 8(5), 2594–2600. https://doi.org/10.1021/pr8009963 (2009).
Maher, A. D. et al. 1H NMR spectroscopy of serum reveals unique metabolic fingerprints associated with subtypes of surgically induced osteoarthritis in sheep. J. Proteome Res. 11(8), 4261–4268. https://doi.org/10.1021/pr300368h (2012).
Bassit, R. A., Sawada, L. A., Bacurau, R. F., Navarro, F. & Costa Rosa, L. F. The effect of BCAA supplementation upon the immune response of triathletes. Med. Sci. Sports Exerc. 32(7), 1214–1219. https://doi.org/10.1097/00005768-200007000-00005 (2000).
Fernandes, J. C., Martel-Pelletier, J. & Pelletier, J. P. The role of cytokines in osteoarthritis pathophysiology. Biorheology 39, 237–246. https://doi.org/10.2741/martel (2002).
Ardawi, M. S. M. & Newsholme, E. A. Metabolism of ketone bodies, oleate and glucose in lymphocytes of the rat. Biochem. J. 221, 255–260. https://doi.org/10.1042/bj2210255 (1984).
Madison, L. L., Mebane, D., Unger, R. H. & Lochner, A. The hypoglycemic action of ketones. II. Evidence for stimulatory feedback of ketones on the pancreatic beta cells. J. Clin. Invest. 43, 408–415. https://doi.org/10.1172/JCI104925 (1964).
Huang, S., Tang, D. & Dai, Y. Metabolic functions of lysine 2-hydroxyisobutyrylation. Cureus 12(8), e9651. https://doi.org/10.7759/cureus.9651 (2020).
Dai, L. et al. Lysine 2- hydroxyisobutyrylation is a widely distributed active histone mark. Nat. Chem. Biol. 10(5), 365–370. https://doi.org/10.1038/nchembio.1497 (2014).
Lafont, J. E., Moustaghfir, S., Durand, A. L. & Mallein-Gerin, F. The epigenetic players and the chromatin marks involved in the articular cartilage during osteoarthritis. Front. Physiol. 14, 1070241. https://doi.org/10.3389/fphys.2023.1070241 (2023).
Cribbs, A. et al. Inhibition of histone H3K27 demethylases selectively modulates inflammatory phenotypes of natural killer cells. J. Biol. Chem. 293(7), 2422–2437. https://doi.org/10.1074/jbc.RA117.000698 (2018).
Yapp, C., Carr, A. J., Price, A., Oppermann, U. & Snelling, S. J. H3K27me3 demethylases regulate in vitro chondrogenesis and chondrocyte activity in osteoarthritis. Arthritis Res. Ther. 18(1), 158. https://doi.org/10.1186/s13075-016-1053-7 (2016).
Heinemann, B. et al. Inhibition of demethylases by GSK-J1/J4. Nature 514, 7520. https://doi.org/10.1038/nature13688 (2014).
Kempson, S. A., Vovor-Dassu, K. & Day, C. Betaine transport in kidney and liver: Use of betaine in liver injury. Cell. Physiol. Biochem. 32(7), 32–40. https://doi.org/10.1159/000356622 (2013).
Ratriyanto, A., Mosenthin, R., Bauer, E. & Eklund, M. Metabolic, osmoregulatory and nutritional functions of betaine in monogastric animals. Asian-Australas. J. Anim. Sci. 22(10), 1461–1476. https://doi.org/10.5713/ajas.2009.80659 (2009).
Horio, M. et al. Apoptosis induced by hypertonicity in Madin Darley canine kidney cells: Protective effect of betaine. Nephrol. Dial. Transpl. 16(3), 483–490. https://doi.org/10.1093/ndt/16.3.483 (2001).
Bush, P. G. & Hall, A. C. The volume and morphology of chondrocytes within non-degenerate and degenerate human articular cartilage. Osteoarthr. Cartil. 11(4), 242–251. https://doi.org/10.1016/s1063-4584(02)00369-2 (2003).
Zhao, G. et al. Betaine in inflammation: Mechanistic aspects and applications. Front. Immunol. 9, 1070. https://doi.org/10.3389/fimmu.2018.01070 (2018).
Craig, S. A. Betaine in human nutrition. Am. J. Clin. Nutr. 80(3), 539. https://doi.org/10.1093/ajcn/80.3.539 (2004).
Parkhitko, A. A., Jouandin, P., Mohr, S. E. & Perrimon, N. Methionine metabolism and methyltransferases in the regulation of aging and lifespan extension across species. Aging Cell 18(6), e13034. https://doi.org/10.1111/acel.13034 (2019).
Stekol, J. A., Anderson, E. I. & Weiss, S. S-adenosyl-L-methionine in the synthesis of choline, creatine and cysteine in vivo and in vitro. J. Biol. Chem. 233(2), 425–429. https://doi.org/10.1016/S0021-9258(18)64777-5 (1958).
Du Vigneaud, V., Chandler, J. P., Moyer, A. W. & Keppel, D. M. The effect of choline on the ability of homocystine to replace methionine in the diet. J. Biol. Chem. 131, 57–76 (1939).
Gao, X., Randell, E., Zhou, H. & Sun, G. Higher serum choline and betaine levels are associated with better body composition in male but not female population. PloS ONE 13, e019314. https://doi.org/10.1371/journal.pone.0193114 (2018).
Taesuwan, S., Vermeylen, F., Caudill, M. A. & Cassano, P. A. Relation of choline intake with blood pressure in the National Health and Nutrition Examination Survey 2007. Am. J. Clin. Nutr. 109, 648–655. https://doi.org/10.1093/ajcn/nqy330 (2019).
Wiklund, P. K. et al. Serum metabolic profiles in overweight and obese women with and without metabolic syndrome. Diabetol. Metab. Syndr. 6, 1–9. https://doi.org/10.1186/1758-5996-6-40 (2014).
Dos SantosFechine, C. P. N. et al. Choline metabolites, hydroxybutyrate and HDL after dietary fiber supplementation in overweight/obese hypertensive women: A metabolomic study. Nutrients 13(5), 1437. https://doi.org/10.3390/nu13051437 (2021).
Chen, Y. et al. Macrophages in osteoarthritis: Pathophysiology and therapeutics. Am. J. Transl. Res. 12(1), 261–268 (2020).
Pérez, S. & Rius-Pérez, S. Macrophage polarization and reprogramming in acute inflammation: A redox perspective. Antioxidants 11(7), 1394. https://doi.org/10.3390/antiox11071394 (2022).
Naughton, D. et al. An investigation of the abnormal metabolic status of synovial fluid from patients with rheumatoid arthritis by high field proton nuclear magnetic resonance spectroscopy. FEBS Lett. 317(1–2), 135–138. https://doi.org/10.1016/0014-5793(93)81508-w (1993).
Naughton, D. P. et al. A comparative evaluation of the metabolic profiles of normal and inflammatory knee-joint synovial fluids by high resolution proton NMR spectroscopy. FEBS Lett. 332(3), 221–225. https://doi.org/10.1016/0014-5793(93)80636-9 (1993).
James, M. J., Cleland, L. G., Rofr, A. M. & Leslie, A. L. Intraarticular pressure and the relationship between synovial perfusion and metabolic demand. J. Rheumatol. 17, 521–527 (1990).
Carlson, A. K. et al. Application of global metabolome profiling of synovial fluid for osteoarthritis biomarkers. Biochem. Biophys. Res. Commun. 499(2), 182–188. https://doi.org/10.1016/j.bbrc.2018.03.117 (2018).
Zizmare, L. et al. Acute and chronic inflammation alter immunometabolism in a cutaneous delayed-type hypersensitivity reaction (DTHR) mouse model. Commun. Biol. 5, 1250. https://doi.org/10.1038/s42003-022-04179-x (2022).
Jha, M. K. et al. Metabolic connection of inflammatory pain: Pivotal role of a pyruvate dehydrogenase kinase-pyruvate dehydrogenase-lactic acid axis. J. Neurosci. 35, 14353–14369. https://doi.org/10.1523/JNEUROSCI.1910-15.2015 (2015).
Dambrova, M. et al. Acylcarnitines: Nomenclature, biomarkers, therapeutic potential, drug targets, and clinical trials. Pharmacol. Rev. 74, 506–551. https://doi.org/10.1124/pharmrev.121.000408 (2022).
Ruiz-Romero, C. et al. Mitochondrial dysregulation of osteoarthritic human articular chondrocytes analyzed by proteomics: A decrease in mitochondrial superoxide dismutase points to a redox imbalance. Mol. Cell Proteomics 8, 172–189. https://doi.org/10.1074/mcp.M800292-MCP200 (2009).
Fontana, L. et al. Decreased consumption of branched-chain amino acids improves metabolic health. Cell. Rep. 16(2), 520–530. https://doi.org/10.1016/j.celrep.2016.05.092 (2016).
Zhou, X. et al. D-mannose alleviates osteoarthritis progression by inhibiting chondrocyte ferroptosis in a HIF- 2α-dependent manner. Cell. Prolif. 54(11), e13134. https://doi.org/10.1111/cpr.13134 (2021).
Yao, X. et al. Chondrocyte ferroptosis contribute to the progression of osteoarthritis. J. Orthop. Transl. 27, 33–43. https://doi.org/10.1016/j.jot.2020.09.006 (2020).
Yajun, W. et al. Betaine attenuates osteoarthritis by inhibiting osteoclastogenesis and angiogenesis in subchondral bone. Front. Pharmacol. 12, 723988. https://doi.org/10.3389/fphar.2021.723988 (2021).
Yang, J. M., Zhou, R., Zhang, M., Tan, H. R. & Yu, J. Q. Betaine attenuates monocrotaline-induced pulmonary arterial hypertension in rats via inhibiting inflammatory response. Molecules 23(6), 1274. https://doi.org/10.3390/molecules23061274 (2018).
Park, S. W. et al. Antiangiogenic effect of betaine on pathologic retinal neovascularization via suppression of reactive oxygen species mediated vascular endothelial growth factor signaling. Vascul. Pharmacol. 90, 19–26. https://doi.org/10.1016/j.vph.2016.07.007 (2017).
Dibaba, D. T. et al. The Association of dietary choline and betaine with the risk of type 2 diabetes: The atherosclerosis risk in communities (ARIC) study. Diabetes Care 43(11), 2840–2846. https://doi.org/10.2337/dc20-0733 (2020).
Ceciora, L. C., Harms, O., Freise, F., Seifert, H. & Fehr, M. Ex vivo evaluation of the cranial tibial artery and its compression through fragment rotation during tibia plateau levelling osteotomy: An angiographic three-dimensional reconstruction. Vet. Comp. Orthop. Traumatol. 35(4), 220–229. https://doi.org/10.1055/s-0042-1745847 (2022).
Viant, M. R., Ludwig, C., Rhodes, S., Günther, U. L. & Allaway, D. Validation of a urine metabolome fingerprint in dog for phenotypic classification. Metabolomics 3(4), 453–463. https://doi.org/10.1007/s11306-007-0092-0 (2007).
Mudumbai, C. S. et al. Development and validation of a predictive model for American society of anesthesiologist physical status. BMC Health Serv. Res. 19, 859. https://doi.org/10.1186/s12913-019-4640-x (2019).
Kohn, M. D., Sassoon, A. A. & Fernando, N. D. Classification in brief: Kellgren-Lawrence classification of osteoarthritis. Clin. Orthop. Relat. Res. 474, 1886–1893. https://doi.org/10.1007/s11999-016-4732-4 (2016).
Brugaletta, G. et al. A multi-omics approach to elucidate the mechanisms of action of a dietary muramidase administered to broiler chicken. Sci. Rep. 12, 5559. https://doi.org/10.1038/s41598-022-09546-6 (2022).
Wishart, D. S. et al. HMDB: The human metabolome database. Nucleic Acids Res. 35, D521–D526. https://doi.org/10.1093/nar/gkl923 (2007).
Sumner, L. et al. Proposed minimum reporting standards for chemical analysis: Chemical analysis working group (CAWG) metabolomics standards initiative (MSI). Metabolomics 3, 211–221. https://doi.org/10.1021/acs.analchem.2c05192 (2007).
R Development Core Team. R: A Language and Environment for Statistical Computing, Vol. 1 (2011).
Dieterle, F., Ross, A., Schlotterbeck, G. & Senn, H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures: Application in 1H NMR metabonomics. Anal. Chem. 78, 4281–4290. https://doi.org/10.1021/ac051632c (2006).
Author information
Authors and Affiliations
Contributions
Conceptualization, A.P.P.; data curation, S.S.; formal analysis, A.D.C., L.L., and C.M.; investigation, A.P.P., S.S., L.P., V.R., C.D.B., M.A., and G.E.M; methodology, A.P.P.; writing—original draft, S.S.; writing—review and editing, S.S., A.P.P., G.R., and G.E.M.; visualization, S.S. and A.D.C.; supervision, A.P.P. and G.E.M. All the authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Piccionello, A.P., Sassaroli, S., Pennasilico, L. et al. Comparative study of 1H-NMR metabolomic profile of canine synovial fluid in patients affected by four progressive stages of spontaneous osteoarthritis. Sci Rep 14, 3627 (2024). https://doi.org/10.1038/s41598-024-54144-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-54144-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.