Abstract
Lipid metabolism plays a key role in erectile dysfunction. Our purpose was to evaluate the influence of lipid-lowering drugs on erectile dysfunction employing a two-sample Mendelian randomization (MR) study. Genetic instruments were employed to represent the exposure of lipid-lowering drugs. Inverse variance-weighted MR (IVWMR) was employed to calculate the estimation of effects. IVW-MR analysis showed that the positive relationship between the expression of HMGCR and the risk of erectile dysfunction (odds ratio [OR] = 1.27, 95% confidence interval [CI] 1.03–1.57; p = 0.028). No significant relationship was detected between NPC1L1, PSK9 expression and erectile dysfunction. This MR study suggested that HMGCR inhibitors are a more desirable treatment modality for patients with ED.
Similar content being viewed by others
Introduction
Erectile dysfunction (ED), defined as the inability to develop or maintain a penile erection during sexual activity, is a common disorder in men. The prevalence increases with age, with a 70% prevalence at age 70 years1. Research has reported an overall ED prevalence of 49.69% in mainland China2, and an increasing number of men under the age of 40 are suffering from ED3. ED can be divided into three categories based on etiology: psychological, organic, and mixed. More than half of ED patients have organic changes4. Because the penis has a special vascular network, vascular factors, especially the status of the penile arteries, are more important in organic ED5. Atherosclerosis, endothelial dysfunction and inflammation, which are pathological factors affecting vascular function, can lead to atherogenic ED6. Therefore ED is also considered as a manifestation of systemic atherosclerosis7.
Dyslipidemia is an important risk factor for endothelial dysfunction8. The low-density lipoprotein (LDL) is considered one of the strongest predictors of atherosclerosis and endothelial dysfunction, and lowering LDL is essential for the prevention and treatment of atherosclerosis9. LDL mediates atherosclerosis by entering the vessel wall through endothelial cells, leading to oxidation of LDL by endothelial cells, which in turn leads to endothelial dysfunction10. LDL elevation can cause endothelial dysfunction in the penile arteries of mice and further induce the development of ED11. Due to the small average diameter of penile arteries, a decrease in blood flow is more likely to occur in the presence of plaque. Moreover, erection of the penis requires a lot of dilation and dysfunction of the vascular endothelium in the cavernous vascular bed has a great impact on the cavernous arteries6. Thus, lipid abnormalities are an important cause of ED.
ED is often accompanied by cardiovascular disease, and dyslipidemia is an important risk factor for cardiovascular disease. Therefore, the use of lipid-lowering drugs has gradually increased in ED patients12. 3-Hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA reductase (HMGCR)) is the rate-limiting enzyme in the valproic acid pathway and is responsible for the synthesis of cholesterol and steroid hormones, and HMG-CoA inhibitors (statins) are commonly used clinically as lipid-lowering drugs to reduce circulating total cholesterol and low-density lipoprotein cholesterol (LDL-C) levels13,14,15. Clinical two other drugs approved for common use that target cholesterol metabolism include ezetimibe (targeting Niemann-pickc1-like protein (NPC1L1)) and proprotein convertase kwashiorkor/kexin type 9 (PCSK9) inhibitors. They enhance LDL-C uptake by reducing intestinal absorption of cholesterol or by increasing cell membrane expression of the ldl receptor (LDLR)16. However, the effect of lipid-lowering drugs on erectile function in patients with or without ED is not known.
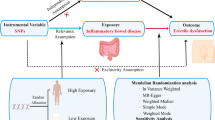
Mendelian randomization (MR) analysis utilizes the inherent properties of common genetic variants to provide a causal validation method that is not susceptible to social, environmental, and behavioral factors17, and has become a common way to explore potential causal relationships between exposure factors and disease18. This article explores the relationship between ED's and three different targets of lipid-lowering drugs using two sample pools: 3-hydroxy-3 methylglutaryl-CoA reductase (HMGCR) inhibitors, Niemann-Pick C1-like 1 (NPC1L1) inhibitors, and PCSK9 inhibitors.
Materials and method
Research methodology
This two-sample MR analysis is predicated upon the utilization of open-access, anonymized, aggregate data extracted from previously conducted Genome-Wide Association Studies (GWASs) (Table 1). All foundational investigations acquired ethical clearance from their respective Institutional Review Boards, with relevant references duly noted, and participating individuals provided informed consent.
In our study, we diligently followed three key assumptions when selecting instrumental variables (IVs) for MR analysis: (1) The SNP (instrument) is correlated with the exposure (lipid-lowering drugs). (2) The SNP is independent of any confounders between the exposure and outcome. 3) The SNP influences the outcome (ED) solely via its relationship with the exposure.
Genetic instrument selection
This investigation encompassed three classifications of FDA-endorsed lipid-lowering pharmaceuticals as exposure factors: HMGCR inhibitors, PCSK9 inhibitors, and the NPC1L1 inhibitor.
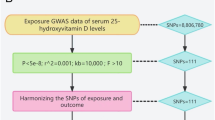
To corroborate the associations discerned through the employment of eQTLs as genetic instruments, we adopted a strategy that involved selecting SNPs situated within 100 kb windows surrounding each drug's target gene. These SNPs were associated with LDL cholesterol concentrations at a genome-wide significance threshold (p < 5.0 × 10−8) and served as proxies for exposure to lipid-lowering medications. The GWAS summary data related to LDL cholesterol levels, procured from the Global Lipids Genetics Consortium (GLGC) and comprising a sample size of 173,082 participants, was employed to pinpoint these SNPs19 (Supplementary Table S1). The screening flowsheet is shown in Fig. 1.
The selection of these genetic instruments was based on their association with LDL cholesterol concentrations at a genome-wide significance threshold (p < 5.0 × 10–8). This criterion ensures that the selected SNPs are robustly associated with the exposure. While each individual SNP might explain a small fraction of the variance in LDL concentration, the combined set of SNPs, especially considering their strong statistical association with LDL cholesterol (evidenced by their p-values), ensures a reliable genetic instrument.
For HMGCR inhibitors, seven prevalent SNPs (MAF > 1%) were pinpointed within ± 100 kb windows encompassing the HMGCR region, exhibiting low linkage disequilibrium (r2 < 0.30) and a significant association (p < 5.0 × 10−8). Similarly, PCSK9 inhibitors had twelve common SNPs, and NPC1L1 inhibitors had three common SNPs, meeting the same criteria within ± 100 kb windows of their respective regions.
The statistical evaluations comprised a primary analysis utilizing inverse-variance-weighted MR (IVW-MR), in addition to sensitivity analyses encompassing the F-Statistic, positive control assessment, linkage disequilibrium assessment, horizontal pleiotropy examination, heterogeneity testing, and MR-Egger regression.
Sources of outcome data
GWAS summary-level data concerning erectile dysfunction outcomes, sourced from the IEU GWAS database, which includes a sample size of 223,805, with 6175 cases and the remaining being controls20.
Statistical analysis
Primary MR analysis
When utilizing genetic variants associated with LDL cholesterol concentrations as instruments, the IVW-MR method was implemented to amalgamate effect estimates. Allele harmonization and subsequent analysis were conducted using version 4.2.3 of the TwoSampleMR package within the R software environment.
Sensitivity analysis
We evaluated the strength of the SNPs used as instruments by calculating the F-statistic, including only SNPs with an F-statistic greater than 10 in order to mitigate the potential for weak instrument bias21. To validate both genetic instruments, we performed positive control analyses. Given that lipid-lowering drugs are known to effectively reduce LDL cholesterol levels, we investigated the relationship between the exposures of interest and LDL cholesterol levels as a positive control study for the eQTLs-based instruments. In the case of the instrument derived from the LDL cholesterol GWAS, we carried out a positive control study by examining the association between the exposures of interest and coronary heart disease. This was done because coronary heart disease is the primary indication for lipid-lowering medications.
In the IVW-MR method, we evaluated heterogeneity using the Cochran Q test, wherein a p-value below 0.05 indicates the presence of heterogeneity22. To assess the potential for horizontal pleiotropy among the SNPs utilized as instrumental variables, we utilized MR-Egger regression and Mendelian Randomization Pleiotropy RESidual Sum and Outlier (MR-PRESSO) analysis. In MR-Egger regression, the intercept term serves as a valuable indicator for assessing directional horizontal pleiotropy, whereby a p-value below 0.05 suggests the presence of horizontal pleiotropy23. MR-PRESSO analysis can identify horizontal pleiotropic outliers and offer adjusted estimates, with a p-value below 0.05 for the Global test indicating the presence of horizontal pleiotropic outliers24. All of the aforementioned analyses were conducted utilizing R software, version 4.2.3.
Results
SNP selection and validation
Using the ED data from the IEU GWAS database, we selected specific SNPs to proxy LDL lowering through the inhibition of HMGCR, NPC1L1, and PCSK9. Specifically: For HMGCR, we identified 7 SNPs to proxy HMG-CoA reductase inhibition: rs10066707, rs10515198, rs12659791, rs12916, rs3804231, rs3857388, rs72633962. For NPC1L1, 3 SNPs were selected to proxy its inhibition: rs2073547, rs217386, and rs7791240. For PCSK9, 12 SNPs were chosen to proxy its inhibition: rs10493176, rs11206510, rs11206514, rs11583974, rs11591147, rs12067569, rs2479394, rs2479409, rs2495495, rs4927193, rs572512, and rs585131 (Supplementary Table S1). Moreover, Supplementary Table S2 results showed that a significant relationship between genetically proxied drug targets and CAD, which were regarded as the positive control analyses, assuring the efficacy of the genetic instruments.
Analysis using the two-sample MR
IVW-MR analysis results showed that the positive relationship between the expression of HMGCR and the risk of erectile dysfunction (odds ratio [OR] = 1.27, 95% confidence interval [CI] 1.03–1.57; p = 0.028), suggesting that lower HMGCR expression reduced the risk of erectile dysfunction (Fig. 2 and Table 2). No significant relationship was detected between NPC1L1, PSK9 expression and erectile dysfunction.
Sensitivity analysis
A lack of causal association remained in all sensitivity analyses (all p > 0.05). Likewise, there was no clear evidence of heterogeneity (all p > 0.05) or pleiotropy (all p values for intercept > 0.05) regarding HMGCR, NPC1L1 and PCSK9 in erectile dysfunction and positive control coronary heart disease (Table 3). Moreover, the leave-one-out analysis plot results certified that the above results were unchanged by the removing of any SNP and were quite robust (Fig. 3).
Discussion
In the present study, increased HMGCR gene expression was associated with an increased risk of erectile dysfunction, suggesting that HMGCR inhibitors may reduce the risk of erectile dysfunction. The expression of NPC1L1 and PCSK9 was not significantly associated with ED.
Statins are the most commonly used HMGCR inhibitors in clinical practice and play an important role in lowering LDL and cholesterol and slowing the progression of atherosclerosis25. Increased production of LDL receptors is thought to be the main mechanism driving the action of statins26. Statins also have an ameliorating effect on platelet and vascular endothelial function27.However, statins still have some side effects, including statin-related muscle symptoms, new-onset type 2 diabetes, neurological and neurocognitive effects28.
Statins may improve penile erectile function in several ways; a reduction in LDL may improve endothelial function, and good endothelial function is important for penile erection. Moreover, statins may increase the availability of nitric oxide, which plays a key regulatory role in penile erection and resistance to oxidative stress. However, statins may also impair penile erectile function because they block reductase in the early stages of cholesterol biosynthesis, thereby reducing testosterone formation29. Testosterone is closely associated with sexual behavior, and it enhances the overall male sexual response30. In patients with reduced testosterone levels, their sexual desire and number of morning erections are significantly reduced31.
However, the effect of statins on ED in men remains controversial in current clinical studies. In 1812 men treated with statins, no association between statin use and the development of ED was found by testing testosterone and luteinizing hormone32. In contrast, in a study by BRUCKERT et al. patients who were treated with statins were more likely to suffer from ED33. And in another individual study, it was also found that some patients experienced decreased libido and significantly lower testosterone after taking different types of statins34. These opposite findings may be due to various differences in the age of initiation of statin therapy, the dose used, and the type of their underlying disease.
As for NPC1L1 and PCSK9 inhibitors, there was no significant correlation between them and ED in this study. We consider that on the one hand, it is because the lipid-lowering effect of NPC1L1 inhibitors is not as strong as that of statins; and PCSK9 inhibitors, although they have a strong lipid-lowering effect, have a low bioavailability and are expensive, which leads to the fact that both of them are usually used only by patients with poor lipids35,36. This problem of poor lipids may make the protective effect of NPC1L1 and PCSK9 inhibitors on ED not significant. On the other hand, the inhibition of the cholesterol biosynthesis process by statins may inhibit multiple actions of downstream products together, which may also be a factor in the ability of statins to exert ED protection, but this needs to be verified by more subsequent studies.
Strengths and limitations
(1) Participants of European ancestry only were enrolled in the study; future studies of populations of other ethnicities are needed to test the generalizability of the current study's findings. (2) We utilized MR to reduce confounding in studying ED's association with lipid-lowering drugs. While stringent SNP selection and sensitivity analyses bolster our study, it's essential to acknowledge that potential biases, like horizontal pleiotropy, can't be fully eliminated. (3) Concerns arose over potential overlaps between IEU GWAS and our eQTL-derived GWASs. Despite verifying distinct cohorts, minor overlaps are possible. This might introduce biases, but our MR-Egger and MR-PRESSO analyses aim to control such biases. (4) Our MR analysis carefully chooses SNPs based on proximity to drug target genes and LDL cholesterol significance. While methods like the Cochran Q test help, the intrinsic variability remains. Despite robust techniques like IVW-MR and MR-Egger regression, potential biases from SNP variability persist. While our results offer valuable insights, they warrant cautious interpretation, suggesting future research could benefit from expanded SNP criteria for enhanced robustness.
Conclusion
In the present study, we reduced the risk of confounding by a Mendelian randomization study and found that HMGCR gene expression was positively associated with ED occurrence, whereas NPC1L1 and PCSK9 gene expression did not correlate with ED occurrence. It is suggested that the use of HMGCR inhibitors may reduce the occurrence of ED and that HMGCR inhibitors are a more desirable treatment modality for patients with cardiovascular disease combined with ED.
Data availability
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
References
Schouten, B. W. et al. Erectile dysfunction in the community: trends over time in incidence, prevalence, GP consultation and medication use—the Krimpen study: trends in ED. J. Sex Med. 7, 2547–2553. https://doi.org/10.1111/j.1743-6109.2010.01849.x (2010).
Wang, W. et al. Meta-analysis of prevalence of erectile dysfunction in mainland China: evidence based on epidemiological surveys. Sex. Med. 5, e19–e30. https://doi.org/10.1016/j.esxm.2016.10.001 (2017).
Nguyen, H. M. T., Gabrielson, A. T. & Hellstrom, W. J. G. Erectile dysfunction in young men-a review of the prevalence and risk factors. Sex Med. Rev. 5, 508–520. https://doi.org/10.1016/j.sxmr.2017.05.004 (2017).
Li, M., Ma, Z., Zhang, X. L., Guo, L. Q. & Yuan, M. Z. Significance of blood lipid parameters as effective markers for arteriogenic erectile dysfunction. Andrology 8, 1086–1094. https://doi.org/10.1111/andr.12776 (2020).
Tal, R. et al. Vasculogenic erectile dysfunction in teenagers: A 5-year multi-institutional experience. BJU Int. 103, 646–650. https://doi.org/10.1111/j.1464-410X.2008.08037.x (2009).
Gandaglia, G. et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur. Urol. 65, 968–978. https://doi.org/10.1016/j.eururo.2013.08.023 (2014).
Shiri, R. et al. Cardiovascular drug use and the incidence of erectile dysfunction. Int. J. Impot. Res. 19, 208–212. https://doi.org/10.1038/sj.ijir.3901516 (2007).
Ghosh, A., Gao, L., Thakur, A., Siu, P. M. & Lai, C. W. K. Role of free fatty acids in endothelial dysfunction. J. Biomed. Sci. 24, 50. https://doi.org/10.1186/s12929-017-0357-5 (2017).
Di Angelantonio, E. et al. Lipid-related markers and cardiovascular disease prediction. JAMA 307, 2499–2506. https://doi.org/10.1001/jama.2012.6571 (2012).
Castelli, W. P., Anderson, K., Wilson, P. W. & Levy, D. Lipids and risk of coronary heart disease: The Framingham Study. Ann. Epidemiol. 2, 23–28. https://doi.org/10.1016/1047-2797(92)90033-m (1992).
Skålén, K. et al. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature 417, 750–754. https://doi.org/10.1038/nature00804 (2002).
Kostis, J. B. & Dobrzynski, J. M. The effect of statins on erectile dysfunction: A meta-analysis of randomized trials. J. Sex Med. 11, 1626–1635. https://doi.org/10.1111/jsm.12521 (2014).
Hu, M., Cheung, B. M. & Tomlinson, B. Safety of statins: An update. Ther. Adv. Drug. Saf. 3, 133–144. https://doi.org/10.1177/2042098612439884 (2012).
Audi, S. et al. The “top 100” drugs and classes in England: An updated “starter formulary” for trainee prescribers. Br. J. Clin. Pharmacol. 84, 2562–2571. https://doi.org/10.1111/bcp.13709 (2018).
Istvan, E. S. & Deisenhofer, J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science 292, 1160–1164. https://doi.org/10.1126/science.1059344 (2001).
Ference, B. A. et al. Association of genetic variants related to CETP inhibitors and statins with lipoprotein levels and cardiovascular risk. JAMA 318, 947–956. https://doi.org/10.1001/jama.2017.11467 (2017).
Evans, D. M. & Davey Smith, G. Mendelian randomization: New applications in the coming age of hypothesis-free causality. Annu. Rev. Genomics Hum. Genet. 16, 327–350. https://doi.org/10.1146/annurev-genom-090314-050016 (2015).
Long, Y., Tang, L., Zhou, Y., Zhao, S. & Zhu, H. Causal relationship between gut microbiota and cancers: A two-sample Mendelian randomisation study. BMC Med. 21, 66. https://doi.org/10.1186/s12916-023-02761-6 (2023).
Willer, C. J. et al. Discovery and refinement of loci associated with lipid levels. Nat. Genet. 45, 1274 (2013).
Bovijn, J. et al. GWAS identifies risk locus for erectile dysfunction and implicates hypothalamic neurobiology and diabetes in etiology. Am. J. Hum. Genet. 104, 157–163. https://doi.org/10.1016/j.ajhg.2018.11.004 (2019).
Burgess, S., Thompson, S. G. & Collaboration, C. C. G. Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 40, 755–764 (2011).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003).
Burgess, S. & Thompson, S. G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 32, 377–389 (2017).
Verbanck, M., Chen, C.-Y., Neale, B. & Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 50, 693–698 (2018).
Dagli-Hernandez, C. et al. Pharmacogenomics of statins: lipid response and other outcomes in Brazilian cohorts. Pharmacol. Rep. 74, 47–66. https://doi.org/10.1007/s43440-021-00319-y (2022).
Goldstein, J. L. & Brown, M. S. A century of cholesterol and coronaries: From plaques to genes to statins. Cell 161, 161–172. https://doi.org/10.1016/j.cell.2015.01.036 (2015).
Kumai, T. et al. Pleiotropic effects of 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors: Candidate mechanisms for anti-lipid deposition in blood vessels. Curr. Med. Chem. Cardiovasc. Hematol. Agents 3, 195–201. https://doi.org/10.2174/1568016054368223 (2005).
Ward, N. C., Watts, G. F. & Eckel, R. H. Statin toxicity. Circ. Res. 124, 328–350. https://doi.org/10.1161/circresaha.118.312782 (2019).
Kostis, J. B. & Dobrzynski, J. M. Statins and erectile function. Can. J. Cardiol. 34, 813.e815. https://doi.org/10.1016/j.cjca.2018.02.014 (2018).
Rastrelli, G., Corona, G. & Maggi, M. Testosterone and sexual function in men. Maturitas 112, 46–52. https://doi.org/10.1016/j.maturitas.2018.04.004 (2018).
Rastrelli, G., Corona, G., Tarocchi, M., Mannucci, E. & Maggi, M. How to define hypogonadism? Results from a population of men consulting for sexual dysfunction. J. Endocrinol. Invest. 39, 473–484. https://doi.org/10.1007/s40618-015-0425-1 (2016).
Hall, S. A. et al. Do statins affect androgen levels in men? Results from the Boston area community health survey. Cancer Epidemiol. Biomark. Prev. 16, 1587–1594. https://doi.org/10.1158/1055-9965.Epi-07-0306 (2007).
Bruckert, E., Giral, P., Heshmati, H. M. & Turpin, G. Men treated with hypolipidaemic drugs complain more frequently of erectile dysfunction. J. Clin. Pharm. Ther. 21, 89–94. https://doi.org/10.1111/j.1365-2710.1996.tb00006.x (1996).
de Graaf, L., Brouwers, A. H. & Diemont, W. L. Is decreased libido associated with the use of HMG-CoA-reductase inhibitors?. Br. J. Clin. Pharmacol. 58, 326–328. https://doi.org/10.1111/j.1365-2125.2004.02128.x (2004).
Ali, A. H. et al. Lipid-lowering therapies for atherosclerosis: Statins, fibrates, ezetimibe and PCSK9 monoclonal antibodies. Curr. Med. Chem. 28, 7427–7445. https://doi.org/10.2174/0929867328666210222092628 (2021).
Gallego-Colon, E., Daum, A. & Yosefy, C. Statins and PCSK9 inhibitors: A new lipid-lowering therapy. Eur. J. Pharmacol. 878, 173114. https://doi.org/10.1016/j.ejphar.2020.173114 (2020).
Funding
This work was supported by Hunan Provincial Natural Science Foundation-Youth Fund Project (Nos. 2023JJ40972, 2023JJ41029).
Author information
Authors and Affiliations
Contributions
Study design: L.Q.L. Data collection, analysis and interpretation: Q.Z., Y.T., and X.Y.Z. Writing and review of the manuscript: Q.Z., L.Q.L., and X.Y.Z.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, Q., Tan, Y., Zou, X. et al. Association of high LDL concentrations with erectile dysfunction from a Mendelian randomization study. Sci Rep 13, 22252 (2023). https://doi.org/10.1038/s41598-023-49771-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-49771-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.