Abstract
Infections, such as mucormycosis, often result from inhaling sporangiospore present in the environment. Surprisingly, the extent of airborne Mucormycetes sporangiospore concentrations remains inadequately explored. This study aimed to assess the influence of UV radiation on microbial populations and Mucormycetes spore levels within a hospital environment in northern Iran. A comprehensive dataset comprising 298 air samples collected from both indoor and outdoor settings was compiled. The culture was conducted using Blood Agar and Dichloran Rose Bengal Chloramphenicol (DRBC) culture media, with Chloramphenicol included for fungal agents and Blood Agar for bacterial. Before UV treatment, the average count of Mucormycetes ranged from 0 to 26.4 ± 25.28 CFU m−3, fungal agents from 2.24 ± 3.22 to 117.24 ± 27.6 CFU m−3, and bacterial agents from 29.03 ± 9.9 to 359.37 ± 68.50 CFU m−3. Following UV irradiation, the averages were as follows: Mucormycetes ranged from 0 to 7.85 ± 6.8 CFU m−3, fungal agents from 16.58 ± 4.79 to 154.98 ± 28.35 CFU m−3, and bacterial agents from 0.38 ± 0.65 to 43.92 ± 6.50 CFU m−3. This study, notably marks the pioneering use of UV light to mitigate Mucormycetes spore counts and bacterial agents in northeastern Iran, contributing to the advancement of environmental health and safety practices in hospital settings.
Similar content being viewed by others
Introduction
The air in hospital environments contains a wide variety of microbial contaminants, and the levels of these contaminants vary from one department to another within a hospital1. In the United States, approximately 2 million people suffer from hospital-acquired infections, and 90,000 people die from these infections every year2. Different diseases can be caused by pathogenic infections that are transmitted through the air, posing a significant threat to human health and life3. Ventilation, heating, and air conditioning systems can contribute rather to the spread of microorganisms that induce infections4. Coughing and talking for five minutes can produce 3000 droplets, while sneezing can produce 40,000 droplets5. Sizes larger than 1 µm and smaller than 50 µm are typical for bioaerosols6. Biological particles in the air have an aerodynamic diameter that ranges from 0.001 to 100 µm7. Inhalation is one of the primary routes through which people are exposed to bacterial, and it can have several adverse health effects, such as cancer, allergies, acute toxicity, and respiratory disorders8. Bioaerosols, which can have harmful health effects, are exposed to hospital employees, patients, and visitors9,10. A collection of filamentous molds from the orders Mucorales and Entomophthorales are the source of the infection known as mucormycosis. Mucorals can be found in soil, rotting food, bread, dust, and other environmental habitats11. Mucormycosis infections can be caused by contact with the skin or open wounds, ingestion of contaminated food, or inhalation of spores in the respiratory system12. In affluent nations, mucormycosis mostly affects individuals with severe immunodeficiencies13. However, patients with uncontrolled diabetes mellitus or individuals who have had an injury account for a significant portion of mucormycosis cases in impoverished nations14. Mucomycosis has a strong tendency to affect blood vessels, leading to thrombosis, necrosis, and tissue infarction, all of which can be fatal15. Numerous taxa, including Mucor, Rhizopus, Lichtheimia (formerly Absidia), Rhizomucor, Cunninghamella, Apophysomyces, and Saksenaea, are members of the order Mucorales. Mucor, Lichtheimia, and Rhizopus are the three genera most commonly associated with Mucormycosis16. Other genera are typically less prevalent. According to research, the most lethal Mucormycetes species in humans are those belonging to the genus Cunninghamella17. Providing medical services to patients is the fundamental objective of hospital administrators18. The hospital should be cleaned up first, though Engineering control measures should be used to reduce the risk of infection19. Filtration or dilution, in which fresh ventilation air is introduced into the space to replace any contaminated air, is a traditional remedy20. There is a significant demand for efficient indoor air disinfection solutions due to the harmful effects that exposure to airborne infections can have on human health21. Strong germicidal effects are known to be caused by ultraviolet germicidal radiation (UVGI), particularly at wavelengths between 180 and 280 nm22 . Studies on the effectiveness of conventional UVC in hospital room air disinfection, however, have only been published occasionally23. The following describes how UV radiation reduces microorganisms: Microorganisms are exposed to ultraviolet radiation, which penetrates their cell walls and affects nucleic acids and other essential cellular substances, resulting in damage and destruction of cells24. Several studies have investigated the effect of photolysis on the removal of microorganisms and found that UV radiation does not significantly affect the destruction of fungi spores, but is effective in eradicating bacterial. Among them, in the study of Maleki and Nazari (2018), which was conducted at Imam Hossein Hospital in Tehran. The ICU department has the highest rate of bacterial contamination. The most abundant bacterial observed were Enterococcus, Pseudomonas species, coagulase negative Staphylococcus, Klebsiella species and group D non-enterococcal streptococcus respectively25. Also, in the study by Mokhtari et al. It was observed in the burn department, operating theater and emergency department. Fungal contamination was more in the skin section than in other sampling sites. The dominant genus of Gram-positive bacterial was Staphylococcus epidermidis and the dominant species of Gram-negative bacterial was Citrobacter freundii. The most fungal genera isolated from hospital air samples were Penicillium, Aspergillus niger and Aspergillus flavus26. However, a limited number of studies specifically investigate the presence of Mucormycetes and other microorganisms in hospital settings. Therefore, there is an urgent need for more research in this field, especially considering the various conditions and environmental factors that may be associated with the occurrence of Mucormycetes in the air. In this study, our aim is to investigate the presence of Mucormycetes in the indoor air of Mashhad educational, research and therapeutic hospital. In addition, we will assess the density and variety of other airborne microbial agents. By conducting this comprehensive investigation, we hope to gain valuable insights into the prevalence and distribution of Mucormycetes and other microorganisms in the hospital environment. Such information will be crucial to implement effective measures to control and prevent the spread of these potentially harmful pathogens.
Materials and methods
Sampling sites
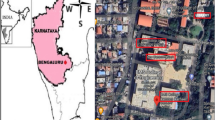
The sampling site for this study was located in Mashhad, which serves as the capital of Razavi Khorasan Province and ranks as the second-largest city in Iran. The local climate is characterized as cold and semi-arid, featuring chilly winters and moderate summers. Mashhad encompasses an area of approximately 280 square kilometers and boasts a population of 3,131,586 people, as per the latest census data. This cross-sectional descriptive study was carried out at the Specialized Teaching and Research Hospital in the year 2021. Presently, the Mashhad University of Medical Sciences operates a sizable and state-of-the-art medical facility with 848 operational beds. This medical center extends its services to patients not only from Mashhad but also from neighboring provinces. Within the hospital, the Ear, Nose, and Throat (ENT) section comprises 30 active beds, encompassing 23 beds designated for adult patients and 7 beds allocated for pediatric patients. The bed occupancy rate in this section stands at an impressive 87%. Given the frequent performance of reconstructive surgeries and examinations conducted under anesthesia, it became imperative to assess the pathogenic factors impacting both patients and the hospital staff. For reference, Fig. 1 provides a schematic representation of the sampling site27,28,29.
Sample size determination and sampling method
In this study, we employed American-made Biostage, following the NIOSH standard method, and utilized Anderson single-stage sampling (active sampling) with a calibrated American-made SKC pump, as outlined by Yang et al.30. Air samples were collected for a duration of 90 min, maintaining a flow rate of 15 L per minute, within specialized controlled environments for culturing31. The sampling process encompassed various areas within the hospital, including the operating room corridor, washroom, clean room, restroom, and recovery room. Specifically, sampling was carried out in the inpatient section, comprising 8 rooms and a connecting hallway. Systems exhaust fan, ceiling fan and air conditioner have been used for the operating room section and systems fan, ceiling fan and air conditioner for air conditioning the hospital room section. Additionally, we conducted outdoor air sampling for Mucormycetes from two distinct locations surrounding the hospital premises. Overall, a total of 298 samples were gathered, comprising 108 samples for bacterial and 190 samples for fungi analysis. These samples were collected both before and after the implementation of the UV device. The main components of the UV disinfection system include the reactor, lamps, lamp power supply and ballast, a mechanical system to maintain the lamps and the control system. The device had 5 UV lamps, with each lamp emitting 30 watts of radiation and a luminous flux of 1900 lumens (Fig. 2). The operating room space was 30 square meters. The UV disinfection device was activated for 30 min. For the ENT section, the sampling site configuration is depicted in Fig. 1. Notably, the sampling apparatus and connections underwent rigorous cleaning and drying using 70% alcohol before each sample collection to eliminate any potential initial contamination. The sampling device was positioned at a height corresponding to the breathing level of patients and was positioned more than one meter away from walls and other obstructions to ensure accurate data acquisition32.
Enumeration and computation of bacterial and fungal agents
The gathered Bacterial samples underwent incubation within a controlled temperature range of 35 to 37 degrees Celsius. After a 48-h incubation period for water-based culture media, the detection of fungi colonies, such as Cladosporium and Penicillium, usually occurred within a temperature range of 25 °C over a period of 5 to 6 days. There might be slight variations in the preferred temperature ranges for growth and colony formation among different species within these genera. Following the incubation period of 5 to 6 days for fungi culture media, the culture plates were examined, and the resulting colonies were quantified.
Enumeration and computation of mucormycosis conidia
Active sampling of conidia from both indoor and outdoor environments, utilizing a pump, was conducted on Dichloran Rose Bengal Chloramphenicol (DRBC) culture media and Blood agar. Blood agar as the culture medium for sampling bacterial bioaerosols, supplemented with the antifungal compound Nystatin. DRBC agar is used for environmental monitoring and assessment of fungal contamination in various samples. Subsequently, the collected samples were transported to a dedicated laboratory for Mucormycetes identification, employing standardized protocols. To estimate colony-forming units (CFU), we applied a modification of the Peto and Powell protocol33.
Ethical considerations
The authors affirm that they have fully adhered to ethical considerations, encompassing aspects such as plagiarism, informed consent, research misconduct, data fabrication or falsification, double publication or submission, redundancy, and other related concerns.
Results and discussion
The spore concentration of Mucormycetes and Bacterial agents in the Ear, Nose, and Throat (ENT) department was evaluated before the implementation of UVC rays. As indicated in Table 1, approximately 56% of the samples yielded positive results.
Table 2 outlines the prevalence of Mucormycetes and bacteria agents in the ear, nose, and throat (ENT) section before exposure to UV rays. The findings reveal that the highest concentration of Mucormycetes was discovered in operating room 1 within the hospital, while the greatest distribution was observed in the outdoor area. Concerning bacteria agents, the highest distribution was noticed in operation rooms 3 and 4, as well as the dirty room.
According to Table 3, about 46% of the samples tested positive after exposure to UV rays. Additionally, the average count of Mucormycetes in the ENT ward, post-UV ray treatment, ranged from 0 to 7.85 ± 6.8. The bacteria load varied between 4.79 ± 16.58 and 28.35 ± 154.98, while the fungi load ranged from 0.65 ± 0.38 to 43.92 ± 6.50. These variations indicated a significant decrease (Table 1).
Table 2 illustrates the prevalence of Mucormycetes and bacteria agents in the ear, nose, and throat (ENT) section after the utilization of UV rays. The results showcased a notable reduction in the occurrence of Mucormycetes and fungal agents. Moreover, bacteria agents exhibited decreased levels with various fluctuations.
The average count of Mucormycetes in the ENT ward and the external environment ranged from 0 to 26.4 ± 25.28 and 69.08 ± 59 to 60.8 ± 48.59, respectively. The results of the bioaerosol density measurements are categorized by bioaerosol type and specific areas (Table 2). According to Table 2, Inpatient Room 5 exhibited the highest bacterial contamination load, registering a density of 359.37 ± 68.5. Conversely, Operating Room 1 demonstrated the lowest bacterial contamination load, with a density of 29.03 ± 9.9. Additionally, Inpatient Room 7 exhibited the highest fungi contamination level, with a density of 117.24 ± 27.6, whereas Operating Room Number 4 had the lowest fungal contamination level, measuring 3.22 ± 2.24 in density.
This finding enabled researchers to investigate the effect of UVA radiation on bacterial removal34. The results of a study assessing the inactivation of gram-negative and gram-positive bacterial using fluorescent light revealed that ultraviolet radiation (4.28 mW/cm2) significantly affects the inactivation of Bacillus subtilis. The rate of bacterial destruction for this organism was found to be 0.1279 per minute Aspergillus and Penicillium can be largely eliminated and destroyed without the application of UVA35. This research evaluated the difference in Aspergillus and Penicillium concentrations after exposure to UVA radiation at 0.85 mW/cm2, but no significant results were found. However, in the current investigation, UVC radiation was applied for 15 min, and a significant difference in the concentration of fungi load was observed36. Different types, intensities, and durations of radiation may have varying effects on the reduction of bacterial and fungi burden. As is well known, UVC rays have a shorter wavelength and more energy than UVA rays. As a result, it causes more harm than UVA. Therefore, due to their low intensity, UVA rays are effective in killing vegetativecells. However, due to its higher intensity, UVC has a significant impact on spore destruction37. mucormycosis is an invasive and destructive fungal infection that is widely recognized as posing a severe health risk38. It is unknown how this disease actually spreads and which types of fungi cause it on an ecological level. Numerous species of Mucormycetes have been isolated from soil, as reported in a recent study conducted in India39. Mucormycosis are known to produce numerous conidia to obtain nutrients, facilitate growth, and spread through the air40. The amount of Mucormycetes in both indoor and outdoor air is not well understood based on environmental data41. Air samples from outdoor areas in the current investigation revealed extremely high Mucormycetes spore counts, ranging from 69,085.7 to 60,848.59 CFU m−3. Although the calculation of the number of Mucormycetes in the ambient air has already been assessed, the present investigation found that the Mucormycetes spores load ranged from 0.73 ± 0.96 to 8.60 ± 5.70 CFU m−3. In addition, the limited number of studies that have examined the prevalence of different airborne fungi in the open air in the United States of America, Zagreb, Croatia, Iran, and India have revealed a very low occurrence of Mucormycete42. The low Mucormycosis load calculated in the current study does not agree with these findings. In the current investigation, we found that the spore burden was greater in the environment outside the hospital. It's interesting to note that the autumn season had a higher spore count.
This might be due to the onset of the growing season near Mashhad, which could lead to the dispersal of Mucormycetes from plant and soil fungi. Although Punjab has similar farming practices, no similar trend was noticed there. Therefore, the validity of this claim is questionable. The findings of sampling can also be influenced by variables such as air speed, ambient temperature, relative humidity, time of day, and activity level. In order to reduce the risk of aerosol-borne infections, it is necessary to implement robust procedures for managing the quality of ventilated air. This is particularly important in hospitals, where the patient population includes a variety of immunocompromised individuals who are at risk of developing invasive fungal infections. Lack of routine cleaning and maintenance could make the department's air conditioning ducts a potential source of disease. High-efficiency particulate air (HEPA) filters and other effective measures for managing nosocomial infections should be used to purify the air. This is particularly important due to the increasing number of reports of nosocomial mucormycosis cases originating from India43. The use of both coarse and fine air filters in central air conditioning, along with regular cleaning, can be beneficial. This is because installing and maintaining high-efficiency particulate air filtration systems may not be practical in most hospital environments due to their high cost44. In the operating room, we also observed a high count of Mucormycetes, which significantly decreased after the completion of the outdoor construction activities. It is common for hospitals to experience air pollution from fungus conidia during building or remodeling45. Therefore, to prevent airborne fungal infections during construction or renovation in hospitals, it is necessary to have a multidisciplinary team, engage in careful planning, and conduct environmental monitoring in high-risk units46. This is particularly significant because outbreaks of mucormycosis associated with construction-related fungal infections have already been documented44.
Conclusion
High spore loads of Mucormycetes were found in indoor environments in the current investigation, despite the fact that the majority of mucormycosis cases are reported from the community (outdoors). Therefore, further research focused on mucormycosis in community settings is required to gain a proper understanding of the disease epidemiology. Estimating the airborne spore load can also help predict and control a potential outbreak of mucormycosis in hospitals. The application of UV radiation reduces the spore load of Mucormycetes and other bacteria and fungal agents, according to the data obtained from analyzing the indoor air. The occurrence of various hospital infections in patients referred to and hospitalized in the studied departments not only disrupts the healing process, but also leads to the development of diseases, resulting in exorbitant treatment costs. The occurrence of different hospital-acquired infections in patients admitted to and treated in the examined departments hinders their healing process, facilitates the transmission of diseases, and ultimately increases the cost of healthcare. The importance of improving air microbiology conditions in the departments under study, especially in the hospital's operating rooms, which are particularly sensitive, should be recognized. This includes addressing Mucormycetes and other bacterial and fungal agents. By implementing filters that have high performance and efficient operation, utilizing UV disinfection, and ensuring proper hygiene practices among staff and patients, hospital-acquired infections, including mucormycosis, can be prevented.
Data availability
The datasets generated and analyzed during the current study are not publicly available due to confidentiality concerns and institutional regulations. However, reasonable requests for data access may be considered by contacting the corresponding author.
Abbreviations
- DRBC:
-
Dichloran Rose Bengal Chloramphenicol
- UVGI:
-
Ultraviolet germicidal radiation
- ICU:
-
Intensive Care Unit
- ENT:
-
Ear, nose, and throat diseases
- GIS:
-
Geographic information system
- CFU/m− 3 :
-
Colony forming units per cubic meter
- HEPA:
-
High-efficiency particulate air
- NIOSH:
-
National Institute for Occupational Safety and Health
References:
Jung, C.-C. et al. Indoor air quality varies with ventilation types and working areas in hospitals. Build. Environ. 85, 190–195 (2015).
Abraham, J., Dowling, K. & Florentine, S. Can copper products and surfaces reduce the spread of infectious microorganisms and hospital-acquired infections?. Materials 14(13), 3444 (2021).
Lian, X. et al. Heat waves accelerate the spread of infectious diseases. Environ. Res. 231, 116090 (2023).
Lipinski, T. et al. Review of ventilation strategies to reduce the risk of disease transmission in high occupancy buildings. Int. J. Thermofluids 7–8, 100045 (2020).
Giovannini, G., Haick, H. & Garoli, D. Detecting COVID-19 from breath: A game changer for a Big challenge. ACS Sensors 6(4), 1408–1417 (2021).
Polednik, B. Exposure of staff to aerosols and bioaerosols in a dental office. Build. Environ. 187, 107388 (2021).
Huffman, J. A., Treutlein, B. & Pöschl, U. Fluorescent biological aerosol particle concentrations and size distributions measured with an Ultraviolet Aerodynamic Particle Sizer (UV-APS) in Central Europe. Atmos. Chem. Phys. 10(7), 3215–3233 (2010).
Sadigh, A. et al. Bacteria bioaerosol in the indoor air of educational microenvironments: Measuring exposures and assessing health effects. J. Environ. Health Sci. Eng. 19(2), 1635–1642 (2021).
Mousavi, M. S. et al. Investigating the effect of several factors on concentrations of bioaerosols in a well-ventilated hospital environment. Environ. Monit. Assess. 191(7), 407 (2019).
Zhai, Y. et al. A review on airborne microorganisms in particulate matters: Composition, characteristics and influence factors. Environ. Int. 113, 74–90 (2018).
Sharma, A. & Goel, A. Mucormycosis: risk factors, diagnosis, treatments, and challenges during COVID-19 pandemic. Folia Microbiologica 67(3), 363–387 (2022).
Daniels, J. B. & Sykes, J. E. 52 - Miscellaneous Gram-Positive Bacterial Infections. In Greene’s Infectious Diseases of the Dog and Cat (Fifth Edition) (ed. Sykes, J. E.) 627–642 (W.B. Saunders, 2021).
Reid, G. et al. Mucormycosis. Semin. Respir. Crit. Care Med. 41(01), 099–114 (2020).
Azhar, A. et al. Mucormycosis and COVID-19 pandemic: Clinical and diagnostic approach. J. Infect. Public Health 15(4), 466–479 (2022).
Siluvai Arulappan, L. A. Incidence of mucormycosis in a tertiary care hospital during Covid first wave-a retrospective study. Indian J. Otolaryngol. Head Neck Surg. 74(2), 3463–3468 (2022).
Gomes, M. Z. R., Lewis, R. E. & Kontoyiannis, D. P. Mucormycosis caused by unusual mucormycosis, non-rhizopus, -mucor, and -lichtheimia species. Clin. Microbiol. Rev. 24(2), 411–445 (2011).
Panayidou, S., Ioannidou, E. & Apidianakis, Y. Human pathogenic bacteria, fungi, and viruses in Drosophila. Virulence 5(2), 253–269 (2014).
Cavicchi, C. Healthcare sustainability and the role of intellectual capital. J. Intellectual Capital 18(3), 544–563 (2017).
Mehta, Y. et al. Guidelines for prevention of hospital acquired infections. Indian J. Crit. Care Med. 18(3), 149–163 (2014).
Zhang, Y. et al. A review of facilities management interventions to mitigate respiratory infections in existing buildings. Build. Environ. 221, 109347 (2022).
Wolkoff, P., Azuma, K. & Carrer, P. Health, work performance, and risk of infection in office-like environments: The role of indoor temperature, air humidity, and ventilation. Int. J. Hygiene Environ. Health 233, 113709 (2021).
Liu, C.-Y. et al. The study of an ultraviolet radiation technique for removal of the indoor air volatile organic compounds and bioaerosol. Int. J. Environ. Res. Public Health 16(14), 2557 (2019).
Boyce, J. M. & Donskey, C. J. Understanding ultraviolet light surface decontamination in hospital rooms: A primer. Infect. Control Hosp. Epidemiol. 40(9), 1030–1035 (2019).
Kang, J.-W., Kim, S.-S. & Kang, D.-H. Inactivation dynamics of 222 nm krypton-chlorine excilamp irradiation on Gram-positive and Gram-negative foodborne pathogenic bacteria. Food Res. Int. 109, 325–333 (2018).
Maleki, R. and S. Nazari, Investigation of type and density of bacterial bioaerosols in the air of Imam Hossein hospital in Tehran in 2018. J. Air Pollut. Health, 2022.
Montazeri, A. et al. Microbiological analysis of bacterial and fungal bioaerosols from burn hospital of Yazd (Iran) in 2019. J. Environ. Health Sci. Eng. 18(2), 1121–1130 (2020).
Yang, X. et al. Particulate matter in Swine Barns: A comprehensive review. Atmosphere 13(3), 490 (2022).
Sarkhosh, M. et al. Indoor Air Quality associations with sick building syndrome: An application of decision tree technology. Build. Environ. 188, 107446 (2021).
Mirhoseini, S. H. et al. Indoor exposure to airborne bacteria and fungi in sensitive wards of an academic pediatric hospital. Aerobiologia 36, 225–232 (2020).
William, M. A. et al. Evaluating heating, ventilation, and air-conditioning systems toward minimizing the airborne transmission risk of Mucormycosis and COVID-19 infections in built environment. Case Stud. Therm. Eng. 28, 101567 (2021).
Chuaybamroong, P. et al. Efficacy of photocatalytic HEPA filter on microorganism removal. Indoor Air 20(3), 246–254 (2010).
Dehghani, M. et al. Concentration and type of bioaerosols before and after conventional disinfection and sterilization procedures inside hospital operating rooms. Ecotoxicol. Environ. Saf. 164, 277–282 (2018).
Chotigawin, R. et al. Airborne microorganism disinfection by photocatalytic HEPA filter. EnvironmentAsia 3(2), 1–7 (2010).
Le, T. T. N. et al. Sterilization effect of UV light on Bacillus spores using TiO2 films depends on wavelength. J. Med. Invest. 59(1,2), 53–58 (2012).
Chakrabarti, A. & Singh, R. Mucormycosis in India: Unique features. Mycoses 57, 85–90 (2014).
Prakash, H. et al. The environmental source of emerging Apophysomyces variabilis infection in India. Med. Mycol. 54(6), 567–575 (2016).
Amiri Fahliyani, S. et al. Human Fungal Pathogens: Diversity, Genomics, and Preventions. In Recent Trends in Mycological Research: Volume 1: Agricultural and Medical Perspective (ed. Yadav, A. N.) 371–394 (Springer International Publishing, 2021).
Meklin, T. et al. Comparison of mold concentrations quantified by MSQPCR in indoor and outdoor air sampled simultaneously. Sci. Total Environ. 382(1), 130–134 (2007).
Shams-Ghahfarokhi, M. et al. Investigation on distribution of airborne fungi in outdoor environment in Tehran, Iran. J. Environ. Health Sci. Eng. 12(1), 54 (2014).
Eames, I., et al., Airborne transmission of disease in hospitals. J. R. Soc. Interface 2009. 6(suppl_6): S697–S702.
Prakash, H. et al. An aero mycological analysis of Mucormycosis in indoor and outdoor environments of northern India. Med. Mycol. 58(1), 118–123 (2019).
Kanamori, H. et al. Review of fungal outbreaks and infection prevention in healthcare settings during construction and renovation. Clin. Infect. Diseases 61(3), 433–444 (2015).
Talento, A. F. et al. Prevention of healthcare-associated invasive aspergillosis during hospital construction/renovation works. J. Hosp. Infect. 103(1), 1–12 (2019).
Acknowledgements
The authors would like to thank Mashhad University of Medical Sciences for their support in conducting this research (Code Number: 4000926).
Author information
Authors and Affiliations
Contributions
M.S.K. and S.S.T. conceived of the presented idea. M.A.N. and M.R.Y. participated in the literature search, data collection. V.G.H. contributed to statistical analysis. M.S.K., S.S.T., M.S. and F.J. wrote the main manuscript text. M.S. supervisor of the environmental microbiology and respond reviewers. The final manuscript was read and approved by all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sarkhoshkalat, M., Nasab, M.A., Yari, M.R. et al. Assessment of UV radiation effects on airborne mucormycetes and bacterial populations in a hospital environment. Sci Rep 14, 2708 (2024). https://doi.org/10.1038/s41598-024-53100-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53100-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.