Abstract
Bile acids (BA) are key for liver regeneration and injury. This study aims at analyzing the changes in the BA pool induced by ischemia-reperfusion (IRI) and investigates the impact of hypothermic oxygenated perfusion (HOPE) on the BA pool compared to static cold storage (SCS). In a porcine model of IRI, liver grafts underwent 30 min of asystolic warm ischemia followed by 6 h of SCS (n = 6) ± 2 h of HOPE (n = 6) and 2 h of ex-situ warm reperfusion. The BA pool in bile samples was analyzed with liquid chromatography coupled with tandem mass spectrometry. We identified 16 BA and observed significant changes in response to ischemia-reperfusion, which were associated with both protective and injury mechanisms. Second, HOPE-treated liver grafts exhibited a more protective BA phenotype, characterized by a more hydrophilic BA pool compared to SCS. Key BA, such as GlycoCholic Acid, were identified and were associated with a decreased transaminase release and improved lactate clearance during reperfusion. Partial Least Square-Discriminant Analysis revealed a distinct injury profile for the HOPE group. In conclusion, the BA pool changes with liver graft IRI, and preservation with HOPE results in a protective BA phenotype compared to SCS.
Similar content being viewed by others
Introduction
Ischemia-reperfusion injury (IRI) impairs graft function recovery1,2 but also triggers bile duct injury3 in liver grafts after transplantation. Thus, investigating the impact of IRI on bile composition may allow to further refine the mechanisms of post-transplant bile duct injury4,5. In this context, a key target are bile acids (BA) which play an important role in various homeostatic metabolic processes in the liver6 through specific signaling pathways such as FXR or TGR57. Indeed, BA have been shown to contribute to liver regeneration8, promote anti-inflammatory or pro-inflammatory responses and trigger liver injury as well as cholangiocyte injury9,10. Thereby, impairment of BA homeostasis by IRI may contribute to post-LT bile duct injury11 and the accumulation of “toxic” BA may be associated with hepatocyte and cholangiocyte injury12,13. A comprehensive analysis of BA associated with IRI is even more relevant in the current era of machine perfusion strategies, aiming to mitigate IRI, improving post-LT outcomes14,15,16 and allowing for viability assessment of liver grafts prior to LT17,18.
The primary objective of this study was therefore to conduct an extensive analysis of the BA pool using mass spectrometry in a porcine model of liver graft IRI. The specific aims of the study were to (a) investigate the alterations in the BA pool induced by IRI and (b) evaluate the potential impact of hypothermic oxygenated perfusion (HOPE) on the BA pool.
Materials and methods
Study aim
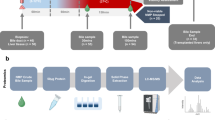
First, we analyzed the changes in the bile acid pool induced by IRI in a porcine model of liver grafts with prolonged warm and static cold ischemia. Second, we investigated the impact of HOPE on the BA pool in comparison to static cold storage (SCS) and correlated these data with surrogate markers of hepatocyte and cholangiocyte IRI (Fig. 1).
Study design
We used a previously reported porcine model of liver grafts to simulate early IRI19,20 and to determine the BA pool in bile samples after 2 h of ex-situ warm reperfusion in liver grafts subjected to SCS or SCS + HOPE (Fig. 1).
Briefly, liver grafts underwent 30 min of asystolic warm ischemia (AWI) prior to organ procurement followed by either static cold storage preservation for 6 h (n = 6, SCS group) or SCS + 2 h of end-ischemic HOPE (n = 6, HOPE group). The SCS group was used as control with expected higher IRI in comparison to the HOPE group21.
HOPE was performed ex-situ using the Liver Assist© device (XVIVO©, Sweden) through the portal vein only. The perfusion pressure was limited to 3 mmHg in the portal vein to reach a target portal flow ranging from 150 to 300 ml/min.
Finally, ex-situ warm reperfusion was performed at 37 °C for 2 h using the Liver Assist© device (XVIVO©, Sweden) to mimic liver transplantation and to focus on the early acute phase of IRI22. During reperfusion, no sodium bicarbonate, parenteral nutrition or glucose/insulin was added to avoid bias in evaluating graft function recovery.
Ethics
This study is reported in accordance with the ARRIVE guidelines for reporting experiments involving animals. All animals received human care following the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication 86–23, revised in 1985).
The experimental protocol was approved by the Institutional Animal Experimentation Committee of Claude Bernard University Lyon 1 (CEEA-55A; project number DR2020-27).
LC-MS/MS bile acid analysis
Bile samples were procured in the pig donor prior to liver procurement (baseline sample) and at the end of 2 h of warm reperfusion (last 10 min of reperfusion). All bile samples were snap-frozen and stored at − 80 °C. Prior to Liquid Chromatography coupled with tandem Mass Spectrometry (LC-MS/MS) analysis, methanol (MeOH) was added to bile samples with a ratio of 2.3/1 (70% bile, 30% MeOH). After centrifugation at 10000g at 4 °C during 10 min for protein precipitation and removal, the supernatant was collected and diluted at tenfold for LC-MS/MS analysis. LC-MS/MS analysis was conducted on a QTRAP® 5500 LC-MS/MS system hybrid triple quadrupole/linear ion trap mass spectrometer (Sciex) equipped with a Turbo V™ ion source coupled with a 1290 series HPLC device (Agilent Technologies, Waldbronn, Germany). Chromatographic separation was performed by Reversed Phase Liquid Chromatography (RPLC) on a Kinetex F5 column (150 mm × 2.1 mm × 2.6 µm). MS analysis was configured in negative ionization mode using a spray voltage of − 4500 V. The nebulizer and the curtain gas flow were set at 40 and 50 psi respectively using nitrogen. The TurboV™ ion source was set at 450 °C with the auxiliary gas flow (nitrogen) set at 40 psi. Data acquisition was performed using Analyst 1.6.3 software® (Sciex) and data processing with MultiQuant™ analysis software (vs 2.1.1., Sciex). The acquisition was done in a targeted fashion by Multiple Reaction Monitoring (MRM) and the method was developed and optimized on pure bile acid standards (BACSMLS, Sigma-Aldrich, France). Stability and reproducibility of LC-MS/MS experiments were monitored by QC samples and signal drift was corrected by Locally Estimated Scatterplot Smoothing (LOESS) using NormalizeMets package under R. Data sets obtained were then analyzed under R (v4.2.1, https://www.r-project.org), using both ropls (v1.28.2, https://bioconductor.org/packages/ropls) and mixomics package (v6.16.3, https://mixomics.org).
BA concentrations were expressed in percentage of the overall analyzed BA pool providing a more comprehensive perspective on variations in the bile acid phenotype in our study.
Hepatocyte and cholangiocyte injury markers
We correlated the results obtained at the level of the BA pool to IRI markers at the hepatocyte and cholangiocyte level. Blood samples first underwent centrifugation at 1500 rpm for 10 min, and the obtained plasma was analyzed. Plasma levels of transaminase (Aspartate Amino Transferase, AST), pH, lactate and glucose were evaluated during ex-situ warm reperfusion. At the end of reperfusion, total bile production as well as bile pH and glucose were assessed. Based on previously published data23,24, those biochemical parameters reflect both hepatocyte and cholangiocyte damage during IRI.
In addition to these biochemical indicators, histological analysis of hepatocyte and cholangiocyte compartments was also performed. Liver and extrahepatic bile ducts biopsies were formalin fixed, paraffin embedded, and stained with HPS (hematoxylin, phloxine and saffron stain). A histological IRI assessment according to a standardized classification adapted from the UCLA classification and Suzuki classification was performed in a blinded manner by an expert pathologist25,26. In detail, IRI injury focused on apoptosis, ballooning, necrosis and neutrophilic infiltrate and were graded from 0 (none) to 4 (severe).
Both intrahepatic and extrahepatic bile ducts were identified using CK7 staining (Dako M7018) to evaluate IRI injury based on op den Dries et al.27 in a blinded manner by an expert pathologist. Histological analysis of bile duct injury focused on mural loss and mural necrosis graded from 0 (none) to 3 (severe) and loss of cell in peri-luminal and deep peri-biliary gland graded from 0 to 2 (severe) in both intrahepatic and extrahepatic bile ducts28.
Statistical analysis
Continuous variables are expressed as median with interquartile range [IQR]. Continuous variables were compared using the non-parametric Mann–Whitney univariate test. Categorical variables were compared using the chi-square test or the Fisher’s exact test.
A descriptive and unsupervised approach using Principal Component Analysis (PCA)29 was used to evaluate sample distribution and separation among groups based on bile acid composition. Then, a supervised strategy by Partial Least Square Discriminant Analysis (PLS-DA)30 was performed to model and highlight differences between injury groups. R2Y values represents goodness-fit measure and Q2 values estimated the predictive ability of the model. The quality of the model was assessed by bootstrap resampling (p = 1000). In addition, variable importance in projection (VIP) scores derived from PLS-DA were used for the selection of significant BA. VIP values > 1 were used as variable selection criterion31. Of note, in multivariable analysis, the use of numerous data as independent variables is prone to a high degree of correlation between those variables. In this particular situation, PCA and PLS-DA allow for a reduction of this co-linearity, hence their application in this study32.
Variables associated with a p-value < 0.05 were considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics for Windows (Version 26.0. Armonk, NY: IBM Corp) and GraphPad Prism (Version 8.0.0 for Windows, GraphPad Software, San Diego, California USA, www.graphpad.com).
Results
Baseline bile acid pool
In baseline bile samples prior to liver procurement (n = 12), we identified a total of 16 different bile acids, including both primary and secondary bile acids (Table S1). Primary BA constituted the main BA (56% [48–60]) with a majority of glyco and tauro-conjugated BA (respectively 39% [34–41] and 35% [31–43]). A detailed composition of the baseline BA pool is presented in Fig. S1. Of note, bile composition prior to organ procurement did not differ between HOPE and SCS groups as shown in Fig. S2. The identified baseline BA pool was compared to the BA pool at the end of 2 h of ex-situ warm reperfusion in two different preservation modalities, namely SCS and HOPE.
Impact of ischemia–reperfusion on the bile acid pool
First, upon warm reperfusion after 6 h of SCS we observed a significant increase of primary BA (67.8% [64.5–72.2] vs 56.6% [49.1–58.9]; p = 0.02) with a significant increase of the Primary/Secondary BA ratio (P/S; p = 0.02) compared to the baseline (n = 6) (Fig. 2). We observed an increase in tauro/glyco-conjugated BA (p = 0.30 and p = 0.39 respectively) with a reduction in unconjugated BA (p = 0.28).
BA pool from baseline to the end of reperfusion. Panel (A): PCA displayed separation in BA pool composition. PCA scores with 52% of the variance explained on the first component, 20% on the second component and 12% on the third component. Panel (B): Detailed composition of BA pool from Baseline to the end of reperfusion. Variable are expressed as median [Inter quartile range, 25–75], non-parametric Mann–Whitney univariate test was used.
A detailed analysis of individual BA showed a significant increase in GlycoCholic Acid (GCA, p = 0.01) and ChenoDeoxyCholic Acid (CDCA, p = 0.01) whereas MuroCholic Acid (MCA, p = 0.006), GlycoChenoDeoxyCholic Acid (GCDCA, p = 0.02), GlycoDeoxyCholic Acid (GDCA, p = 0.04), GlycoLithoCholic Acid (GLCA, p = 0.004), TauroLithoCholic Acid (TLCA, p = 0.01) and HyoDeoxyCholic Acid (HDCA, p = 0.004) were significantly decreased (Fig. 2).
PCA presented in Fig. 2 exhibited a good separation between baseline and post reperfusion BA pool. PLS-DA (Fig. S2) was used to select individual BA of particular interest (VIP > 1) and exhibited good discrimination between baseline and post reperfusion BA pool (Q2Y = 0.801, R2X = 0.637, R2Y = 0.976) with a pR2Y < 0.05. In detail, GlycoLithoCholic Acid (GLCA), ChenoDeoxyCholic Acid (CDCA), MuroCholic Acid (MCA), HyoDeoxyCholic Acid (HDCA), GlycoChenoDeoxyCholic Acid (GCDCA), GlycoCholic Acid (GCA) and TauroLithoCholic Acid (TLCA) were the main BA involved in overall BA pool changes (Table S2).
Do HOPE-treated liver grafts display a different bile acid pool?
In comparison to SCS, HOPE-treated livers showed significantly lower GCA (p = 0.01) with higher levels of TauroMuroCholic Acid (TMCA, p = 0.15), TauroDeoxyCholic Acid (TDCA, p = 0.15) and TauroChenoDeoxyCholic Acid (TCDCA, p = 0.11) upon warm reperfusion (Table 1, Fig. 3). The proportion of glyco-conjugated BA was significantly lower in the HOPE group (23% [20–37] vs 37% [32–47], p = 0.01) with a higher P/S ratio (p = 0.10).
BA pool at the end of reperfusion in both groups. Panel (A): PCA displayed separation in BA pool composition between SCS and HOPE. PCA scores with 48% of the variance explained on the first component, 21% on the second component. Panel (B): Detailed BA pool at the end of reperfusion in both groups. Variable are expressed as median [Inter quartile range, 25–75], non-parametric Mann–Whitney univariate test was used. SCS: Static Cold Storage, HOPE: Hypothermic Oxygenated Perfusion, BA: Bile Acids, FC: Fold Change, P/S: Primary/Secondary ratio.
When focusing on fold changes (FC) from the baseline to the end of reperfusion, we observed a significant decrease of glyco-conjugated BA in the HOPE group compared to SCS (FC: 0.7 vs 0.9, p = 0.02; Fig. 3) and an increase of unconjugated BA (1.8 vs 1.1, p = 0.06). We also observed higher P/S ratio in the HOPE group (2.6 vs 1.4, p = 0.08) with a higher proportion of primary BA (1.4 vs 1.1, p = 0.10) and less secondary BA (0.5 vs 0.8 p = 0.08).
PCA based on the BA pool at the end of reperfusion exhibited a separation between the two groups as shown in Fig. 3. PLS-DA was used to identify BA of particular interest (VIP > 1). ChenoDeoxyCholic Acid (CDCA), TauroCholic Acid (TCA), GlycoChenoDeoxyCholic Acid (GCDCA), GlycoCholic Acid (GCA) and TauroUrsoDeoxyCholic Acid (TUDCA), were the main BA involved in group discrimination (Table 1).
Are differences in the bile acid pool associated with liver graft injury?
We observed a positive correlation between GlycoCholic Acid (GCA) levels and biochemical injury markers such as AST or Lactate (R spearman 0.6; p = 0.04 and 0.62; p = 0.03 respectively).
In addition, the BA phenotype observed in the HOPE group was associated with significantly lower transaminase release (63 UI/L/100 g [43–77] vs 114 [105–132], p = 0.004) and a significant increase in cumulative bile production (12.5 ml [9–20] vs 7.5 [4–10], p = 0.04) compared to SCS. Lactate clearance and pH normalization were comparable between both groups during reperfusion with however less lactate release and a higher pH in the HOPE group (3.96 vs 6.96, p = 0.004 and 7.17 vs 7.15, p = 0.02 respectively, Fig. 4). We also observed a higher bile pH (7.47 [7.43–7.6] vs 7.29 [7.1–7.5]; p = 0.13] and a significant lower bile glucose (13.6 mmol/L [12.2–16.5] vs 22.1 [18.09–27.7]; p = 0.04] in the HOPE group.
Biochemical injury markers and histological analysis comparing HOPE to SCS. Variable are expressed as median [Inter quartile range, 25–75], non-parametric Mann–Whitney univariate test was used. SCS: Static Cold Storage, HOPE: Hypothermic Oxygenated Perfusion, AST: Aspartate aminotransferase, IRI: Ischemia–Reperfusion Injury, PNN: Neutrophilic infiltrate, PBG: Peri-Biliary Gland, HPS: Hematoxylin, Phloxine and saffron stain.
We did not observe any difference in histological median hepatocyte injury score between HOPE and SCS groups as shown in Fig. 4 (1.5 vs 2.5; p = 0.35) as well as in median histological bile duct injury score (7.5 vs 8 points for HOPE and SCS respectively, p = 0.88; Fig. 4).
Overall, the HOPE group exhibited a distinct IRI profile compared to SCS based on biochemical data ± BA pool composition as shown by PCA (Fig. S3). As shown in Fig. 5, PLS-DA analysis based on biochemistry alone ± BA pool composition allowed for a good discrimination among groups (Q2Y = 0.601 and Q2Y = 0.721 respectively with a pR2Y < 0.05).
PLS-DA based on biochemical injury markers and BA pool in HOPE and SCS group. R2 values represents goodness-fit-measure and Q2 values estimated the predictive ability of the model. Panel (A) exhibits PLS-DA analysis based on biochemical data alone, 37% of the variance explained on the first component, 19% on the second component. Panel (B) exhibits PLS-DA analysis based on biochemical data and BA pool 31% of the variance explained on the first component, 24% on the second component. SCS: Static Cold Storage, HOPE: Hypothermic Oxygenated Perfusion, BA: Bile Acids.
Discussion
In this study using a porcine model of liver graft ischemia-reperfusion injury, we conducted a comprehensive analysis of the bile acid pool following warm ex-situ reperfusion using LC–MS/MS analysis. First, we identified 16 BA of which 7 exhibited significant changes after IRI namely GlycoLithoCholic Acid (GLCA), ChenoDeoxyCholic Acid (CDCA), MuroCholic Acid (MCA), HyoDeoxyCholic Acid (HDCA), GlycoChenoDeoxyCholic Acid (GCDCA), GlycoCholic Acid (GCA) and TauroLithoCholic Acid (TLCA). Second, we found that HOPE-treated liver grafts exhibited a significant decrease in glyco-conjugated BA along with a more hydrophilic BA pool compared to SCS. The HOPE BA phenotype was associated with a decreased transaminase release and improved lactate clearance upon reperfusion resulting in a distinct liver graft injury profile compared to SCS. Changes in GlycoCholic acid (GCA) and to a lesser extent ChenoDeoxyCholic Acid (CDCA), TauroCholic Acid (TCA), GlycoChenoDeoxyCholic Acid (GCDCA) and TauroUrsoDeoxyCholic Acid (TUDCA) allowed to establish specific BA phenotypes for SCS and HOPE-treated liver grafts.
Investigating bile duct injury mechanisms12,33 is a key challenge with the aim of promoting bile duct integrity16,34 and enable bile duct viability assessment in liver grafts prior to LT5,35. At the level of biliary tree, the IRI cascade is initiated by ATP depletion and mitochondrial succinate accumulation36 during SCS. These early events impair BA secretion and reabsorption in both hepatocytes and cholangiocytes. Upon warm reperfusion in the recipient, ROS (reactive oxygen species) and DAMPs (damaged-associated molecular patterns) release, the accumulation of toxic hydrophobic BA11 associated with a damage bicarbonate umbrella37 trigger cholangiocellular injury. Besides those early IRI mechanisms, lack of regeneration of damaged peribiliary glands27 also leads to the loss of bile duct integrity hence the occurrence of ischemic type biliary lesions. In contrast, preservation of liver grafts with HOPE has been shown to protect from early IRI events by allowing energy recovery prior to reperfusion and reducing early ROS and DAMPs release after LT38. HOPE also improves cellular regeneration and BA secretion, thus decreasing the occurrence of ischemic type biliary lesions5,16. Besides the aforementioned indicators of IRI, BA have also been shown to be major determinants in the liver injury/protection balance8. For instance, bile salt secretion relative to phospholipid concentration has been proposed as a surrogate marker for assessing bile duct ischemic injury39,40. However to date, BA remain seldomly studied in the setting of IRI and liver graft preservation5,11. The aim of this study was thus to identify changes in BA pool induced by liver graft IRI and analyze the impact of different graft preservation strategies.
First, we attempted to characterize the impact of IRI on the BA pool of liver grafts. We identified 16 different BA by LC-MS/MS analysis which underwent significant changes from baseline to the end of the early reperfusion phase. This observation was further supported by PCA and PLS-DA analysis which both showed a clear separation between the BA pool at the 2 different time points. After 2 h of warm ex-situ reperfusion we indeed observed a significant increase of primary BA (p = 0.02), with a switch toward a more hydrophilic BA pool. Primary BA are known to be more hydrophilic BA thereby being more protective against liver injury7. On the other hand, BA overload or a shift toward a more hydrophobic BA pool (reflected by the primary/secondary BA ratio) may promote liver injury7,8. In detail, MuroCholic Acid (MCA) (p = 0.006) and GlycoChenoDeoxyCholic Acid (GCDCA) (p = 0.002) were significantly decreased at the end of reperfusion. MCA is known to be a FXR antagonist potentially impairing liver regeneration and the adaptive response to liver injury41. GCDCA has been associated with hepatocyte cell death and oxidative stress42. Consistent with the increase of ChenoDeoxyCholic Acid (CDCA) (p = 0.01) and the decrease of GlycoDeoxyCholic Acid (GDCA) (p = 0.04) in our study, these findings suggest a protective response from these livers when expose to IRI, in an attempt to mitigate BA mediated injury43. Besides those protective mechanisms, we observed an increase of GlycoCholic Acid (GCA) and a decrease of Tauro/Glyco-conjugated LithoCholic Acid, which are indicative of a higher degree of liver damage44. Finally, HyoDeoxyCholic acid (HDCA) (p = 0.004) was significantly increase at the end of reperfusion. HDCA has been linked to glucose homeostasis45 and bile glucose serves as a known surrogate marker for bile duct injury during normothermic perfusion23, thereby suggesting that HDCA may be more direct marker of bile duct viability. Altogether, these data suggest that the BA phenotype relates to IRI, demonstrating significant changes in line with the extent of liver injury as expressed by GlycoCholic Acid (GCA) or uncovering the protective adaptive response of liver grafts to mitigate liver IRI.
Second, we focused on the impact of two different preservation strategies on the BA pool by comparing the BA phenotype in liver grafts undergoing SCS versus HOPE. PCA revealed a clear separation between the HOPE and SCS groups based on their respective BA phenotype (Fig. 3). Overall, HOPE exhibited a more hydrophilic thus protective BA pool7,8 compare to SCS. HOPE indeed demonstrated a higher P/S ratio (p = 0.08) and a significant reduction in hydrophobic glyco-conjugated BA (p = 0.02) which are associated with the progression of liver injury46. In detail, we identified 5 specific BA that allowed to differentiate HOPE and SCS preserved liver grafts, namely GlycoCholic acid (GCA), ChenoDeoxyCholic Acid (CDCA), TauroCholic Acid (TCA), GlycoChenoDeoxyCholic Acid (GCDCA) and TauroUrsoDeoxyCholic Acid (TUDCA). The main discriminative BA was GlycoCholic Acid (GCA) which was significantly lower in the HOPE group (p = 0.03) compared to SCS and has been shown to be associated with a higher degree of liver damage44,47. In addition, levels of TauroCholic Acid (TCA) and GlycoChenoDeoxyCholic Acid (GCDCA) were both decreased in the HOPE group (p = 0.63 and p = 0.42 respectively) which points to less severe liver injury44,48. On the other hand, TauroUrsoDeoxyCholic Acid (TUDCA) was increased in the HOPE group (p = 0.58). This BA is involved in liver protective mechanisms as it decreases hepatic toxicity, inhibit pro-inflammatory signaling and mitigates cholangiocellular injury49. Altogether, the presented data suggest that HOPE-treated livers exhibited an overall more protective BA pool with GlycoCholic Acid being the main discriminative BA between SCS and HOPE in our study.
Third, we investigated whether the observed differences in BA phenotype were also associated with less severe IRI based on hepatocyte and cholangiocyte injury markers. Our findings demonstrated that HOPE-treated livers exhibited less severe IRI in univariable analysis consistent with previously published data21. We observed a significant reduction in transaminase release (63 UI/L/100 g vs 114, p = 0.004), improved lactate clearance (p = 0.04) and pH normalization (p = 0.02). Besides, PCA revealed a separation between HOPE and SCS groups based on these biochemical data, with a robust discrimination observed in PLS-DA (Q2Y > 0.5; Q2Y = 0.601; R2X = 0.708; R2Y = 0.871; Fig. S3). Moreover, when focusing on the key BA identified in PLS-DA, we found that GlycoCholic Acid positively correlated with transaminase release or lactate clearance (R spearman 0.6; p = 0.04 and 0.62; p = 0.03 respectively). These findings suggest that the changes observed in the HOPE BA phenotype correlated with biochemical markers of cellular injury in our study. In addition to GlycoCholic Acid (GCA), adding selected BA as IRI markers, improved the predictive ability of the PLS-DA model (Q2Y = 0.721, R2X = 0.627, pR2Y < 0.05) resulting in an improved discrimination between the HOPE and SCS group. These results suggest that BA phenotype may provide additional valuable information to refine liver IRI assessment. To further support this assumption, future clinical studies should correlate BA pool during ex-situ normothermic perfusion to relevant clinical endpoints such as post-reperfusion syndrome, ischemic type biliary lesions and graft loss.
Our study was designed as a proof-of-concept study aiming at evaluating the impact IRI and HOPE on BA homeostasis and has thus several limitations primarily the limited sample size. Second, we focused on the early phase of IRI22 which is the key trigger for downstream activation of the liver inflammasome. Thus, we were not able to draw any conclusions on long-term events and clinically relevant complications. However, the identified key BA in IRI pave the way for a rapid translational application. Third, it is important to acknowledge that BA physiology also involves the gut-liver axis which is not included in our isolated ex-situ reperfusion model50. This does however not preclude from analyzing bile composition and provide valuable insights as it takes into account both BA uptake and formation by the hepatocyte and the cholangiocyte11,13. In addition, ex-situ machine perfusion as viability assessment platform is also performed in the absence of a gut-liver axis, highlighting the translational potential of our data. Fourth, while BA composition has been shown to be comparable between tissue and bile, liver tissue biopsy may provide additional useful information regarding BA shifts and should be explored in future studies51. Finally, we performed a quantitative analysis of the BA pool which did not allow for a precise determination of single BA concentrations and their respective variations. The LC-MS/MS method was developed and optimized on pure bile acid standards (BACSMLS, Sigma-Aldrich, France) aiming at performing an exhaustive analysis of 40 BA. We observed 16 BA, and their respective values were expressed as percentages to facilitate understanding and result comparison. However, these percentages should not be used as reference values for future studies. While focusing on specific BA may be of interest from a mechanistic standpoint, the overall BA phenotype may offer more informative insights for assessing IRI profile compared to individual BA8.
In conclusion, in a porcine model of liver ischemia-reperfusion injury, we successfully identified 16 BA in bile samples after 2 h of ex-situ warm reperfusion. We observed significant changes in the BA pool induced by liver graft IRI, reflecting both the liver protective adaptive response and the extent of liver injury. Second, HOPE-treated liver grafts exhibited a more protective BA phenotype characterized by a shift towards a more hydrophilic BA pool and a significant reduction of GlycoCholic Acid (GCA). Notably, The HOPE BA phenotype was associated with a lower overall liver graft IRI profile compared to SCS. Future studies must validate the use of BA phenotype as a liver graft injury marker by correlating the latter to relevant clinical outcomes. Another future application of the presented data is the analysis of BA homeostasis during ex-situ machine perfusion to refine graft viability assessment prior to LT.
Data availability
The datasets generated and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Hartog, H., Hann, A. & Perera, M. T. P. R. Primary nonfunction of the liver allograft. Transplantation 106, 117 (2022).
Buis, C. I., Hoekstra, H., Verdonk, R. C. & Porte, R. J. Causes and consequences of ischemic-type biliary lesions after liver transplantation. J. Hepatobiliary. Pancreat. Surg. 13, 517–524 (2006).
Croome, K. P. et al. Classification of distinct patterns of ischemic cholangiopathy following DCD liver transplantation: Distinct clinical courses and long-term outcomes from a multicenter cohort. Transplantation 106, 1206 (2022).
Matton, A. P. M. et al. Biliary bicarbonate, pH, and glucose are suitable biomarkers of biliary viability during ex situ normothermic machine perfusion of human donor livers. Transplantation 103, 1405–1413 (2019).
Brüggenwirth, I. M. A., Porte, R. J. & Martins, P. N. Bile composition as a diagnostic and prognostic tool in liver transplantation. Liver Transplant. 26, 1177–1187 (2020).
Perino, A., Demagny, H., Velazquez-Villegas, L. & Schoonjans, K. Molecular physiology of bile acid signaling in health, disease, and aging. Physiol. Rev. 101, 683–731 (2021).
Bidault-Jourdainne, V. et al. TGR5 controls bile acid composition and gallbladder function to protect the liver from bile acid overload. JHEP Rep. 3, 100214 (2021).
Merlen, G. et al. Bile acids and their receptors during liver regeneration: ‘Dangerous protectors’. Mol. Aspects Med. 56, 25–33 (2017).
Xie, A.-J., Mai, C.-T., Zhu, Y.-Z., Liu, X.-C. & Xie, Y. Bile acids as regulatory molecules and potential targets in metabolic diseases. Life Sci. 287, 120152 (2021).
Fickert, P. & Wagner, M. Biliary bile acids in hepatobiliary injury—What is the link?. J. Hepatol. 67, 619–631 (2017).
Schlegel, A., Porte, R. J. & Dutkowski, P. Protective mechanisms and current clinical evidence of hypothermic oxygenated machine perfusion (HOPE) in preventing post-transplant cholangiopathy. J. Hepatol. 76, 1330–1347 (2022).
Schlegel, A. & Dutkowski, P. Impact of machine perfusion on biliary complications after liver transplantation. Int. J. Mol. Sci. 19, 3567 (2018).
Di Ciaula, A. et al. Bile acid physiology. Ann. Hepatol. 16, S4–S14 (2017).
Schlegel, A. et al. A multicenter randomized-controlled trial of hypothermic oxygenated perfusion (HOPE) for human liver grafts before transplantation. J. Hepatol. 78, 783–793 (2023).
Nasralla, D. et al. A randomized trial of normothermic preservation in liver transplantation. Nature 557, 50–56 (2018).
van Rijn, R. et al. Hypothermic machine perfusion in liver transplantation—a randomized trial. N. Engl. J. Med. 384(15), 1391–1401 (2021).
Panconesi, R. et al. Viability assessment in liver transplantation—what is the impact of dynamic organ preservation?. Biomedicines 9, 161 (2021).
Watson, C. J. E. & Jochmans, I. From ‘gut feeling’ to objectivity: Machine preservation of the liver as a tool to assess organ viability. Curr. Transplant. Rep. 5, 72–81 (2018).
Brüggenwirth, I. M. A. et al. Extended hypothermic oxygenated machine perfusion enables ex situ preservation of porcine livers for up to 24 hours. JHEP Rep. 2, 100092 (2020).
Muller, X. et al. A single preservation solution for static cold storage and hypothermic oxygenated perfusion of marginal liver grafts: A preclinical study. Transplantation. 108(1), 175–183 (2023).
Schlegel, A., de Rougemont, O., Graf, R., Clavien, P.-A. & Dutkowski, P. Protective mechanisms of end-ischemic cold machine perfusion in DCD liver grafts. J. Hepatol. 58, 278–286 (2013).
van Golen, R. F., van Gulik, T. M. & Heger, M. Mechanistic overview of reactive species-induced degradation of the endothelial glycocalyx during hepatic ischemia/reperfusion injury. Free Radic. Biol. Med. 52, 1382–1402 (2012).
van Leeuwen, O. B. et al. Sequential hypothermic and normothermic machine perfusion enables safe transplantation of high-risk donor livers. Am. J. Transplant. 22, 1658–1670 (2022).
Watson, C. J. E. et al. Predicting early allograft function after normothermic machine perfusion. Transplantation 106, 2391–2398 (2022).
Suzuki, S., Toledo-Pereyra, L. H., Rodriguez, F. J. & Cejalvo, D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation 55, 1265–1272 (1993).
Sosa, R. A. et al. Early cytokine signatures of ischemia/reperfusion injury in human orthotopic liver transplantation. JCI Insight 1, e89679 (2016).
op den Dries, S. et al. Injury to peribiliary glands and vascular plexus before liver transplantation predicts formation of non-anastomotic biliary strictures. J. Hepatol. 60, 1172–1179 (2014).
Karimian, N., Weeder, P. D., Bomfati, F., Gouw, A. S. H. & Porte, R. J. Preservation injury of the distal extrahepatic bile duct of donor livers is representative for injury of the intrahepatic bile ducts. J. Hepatol. 63, 284–287 (2015).
Wold, S., Esbensen, K. & Geladi, P. Principal component analysis. Chemom. Intell. Lab. Syst. 2, 37–52 (1987).
Brereton, R. G. & Lloyd, G. R. Partial least squares discriminant analysis: Taking the magic away. J. Chemom. 28, 213–225 (2014).
Mehmood, T., Liland, K. H., Snipen, L. & Sæbø, S. A review of variable selection methods in partial least squares regression. Chemom. Intell. Lab. Syst. 118, 62–69 (2012).
Maitra, S. & Yan, J. Principle component analysis and partial least squares: Two dimension reduction techniques for regression. Appl. Multivar. Stat. Models 79, 79–90 (2008).
de Jong, I. E. M. et al. Persistent biliary hypoxia and lack of regeneration are key mechanisms in the pathogenesis of posttransplant nonanastomotic strictures. Hepatol. Baltim. Md 75, 814–830 (2022).
de Jong, I. E. M. et al. Restoration of bile duct injury of donor livers during ex situ normothermic machine perfusion. Transplantation. 107(6), 161–172 (2023).
Brüggenwirth, I. M. A., van Leeuwen, O. B., Porte, R. J. & Martins, P. N. The emerging role of viability testing during liver machine perfusion. Liver Transplant. 28, 876–886 (2022).
Martin, J. L. et al. Succinate accumulation drives ischaemia-reperfusion injury during organ transplantation. Nat. Metab. 1, 966–974 (2019).
Hohenester, S. et al. A biliary HCO3—umbrella constitutes a protective mechanism against bile acid-induced injury in human cholangiocytes. Hepatology 55, 173–183 (2012).
Schlegel, A. et al. Hypothermic oxygenated perfusion protects from mitochondrial injury before liver transplantation. EBioMedicine 60, 103014 (2020).
Hoekstra, H. et al. Bile salt toxicity aggravates cold ischemic injury of bile ducts after liver transplantation in Mdr2+/− mice. Hepatology 43, 1022–1031 (2006).
Yska, M. J. et al. The role of bile salt toxicity in the pathogenesis of bile duct injury after non-heart-beating porcine liver transplantation. Transplantation 85, 1625 (2008).
Sayin, S. I. et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 17, 225–235 (2013).
González-Rubio, S. et al. GCDCA down-regulates gene expression by increasing Sp1 binding to the NOS-3 promoter in an oxidative stress dependent manner. Biochem. Pharmacol. 96, 39–51 (2015).
Lake, A. D. et al. Decreased hepatotoxic bile acid composition and altered synthesis in progressive human nonalcoholic fatty liver disease. Toxicol. Appl. Pharmacol. 268, 132–140 (2013).
Ma, Z. et al. Serum metabolome and targeted bile acid profiling reveals potential novel biomarkers for drug-induced liver injury. Med. (Baltim.) 98, 16717 (2019).
Zheng, X. et al. Hyocholic acid species improve glucose homeostasis through a distinct TGR5 and FXR signaling mechanism. Cell Metab. 33, 791-803.e7 (2021).
Chen, T. et al. Altered bile acid glycine: Taurine ratio in the progression of chronic liver disease. J. Gastroenterol. Hepatol. 37, 208–215 (2022).
Dotan, M. et al. Periductal bile acid exposure causes cholangiocyte injury and fibrosis. PloS ONE 17, e0265418 (2022).
Vilca Melendez, H., Rela, M., Setchell, K. D. R., Murphy, G. M. & Heaton, N. D. Bile acids analysis: A tool to assess graft function in human liver transplantation. Transpl. Int. 17, 286–292 (2004).
Van Nieuwkerk, C. M. et al. Effects of ursodeoxycholate and cholate feeding on liver disease in FVB mice with a disrupted mdr2 P-glycoprotein gene. Gastroenterology 111, 165–171 (1996).
Hessheimer, A. J., Vengohechea, J. & Fondevila, C. Metabolomic analysis, perfusate composition, and pseudo-physiology of the isolated liver during ex situ normothermic machine perfusion. Transplantation. 107(5), 125–126 (2023).
Gillard, J. et al. Bile acids contribute to the development of non-alcoholic steatohepatitis in mice. JHEP Rep. Innov. Hepatol. 4, 100387 (2022).
Acknowledgements
The authors would like to thank the entire team from the Ecole de Chirurgie de Lyon for their support.
Funding
This study was co-funded by a research grant of the Prix Poncet of the Surgical Society of Lyon as well as an industry partnership grant with the Institute Georges Lopez, France.
Author information
Authors and Affiliations
Contributions
G.R. and X.M.: *contributed equally as first authors, designed the study, acquired the data, performed the statistical analysis, interpreted the data and wrote the manuscript. T.B. and Y.C. performed the statistical analysis. V.H. performed the histological analysis and critically reviewed the data. V.B. critically reviewed the data. J.Y.M., K.M., S.A. and A.S. designed the study, interpreted the data, critically reviewed the data and drafted a final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rossignol, G., Muller, X., Brunet, T.A. et al. Comprehensive bile acid pool analysis during ex-vivo liver perfusion in a porcine model of ischemia-reperfusion injury. Sci Rep 14, 2384 (2024). https://doi.org/10.1038/s41598-024-52504-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-52504-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.