Abstract
Brown seaweeds have a rich bioactive content known to modulate biological processes, including the mucosal immune response and microbiota function, and may therefore have the potential to control enteric pathogens. Here, we tested if dietary seaweed (Saccharina latissima) supplementation could modulate pig gut health with a specific focus on parasitic helminth burdens, gut microbiota composition, and host immune response during a five week feeding period in pigs co-infected with the helminths Ascaris suum and Oesophagostomum dentatum. We found that inclusion of fermented S. latissima (Fer-SL) at 8% of the diet increased gut microbiota α-diversity with higher relative abundances of Firmicutes, Tenericutes, Verrucomicrobia, Spirochaetes and Elusimicrobia, and lower abundance of Prevotella copri. In the absence of helminth infection, transcription of immune-related genes in the intestine was only moderately influenced by dietary seaweed. However, Fer-SL modulated the transcriptional response to infection in a site-specific manner in the gut, with an attenuation of infection-induced gene expression in the jejunum and an amplification of gene expression in the colon. Effects on systemic immune parameters (e.g. blood lymphocyte populations) were limited, indicating the effects of Fer-SL were mainly localized to the intestinal tissues. Despite previously documented in vitro anti-parasitic activity against pig helminths, Fer-SL inclusion did not significantly affect parasite egg excretion or worm establishment. Collectively, our results show that although Fer-SL inclusion did not reduce parasite burdens, it may modify the gut environment during enteric parasite infection, which encourages continued investigations into the use of seaweeds or related products as novel tools to improve gut health.
Similar content being viewed by others
Introduction
Brown seaweeds are marine macroalgae with bioactive-rich content such as the polysaccharides fucoidan and laminarin (up to > 10% dry matter in some species)1. Brown seaweeds (typically at a dietary inclusion rate of 1–2%) and their compounds may provide a range of health benefits for pigs, such as prebiotic and immune-enhancing effects, increased average daily gain, improved digestibility, and stimulation of beneficial bacteria in the gut2,3,4. Effects of dietary brown seaweeds or their extracts on the porcine gut microbiota (GM) vary depending on the brown seaweed type, seaweed extracts, and the use of extracts alone or in combination. Brown seaweeds can modulate the GM by increasing the abundance of Lactobacillus spp. and reducing E. coli5,6. Likewise, a diet containing the brown seaweed compounds laminarin and fucoidan alone reduces Enterobacterium spp. and increases Lactobacilli spp. in pigs, respectively, yet these effects were not apparent when laminarin and fucoidan were used in combination7,8. However, it has also been shown that dietary inclusion (0.15% of dietary intake), of two different brown seaweed extracts containing both laminarin and fucoidan reduce enterobacteria, bifidobacteria or lactobacilli populations in pigs regardless of their use alone or in combination9. In addition to modulating the GM, dietary seaweed supplementation has also been reported to exert modulatory activity on the host immune system, for example by increasing IgG production, upregulating intestinal mucin, MUC2 transcription, and lowering pro-inflammatory gene expression7,10,11.
Helminth infections are common in livestock, and the interaction between helminths and GM can be critical for the health of the host12. There appears to be a complex relationship between helminth infection, GM, and the immune system, but how such interactions can be modulated by bioactive dietary compounds remains unclear in many cases13. Moreover, whether manipulation of the gut environment by dietary seaweed inclusion can be a novel treatment for helminth infections is unclear, and is of increasing importance as resistance to conventional anthelmintic drugs is widespread14.
In the last two decades, anthelmintic resistance in the porcine nodular worm (Oesophagostomum spp.) against all the major drug classes has developed in Europe15,16,17,18,19. For this reason, a number of alternative helminth control options such as probiotics or bioactive forages have been investigated in pigs, with the inclusion of plants such as chicory in the diet a promising tool to lower worm burdens20,21. In ruminants, where anthelmintic resistance is even more prevalent, many bioactive forages with anti-parasitic properties such as chicory, sainfoin, and birdsfoot trefoil have been demonstrated as a viable alternative option for control of helminth infections22,23.
Fermentation technology may be applied to increase both the bioactive properties of plants and plant medicines24,25 and the nutrient digestibility of feed26,27. Recently, we demonstrated that extracts from fermented and non-fermented brown seaweeds, Saccharina latissima and Laminaria digitata had potent in vitro activity against Ascaris suum infective larvae (L3)28. These findings encouraged the present in vivo trials to determine whether inclusion of fermented seaweed (S. latissima) in the diet could reduce parasite burdens in pigs. Moreover, given the helminth-GM-immune system interaction and the anti-inflammatory and prebiotic properties of (brown) seaweed, we reasoned that seaweed supplementation may also alleviate infection-associated pathology and positively influence gut health. Thus, we investigated the effect of fermented S. latissima in pigs experimentally infected with A. suum and O. dentatum on possible anti-parasitic effects, alterations in GM composition, and modulatory effects on the immune system.
Experimental section
Feed
The seaweed S. latissima was provided by Nordic Seaweed Aps (NS), Grena, Denmark. It was harvested in Fjekkefjord Norway 2019 (Lot: ASNT210119) and fermented aerobically as previously described28. The same lot was used in the two studies described below, but the batches were fermented, frozen and dried four months apart. The inclusion of seaweed was a 1:1 substitution of 5% (w/w) of the basal diet in Study 1 of fermented (Fer-SL) and non-fermented S. latissima (Non-Fer-SL). In Study 2, the seaweed substitution was 8% (w/w) Fer-SL, and the total feed allocated to Fer-SL groups were adjusted to the metabolic energy of the groups fed control diet (Fer-SL groups were allocated 1% more feed, Table 1). Feeding was provided twice daily according to the norm for Danish pig production29 for both Study 1 and 2, and water was provided ad libitum.
Parasites
The batch of O. dentatum L3 (EH-strain) used for both studies was produced from larval faecal cultures and kept in tap water at 10 °C until use30. The A. suum eggs were isolated and embryonated as described previously28.
Animals
Study 1: Study 1 was designed as a preliminary investigation to assess palatability and potential indications of anti-parasitic activity. Nine castrated male pigs [Duroc x (Yorkshire x Landrace)] were obtained from a Danish certified specific pathogen-free (SPF) farm with no previous history of helminth infection. The pigs were ∼9 weeks of age and weighed 25.6 ± 2.5 kg (mean ± standard deviation (SD)) at arrival. All animals were helminth-free as confirmed by absence of helminth eggs in faeces and anti-Ascaris antibodies in serum. During the study, pigs were housed on a group-basis in solid concrete-floored pens with wood shavings. Although we acknowledge that co-housing may not be optimal for assessing gut microbiota changes, individual housing was not possible for practical and ethical reasons. Animal welfare checks were performed daily, and body weight and faecal consistency (scored 1–5 where 3 or above is diarrhoea) were recorded weekly. Separate tools and boots were used in each pen to prevent cross-contamination between groups.
Study 2: This study was designed as larger study to assess in more detail anti-parasitic effects as well as dietary effects on parasite-induced immunity and GM modulation. Thirty-two crossbred [Duroc x (Yorkshire x Landrace)] pigs (16 castrated males, 16 females) were purchased from the same provider listed above. The pigs were ∼9 weeks of age and weighed 26.0 ± 1.7 kg (mean ± SD) on arrival. The animals were housed and managed as in Study 1. All pigs were vaccinated against Lawsonia intracellularis with one dose of a live, attenuated vaccine (Enterisol® Ileitis, Boehringer Ingelheim, DK) on-farm 40 days prior to the first infection and confirmed helminth free by absence of eggs in faeces counts at arrival and serology for A. suum and O. dentatum by ELISA.
Experimental design
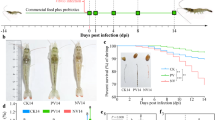
Study 1: The experiment was set up as a parallel study of three diets. After stratification for body weight, the pigs were randomly allocated into three groups (n = 3), housed in 5 m2 pens, with groups fed either control feed (C), control feed added Non-Fer-SL (NSL), or control feed added Fer-SL (FSL). All pigs were provided with their respective diets from arrival. Six days after arrival all animals were inoculated with 6000 O. dentatum third stage larvae (L3) (0 days post infection (dpi)), and 8 days later infected with 2000 A. suum infective eggs (8 dpi) by stomach tube. Twenty-two days post infection with O. dentatum (22 dpi), pigs were euthanized by captive bolt, followed by exsanguination and evisceration (Supplementary Figure 1).
Study 2: This was a 2 × 2 factorial study with diet and infection. After stratification for sex and body weight, the thirty-two pigs were randomly allocated into four groups (housed in 5 m2 pens): UC (uninfected pigs fed control diet), IC (infected pigs fed control diet), USL (uninfected pigs fed Fer-SL diet) and ISL (infected pigs fed Fer-SL diet). The diets were offered from arrival, and groups IC and ISL were one week later inoculated with 6000 O. dentatum L3 (0 dpi) and two weeks later with 2000 A. suum infective eggs (14 dpi), using a stomach tube. Twenty-eight days post infection with O. dentatum (28 dpi), pigs were euthanized (Supplementary Figure 2) as mentioned above. 14 days of A, suum infection was judged optimal to measure larval counts before the onset of larval expulsion which occurs around 17–21 days dpi31 as well as being a time point where strong immune responses and changes in GM composition have been detected in the gut tissues as result of infection32,33,34.
Parasitological techniques
Rectal faecal samples were collected weekly and examined for faecal nematode egg counts (FEC) of by a modified concentration McMaster technique, with an analytical sensitivity of 20 eggs per gram faeces (EPG)30. Additional samples were collected in Study 1 at 19–22 dpi, and for Study 2 at 17, 21, 24 and 28 dpi for FEC. In Study 2, hatching of O. dentatum eggs and larval development were quantified for faecal samples at 24 dpi. Faecal larval cultures of 5 g faeces were set up in triplicates for each infected animal and the L3 were harvested 18 days later30. The larval development percentage was calculated based on the total number of L3 recovered divided by the number of eggs in the faecal culture (i.e. EPG × 5 g).
At necropsy, livers were macroscopically examined for liver spots on the surfaces. The small intestine was rinsed with warm saline and a 50% subsample of the contents and washings embedded in agar (agar powder, product code 20768.292; VWR, Denmark) to isolate minute A. suum larvae35. The larvae were harvested using a 20 µm sieve after 3 h and preserved in 70% ethanol. The same procedure was applied for the large intestine (caecum and colon together) but only two 5% subsamples were embedded in agar and samples were harvested using a 38 µm sieve after 20 h36.
Immunology samples (Blood, PBMCs, CLN, Flow cytometry)
In both studies, blood samples were collected from pigs by jugular venipuncture using vacutainer serum separator tubes (BD Vacutainer ®, SSTTMII Advance) at arrival, 0 dpi, and at necropsy (22 or 28 dpi, for Study 1 and 2, respectively). After storage for 24 h at 5 °C, serum was separated by centrifugation (10 min at 1580 RCF) and stored at − 80 °C until use. At necropsy (28 dpi) in Study 2, blood was also collected in heparin-coated tubes (BD Vacutainer®) and PBMCs were isolated as described37. Cells were isolated from the ileo-caecal lymph nodes (CLN) of the pigs at necropsy. Flow cytometry was performed on both PBMC and CLN cells as described37. Ex vivo cell stimulation was performed on PBMCs to assess the production of inflammatory cytokines. Cells were stimulated for 24 h with Lipopolysaccharides (LPS) (1 µg/mL) and the supernatant was collected and frozen. Secreted tumour necrosis factor-α (TNF-α) and interleukin 1β (IL-1β) were quantified using commercial antibody pairs (Duosets; R&D systems; UK). An ELISA on Immunoglobulin G1 (IgG1) (Study 1) or total IgG (Study 2) levels in serum against A. suum adult body fluid (ABF) on samples at arrival and on day of necropsy. The ELISA was conducted as described37, utilizing mouse anti-pig IgG1 (clone K139 3C8; Bio-Rad, CA, USA) followed by goat anti-mouse IgG:HRP (Bio-Rad), or goat anti-pig IgG:HRP. Study 2 also included an ELISA on total IgG levels in serum against O. dentatum excretory/secretory (E/S) products on samples at arrival and on day of necropsy.
Tissue samples (Histology and Gene expression)
For Study 2 tissue samples were taken from the mid-point of the jejunum and proximal colon (20 cm distal of the ileo-caecal junction). The samples were fixed in 4% formaldehyde, paraffin-embedded, sectioned, and stained with Periodic Acid Schiff stain to evaluate goblet cells and villi/crypt ratios. For determination of goblet cell numbers, 5 random microscopy fields of each tissue section were counted by a single-blinded observer using a calibrated counting grid covering a total area of 0.25 mm2 at 200 × magnification. Cell counts were expressed as cells/mm2 tissue. For villous/crypt ratios, 5 well-orientated villus/crypt units were randomly selected from each tissue section by a blinded observer, imaged using a Leica DFC480 camera and measurements performed using LAS v4.6 software (Leica, Switzerland). Additional jejunum and colon tissue samples were collected for gene expression analysis. The samples were gently washed in PBS and stored in RNA later at − 20 °C until use. RNA was extracted from the samples using a miRNeasy Kit (Qiagen, Denmark) 37. The RNA concentration and purity were measured on a Nanodrop, RNA integrity was measured on a bioanalyzer and stored at − 80 °C until cDNA synthesis. cDNA synthesis of 500 ng total RNA was carried out using Qiagen QuantiTect Reverse Transcription Kit following the manufacturer’s protocol. The expression levels of a panel of genes of interest and reference genes were examined by microfluidic qPCR (BioMark, Fluidigm, San Francisco, CA, USA) after 19 cycles of preamplification37. Microfluidic qPCR was performed in a 96.96 Dynamic Array (Fluidigm), using the following cycle conditions: 2 min at 50 °C and 10 min at 95 °C for thermal mix, followed by 35 cycles with denaturation for 15 s at 95 °C and annealing/elongation for 1 min at 60 °C. After each run, melting curves were generated to confirm a single PCR product (increasing 1 °C/3 s). After data pre-processing 75 genes of interest for proximal colon and 80 genes of interest for jejunum were statistically analyzed. The data pre-processing and normalization to three validated reference genes B2M, PL13A and TBP were carried out as described38. The list of genes and primers is presented in Supplementary Table 1.
Gut microbiota analysis (Digesta sampling, DNA Extraction, 16S rRNA gene amplicon sequencing and data processing)
Fresh intestinal digesta samples were removed from the proximal colon (20 cm distal of ileo-caecal junction) of each pig for GM sampling in Study 2. The samples were snap-frozen in liquid nitrogen and stored at − 80 °C. Additional digesta samples were taken for pH measurement using a Hi2211 pH/ORP Meter (Hanna Instruments). DNA extraction from digesta samples was performed by using the Qiagen QIAmp Fast DNA Stool Mini Kit (Cat. No. 51604), following the manufacturer’s protocol. The extracted DNA was stored at − 20 °C until further analysis. Nanodrop 1000 Spectrophotometer (Thermo Fisher Scientific, USA) and Varioskan Flash (Thermo Fisher Scientific, USA) were used for DNA purity and concentration measurements, respectively.
To determine GM composition, near full-length 16S rRNA gene was amplified with protocol involving multiple forward and reverse primers (Supplementary Table 2). Two-step PCR was performed for the amplification. First PCR conditions were as follows: 95 °C for 5 min, 2 cycles of 95 °C for 20 s, 48 °C for 30 s, 65 °C for 10 s, 72 °C for 45 s, and a final extension at 72 °C for 4 min. A second PCR step was done to barcode the first PCR products with the following conditions: 95 °C for 2 min followed by 33 cycles of 95 °C for 20 s, 55 °C for 20 s, 72 °C for 40 s, and a final extension at 72 °C for 4 min. PCR products were cleaned up using SpeedBeads magnetic carboxylate (obtained from Sigma Aldrich) after each PCR step. The size of barcoded PCR products was checked on 1.5% agarose gel.
The Nanopore sequencing library was constructed according to the ligation sequencing kit SQK-LSK109 protocol. The library was loaded on R9.4 flowcell and sequenced with GridIONX5 platform (Oxford Nanopore Technologies, Oxford, UK). Sequencing data was collected using Nanopore sequencing software GridION version 21.02.5 (https://nanoporetech.com). Guppy version 4.5.2 (https://nanoporetech.com) was used for base calling and demultiplexing. Next, filtering and trimming of demultiplexed sequences (min = 1300 bp, max = 1600 bp, q score ≥ 10) were performed by Nanofilt version 2.7.139. Taxonomy assignment was done by parallel_assign_taxonomy_uclust.py script of Quantitative Insights into Microbial Ecology (Qiime) 1 version 1.8.040. Greengenes database version 13.841 was used as a reference database. The reads classifications did not contain UMI correction because of the low coverage of UMI clusters.
Statistical analysis
GraphPad Prism 7 (GraphPad Software Inc., CA, USA) or R (version R i386 3.5.2) was used for statistical analysis. Normally distribution of the data was tested using Shapiro–Wilk’s normality test. Normally distributed data was tested using one-way ANOVA or T-test (Study 1) or 2-way ANOVA or T-test (Study 2). Non-normally distributed data was analysed using Mann–Whitney test. FEC area under the curve (AUC) was calculated in Prism using the formula ΔX*([(Y1 + Y2)/2]-Baseline]) where ΔX refers to the time period being assessed, Y1 and Y2 are the egg counts at the start and end of the specified period, and baseline is the minimum FEC value in the area being assessed (0 in this case). An individual value was obtained for each pig, and groups compared using t-test. Statistical significance was assigned at P ≤ 0.05.
For bioinformatics of 16S rRNA gene amplicon sequencing data, Qiime 2 version 2020.6.042 was used to rarefy data to 15,000 reads per sample, and rarefied data were then processed in RStudio version 1.3.107343 using R version 4.0.244 and R packages phyloseq45, tidyverse46, ggpubr47, and reshape248. Alpha diversity was evaluated based on observed features and Shannon index. Principal coordinate analysis (PCoA) plots were generated based on Bray–Curtis and Jaccard distances dissimilarity metrics. Analysis of differentially abundant bacteria was performed by using the DESeq2 package49 and DESeq2-based heatmaps showing the significantly differed bacteria (adjusted p value < 0.05) were plotted using pheatmap50 and RColorbrewer51 packages. Pairwise Wilcoxon rank-sum test with Benjamini–Hochberg method from R stats package44 was used to obtain adjusted P-values for alpha diversity measures. For beta diversity, adjusted P-values were calculated by pairwise comparisons using permutation MANOVAs on a distance matrix with Holm method from R package RVAideMemoire52.
Results
Clinical observations
No clinical signs of infections were observed in the pigs, except for a mild cough around 7–9 days after A. suum infection, which is associated with migration of the larvae to the lungs. There were no significant differences in daily weight gains between groups in either Study 1 when pigs were fed a 5% inclusion or Study 2 when fed an 8% inclusion (Tables 2, 3). One pig in the IC group in Study 2 had mild diarrhoea at 14 dpi.
Seaweed does not significantly reduce parasite burdens
Study 1: A. suum and O. dentatum worms were recovered from all animals, with no significant differences between groups (Table 2). However, O. dentatum FEC based on individual EPG at 19 to 22 dpi showed a decrease of 46.0% and 42.0% for FSL and NSL, respectively, as compared to the controls (Fig. 1). However, this was not significant, either by comparing the control group with the two individual seaweed groups (p = 0.12 and 0.15 by AUC analysis for NSL and FSL, respectively; Table 2), or by pooling the seaweed groups and comparing to the control (p = 0.09 by unpaired t-test).
Area under the curve (AUC) of faecal egg counts (FEC), based on eggs per gram faeces (EPG) of individual pigs on days 19, 20, 21 and 22 post infection with Oesophagostomum dentatum in Study 1 (n = 3) for control group (C), non-fermented seaweed inclusion group (NSL) and fermented seaweed inclusion group (FSL). Data are presented as mean ± SEM.
Study 2: A. suum and O. dentatum worms were recovered from all infected animals while no worms or liver spots were found in uninfected animals. Infected pigs had significantly higher Ascaris-specific and Oesophagostomum-specific IgG levels in serum than uninfected pigs within the two feeding groups (P < 0.01), but no difference was found in specific IgG levels between the ISL group and the IC group for either A. suum or O. dentatum. No significant differences were found in any of the parasitological/pathological parameters between the IC and the ISL group (Table 3).
Seaweed changes the gut microbiota composition
To explore the effects of Fer-SL inclusion on the intestinal environment and GM, we first measured pH values of the intestinal content in jejunum and proximal colon. Average pH values were 6.4 (jejunum) and 6 (proximal colon), but no significant effects of either diet or infection were found in either study (data not shown). We next profiled the GM composition in the proximal colon of pigs from Study 2 using Oxford Nanopore based 16S rRNA gene amplicon sequencing.
We observed increases in GM alpha diversity as a result of feeding seaweed, both with observed features and Shannon Index, whereas infection had no significant effect (Fig. 2A, B). Beta diversity analysis based on Bray–Curtis dissimilarity (Fig. 2C) and Jaccard (Fig. 2D) indices showed no clear differences between GM composition between uninfected (UC and USL) and infected (IC and ISL) groups (P > 0.05), respectively. However, there was a marked effect of Fer-SL inclusion on both beta-diversity metrics (Fig. 2C, D; P = 0.002). All pairwise group comparisons results for beta diversity are listed in Fig. 2E. Notably, the effect of seaweed was identical across pens (regardless of parasite infection), suggesting a biological effect of seaweed consumption rather than a pen effect.
Gut microbiota diversity of pigs in Study 2. Upper panel: Box plot of α-diversity based on (A) observed features and (B) Shannon index of gut microbiota from proximal colon of groups of pigs (n = 8) in Study 2: control group (UC), fermented Saccharina latissima supplementation (USL), Oesphagostomum dentatum and Ascaris suum infection (IC), or O. dentatum and A. suum infection combined with S. latissima supplementation (ISL). The horizontal line in each box shows the median value. The lower and upper boundaries of boxes are the 25th and 75th quartiles, respectively. The significance of difference was assessed by pairwise Wilcoxon rank-sum test with Benjamini–Hochberg method (*P < 0.05, ** P < 0.01, ***P < 0.001). Lower panel: Principal coordinates analysis (PCoA) plots based on Bray–Curtis (C) and Jaccard (D) distances for beta diversity. Each point on PCoA plots shows the sample, which was coloured and shaped by Fer-SL diet and parasite infection, respectively. The percentage in parenthesis is the percentage variation captured by first and second PCoA axes. Ellipses represent 95% confidence interval. (E) Statistical pairwise group comparisons for beta diversity using permutation MANOVAs on a distance matrix with Holm adjustment method. P value < 0.05 was considered as significant.
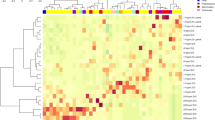
We next assessed the changes in GM composition at phylum level and found that the GM of all groups was mainly dominated by the Bacteroidetes and Firmicutes phyla (Fig. 3). To identify bacteria with significantly different abundance between pigs fed the control diet (UC and IC) or Fer-SL diet (USL and ISL), we performed DESeq2-based heatmap analysis of the GM. At phylum level, our findings revealed that Fer-SL diet inclusion resulted in the increased levels of Elusimicrobia (P = 0.04), Tenericutes (P < 0.0001), Spirochaetes (P = 0.02) and Verrucomicrobia (P = 0.02), but it should be noted that all these taxa were of relatively low abundance. The abundance of Proteobacteria tended to be reduced by Fer-SL inclusion in diet (P = 0.06). At the species-level, analysis showed a clear grouping as a result of diet (Fig. 4). The pigs fed the control diet had a higher relative abundance of Prevotella copri and an unclassified Phascolarctobacterium. The GM of pigs fed Fer-SL were characterised by increased relative abundance of Oscillospira, Desulfovibrionaceae, RF16, Clostridiales, Bacteroidales, Bacteroides, Treponema, Coprococcus, Anaeroplasma, and Anaeroplasmataceae spp.
Phylum-level gut microbiota composition of pigs in Study 2. In the left panel, (A) Stacked bar plots show relative abundance of phyla in groups of pigs (n = 8) for control fed pigs with (IC) or without (UC) parasite infection (Oesphagostomum dentatum and Ascaris suum) or pigs fed fermented Saccharina latissima (Fer-SL) with (ISL) or without (USL) parasite infection (n = 8). “Other” indicates the phyla with median relative abundance below 1%. In the right panel, bar plots show alterations induced by diet and parasite infection for (B) Bacteroidetes, (C) Firmicutes, (D) Proteobacteria, and (E) Spirochaetes. Data for B-E are presented as mean ± SEM.
DeSeq2-based heatmap for Study 2. Heatmap shows the significantly differed bacteria at species level in pigs fed with diet with fermented Saccharina latissima (Fer-SL) (red) and without Fer-SL diet (blue). Rectangles on heatmap comprise the increased bacteria in groups. Heat map was created using pheatmap, v1.0.12, https://cran.r-project.org/web/packages/pheatmap/index.html50.
Overall, these data indicate that Fer-SL diet inclusion increased alpha diversity of the GM and modulated the GM composition, and that this effect was largely independent of underlying helminth infection.
Seaweed modulates the immune response to parasite infection
We next investigated how Fer-SL inclusion affected intestinal morphology and expression of genes related to immune responses and epithelial barrier function. We found no significant effect of diet or infection on goblet cell numbers, nor the villi/crypt ratio or crypt depth in either the jejunum or proximal colon in Study 2. However, goblet cell numbers in the jejunum tended to increase in Fer-SL-fed, uninfected animals (USL vs UC, P = 0.09), whilst infection in control-fed pigs tended to increase villus/crypt ratios (UC vs IC, P = 0.08) in the jejunum (Supplementary Figure 3).
To explore whether the dietary inclusion of Fer-SL modulated the intestinal transcriptional response during infection, the expression of a panel of genes covering lipid metabolism, immune and mucosal barrier function was assessed in jejunum and colon tissue. In the jejunum, the diet had only a modest effect on gene expression, but we did note significantly decreased expression of the pro-inflammatory genes IL12A and PTGS2 (Fold change − 1.2 and − 1.5, respectively) (Supplementary Figure 4). Infection resulted in increased expression of immune-related genes in the jejunum, particularly those involved in type-2 responses (Fig. 5). In particular, the expression of IL13 and RETNLB was significantly increased in infected pigs (Supplementary Figure 5), regardless of diet (main effect of infection with a fold change of 3.7 and 1.7, respectively), and the expression of CCL26, IL10 and TFF2 also tended to be increased (Table 4). Conversely, expression of the pro-inflammatory IL18 in the jejunum was significantly decreased by infection (fold change − 1.2), and so were type-2 associated gene CCL22 (fold change − 2.1) and adipocyte regulator PPARG (fold change − 1.3). Interestingly, we noted that, across the genes we measured, expression in the jejunum was consistently lower in the ISL group, relative to IC (Fig. 5). This was evidenced by significant, or close to significant, interactions between diet and infection for the expression of CXCL9, CXCL10, and TNF (Table 4; Supplementary Figure 6), indicating that infection-induced changes in gene expression were attenuated by inclusion of dietary Fer-SL. Thus, although Fer-SL inclusion had limited effects on the gene expression in the jejunum in uninfected pigs, it tended to modulate the response to infection.
Expression of genes involved in different immunological functions in groups of pigs (n = 8) as a result of Oesphagostomum dentatum and Ascaris suum infection (IC), fermented Saccharina latissima supplementation (USL), or O. dentatum and A. suum infection combined with fermented S. latissima supplementation (ISL). The control group (UC) received no infection or S. latissima inclusion. Data presented as Z-scores of relative gene expression data for (A) jejunum and (B) proximal colon. Heat maps were created using GraphPad Prism 7 (GraphPad Software Inc., CA, USA), https://www.graphpad.com.
In the proximal colon, we also found that diet only had modest effects, with a decrease in the expression of the pro-inflammatory cytokine-encoding gene IL7 (fold change − 1.2), as well as increases in genes encoding innate immune inhibitors (NFKBIA) and chemokine receptors (CCR4) (fold change 1.3 and 1.4, respectively) (Supplementary Figure 4). When looking at the effects of infection on expression of immune-related genes in the proximal colon we found an upregulation of genes related to type-2 responses such as CCL26, CCR4, and RETNLB (fold changes 1.4, 1.5 and 1.4, respectively) (Supplementary Figure 5). Furthermore, there was a tendency for increased expression of IL4 and EPX in infected pigs, regardless of diet (Table 5). However, infected pigs also had increased expression of the pro-inflammatory gene STAT4 (fold change 1.2), and IL12A, TLR5 and CD86 expression tended to be increased. Most notably, we found that the ISL gene expression was consistently higher, relative to IC (Fig. 5), in contrast to the effect seen in the jejunum. Thus, we found significant interactions of diet and infection for several genes, especially those related to the mucosal barrier (Table 5), such as DCLK1, HDAC9, IL13RA1, MUC2 and HDAC1 (Fold change 2.5, 1.9, 1.3, 1.3 and − 1.2, respectively). The same effect was seen for pro-inflammatory genes ILIB2, IL1R1, NFKB1, STAT3 (fold change 1.3, 1.7, 1.3 and 1.2, respectively), and type-2 response genes ARG1 and CCL22 (Fold change 1.3 and 1.7, respectively) (Supplementary Figure 6).
Taken together, these data suggest that Fer-SL inclusion only had a slight regulatory effect on the immune genes in the intestinal tract in uninfected pigs, with decreased expression of some pro-inflammatory immune genes. However, during a helminth infection, the inclusion of Fer-SL induced an attenuation of immune genes in the jejunum and upregulation of immune genes in the proximal colon.
Limited effects of seaweed or parasite infection on systemic immune parameters
Further investigations of the systemic immune-modulating effects of Fer-SL inclusion in diet were conducted on peripheral blood mononuclear cells (PBMCs) and cells from the ileo-caecal lymph nodes (CLN). In Study 2, we found slight modulatory effects of dietary Fer-SL inclusion on PBMCs, with a small reduction in the proportion of CD14+ onocytes within the PBMC population in the USL group compared to the UC group (− 7.7%, P = 0.07). Furthermore, we found a small increase in the proportion of CD3+ T-cells (8.8%, P = 0.05) (Supplementary Figure 7). Infection did not show any significant effects on PBMCs, apart from a reduced amount of CD14hi cells in infected pigs (P = 0.03) (Supplementary Figure 8). In the lymph nodes, we found no significant effects of Fer-SL diet inclusion, but an effect of infection, with a reduction in the proportion of CD3+ T-cells in infected vs non-infected animals (P = 0.03), and a small increase in CD14+ monocytes in infected vs non-infected animals, although this was not significant (P = 0.08). A significant difference was found between the UC and ISL group (P = 0.04) (Supplementary Figure 9). Ex vivo stimulation of PBMCs showed no significant differences in LPS-induced TNF-α or IL-1β secretion in the different treatment groups (data not shown).
Overall, Fer-SL inclusion showed very limited effects on the systemic immune response to helminths with only a trend of a decrease of CD14+ monocytes and an increase of CD3+ T-cells in PBMCs.
Discussion
Here, we investigated the effects of seaweed dietary inclusion during helminth infection in pigs. It is normally advised to include seaweed at low levels (1–2%) in pig feed to achieve the beneficial prebiotic effects without adverse effects, such as weight loss2,53, and avoid high levels of trace elements, such as iodine4. However, our study highlights that there were no apparent adverse effects on growth or health at short-term inclusion levels of 5 or 8%. Although S. latissima showed promising effects on egg excretion at a 5% inclusion level of both Fer-SL and Non-Fer-SL, it did not significantly affect worm burdens and egg excretion at 8% dietary inclusion of Fer-SL. The reason for the lack of effect on worm burden in vivo with Fer-SL inclusion, despite promising results in vitro, could be that the omega-3 fatty acids, which were the active compounds found in vitro28, were not able to reach the nematodes in high enough concentrations locally. This could be explained by the metabolism of fatty acids, as free fatty acids are quickly absorbed and metabolised54. Another factor could be interactions with the digesta, which have previously been shown to reduce the effects of natural dietary compounds against A. suum migration55. The reasons for an apparent discrepancy between the preliminary data and the larger trial may be numerous. Potentially, minor differences in the fermentation efficacy between the two diets, small differences in feed intake, or differences related to pig cohorts, e.g. basal GM composition in the two studies may have contributed.
Despite not showing significant anti-parasitic effects, Fer-SL inclusion had a modulatory effect on GM composition with an increase in both Observed features and Shannon index diversity in the GM of the proximal colon. Our data have to be interpreted with some caution as pigs were group-housed, which allowed normal social behaviour but does induce a confounding factor of pen effect. However, we found consistent GM changes in the two pens fed Fer-SL (regardless of infection status), and the results of GM changes are in accordance with previous studies on the effect of seaweed-derived components on the pig GM, which have also found decreases in Enterobacteriaceae (Proteobacteria) 7. Collectively, the GM analysis showed that there was limited effect of parasite infection on the GM composition, regardless of dietary treatment, and that Fer-SL inclusion was the driving factor behind the changes in altered GM diversity. These changes were manifested by the increase in the abundance of taxa which have been putatively linked to nutrient digestibility (e.g., Anaeroplasma and Treponema)56, butyrate production by fermentation of seaweed components (e.g., Oscillospira57 and Clostridiales58), and the seaweed epiphytic bacterial communities (Desulfovibrionaceae)59. Furthermore, the abundance of the bacteria Prevotella copri was reduced. Some studies have found this bacteria associated with modified mucus layer permeability60 and increased sensitivity to chemically induced colitis in mice61. However, a positive correlation between Prevotella spp. on production and health in pigs has also been found62. Taken together, these data show that Fer-SL was able to modulate GM composition and increase the relative abundance of taxa putatively associated with gut health. Importantly, these changes were also apparent in helminth-infected pigs, indicating a stable and robust prebiotic effect of Fer-SL in pigs either with or without an acute enteric infection.
Pig feeding trials with compounds from brown seaweeds have in general been found to stimulate Lactobacillus growth and reduce Enterobacteriaceae or E.coli4, and laminarin from L. digitata has been found to decrease Enterobacteriaceae in the colon63,64. The GM modulating effect of including Fer-SL in the diet could have been induced by polysaccharides, such as laminarin, working as dietary fibres65, but inhibitory effects of S. latissima extract directly on bacteria have also been found66, indicating a direct effect of S. latissima on the gut bacteria. Interestingly, we did not see any significant effects on GM composition by the parasite infections, even though many helminth infections may result in expansion of e.g. lactobacilli12. A recent study found infection with A. suum reduced Lactobacillus and Ruminicoccus, and gave non-significant increases in Prevotella55, unlike what was seen in our study.
We speculate that the observed lack of effect on the GM by infection could be due to the co-infection with two parasites (A. suum and O. dentatum). Previous studies have found that O. dentatum is able to change the immune response toward other parasites during co-infection67,68. Therefore, it can be speculated that an interaction between the two parasites on GM is occurring, and the co-infection of the two parasites may cancel out each other's effects on the GM. Although, one study indicates that O. dentatum may be able to suppress the effects of probiotics on GM69, studies of the effects of O. dentatum infection on the GM are scarce.
Brown seaweeds, such as S. latissima, contain storage polysaccharides such as laminarin1,70, and seaweed polysaccharides can be fermented by the GM in the large intestine71. However, the S. latissima we used was fermented pre-feeding, thus it may be likely that the bioactivity we observed on gene expression and GM composition derives from fermentation products present in the diet, rather than active metabolism of the seaweed polysaccharides in the gut. It is currently unclear which active compounds within seaweed lead to the modulatory effects on the GM and immune function, and whether they are produced in the pre-feeding fermentation or in vivo following seaweed metabolism. Identification of such compounds could lead to a more standardized approach to design seaweed-based supplements that have specific health benefits. However, it should be noted that the biological activity is unlikely to derive from a single compound, and instead is most probably due to a synergistic interaction between diverse bioactive molecules, which may present some difficulties for standardization.
In general, Fer-SL inclusion tended to decrease expression of pro-inflammatory genes in both the jejunum and proximal colon and increase expression of type-2 response genes in the proximal colon. Seaweed extract inclusion in the feed has been found to downregulate Th1 pro-inflammatory genes such as IL1B and IL6, but also IL17A7, and ex vivo results with ethanol extracts on porcine colonic tissue also showed decrease in IL6, IL10, and CCL2 expression, as well as an increase in APO1 expression72. Cytokine gene expression of pro-inflammatory genes IL1α, IL10, TNFα and IL17A was down-regulated in the colon following exposure to laminarin from L. digitata, L. hyperborea and Saccharomyces cerevisiae, and a significant increase in IL8 gene expression in the colon following an LPS challenge ex vivo when exposed to laminarin from L. digitata63. However, these studies were carried out using different species of brown seaweed and pigs of a different age group compared to the current study. Infection also affected the gene expression in the intestinal segments, with an increase in expression of type-2 associated genes in the colon and jejunum, which is in line with previous studies showing increases in type-2 response-related genes with both Ascaris and Oesophagostomum infections in pigs33,67. Fer-SL appeared to modulate gene expression during infection, albeit with relatively modest fold changes. Thus, it is not clear whether Fer-SL or related dietary interventions can modulate immune function and/or reduce inflammation during mucosal pathogen infection. However, such immune-modulation would have important implications for animal production in an era of reduced antimicrobial drug usage, and thus further studies to assess whether Fer-SL administered under large scale, commercial conditions may impact immune function and disease resistance are warranted.
Fer-SL inclusion by itself tended to positively affect gut morphology, with increased villi/crypt ratios in the jejunum in uninfected pigs (UC vs USL), unlike what was found in a study with piglets offered L. digitata extract, which resulted in reduced villus height in the small intestine9. Reduced villi height has been found to have negative effects on absorption capability73. We also saw that goblet cells in the jejunum tended to increase with Fer-SL inclusion in non-infected pigs (UC vs USL), which correlates well with a higher abundance of the mucus encoding gene MUC2 in pigs feed seaweed extracts, despite it originating from another seaweed, Laminaria spp.10,11,64.
Conclusion
Inclusion of 8% Fer-SL in the diet of growing pigs did not significantly alter parasite burdens or associated pathology. However, Fer-SL inclusion showed a moderate local modulatory effect on the immune system, with general down-regulation of pro-inflammatory genes and upregulation of type 2 response genes. Interestingly, Fer-SL induced an overall decrease in the expression of immune-related genes in the jejunum of infected pigs. In contrast, the expression of immune regulating genes was increased in colon tissue during infection. In addition, microbial composition in the proximal colon was not distinctly affected by infection, but Fer-SL inclusion did in both groups show significantly increased diversity of the GM in the proximal colon, increasing the relative abundance of taxa putatively associated with gut health. These results indicate Fer-SL inclusion, despite not displaying any substantial anti-parasitic effects, may modulate GM composition and hence potentially also gut health regardless of the presence of intestinal parasites.
Ethical approval
All experimentation was approved by the Danish Animal Experimentation Inspectorate (License number 2015-15-0201-00760), and conducted at the Experimental Animal Unit, University of Copenhagen according to FELASA guidelines and recommendations. The study is reported in accordance with ARRIVE guidelines (https://arriveguidelines.org).
Data availability
16S rRNA gene amplicon sequencing raw data are available at Sequence Read Archive (www.ncbi.nlm.nih.gov/sra/) under the accession number PRJNA817076.
References
Holdt, S. L. & Kraan, S. Bioactive compounds in seaweed: functional food applications and legislation. J. Appl. Phycol. 23, 543–597 (2011).
Makkar, H. P. S. et al. Seaweeds for livestock diets: A review. Anim. Feed Sci. Technol. 212, 1–17 (2016).
Øverland, M., Mydland, L. T. & Skrede, A. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J. Sci. Food Agric. 99, 13–24 (2019).
Corino, C., Modina, S. C., Di Giancamillo, A., Chiapparini, S. & Rossi, R. Seaweeds in pig nutrition. Animals 9, 1126 (2019).
Dierick, N., Ovyn, A. & De Smet, S. Effect of feeding intact brown seaweed Ascophyllum nodosum on some digestive parameters and on iodine content in edible tissues in pigs. J. Sci. Food Agric. 89, 584–594 (2009).
Shimazu, T. et al. Addition of Wakame seaweed (Undaria pinnatifida) stalk to animal feed enhances immune response and improves intestinal microflora in pigs. Anim. Sci. J. 90, 1248–1260 (2019).
Walsh, A. M., Sweeney, T., O’Shea, C. J., Doyle, D. N. & O’Doherty, J. V. Effect of dietary laminarin and fucoidan on selected microbiota, intestinal morphology and immune status of the newly weaned pig. Br. J. Nutr. 110, 1630–1638 (2013).
Lynch, M. B., Sweeney, T., Callan, J. J., O’Sullivan, J. T. & O’Doherty, J. V. The effect of dietary Laminaria derived laminarin and fucoidan on intestinal microflora and volatile fatty acid concentration in pigs. Livest. Sci. 133, 157–160 (2010).
Reilly, P. et al. The effects of seaweed extract inclusion on gut morphology, selected intestinal microbiota, nutrient digestibility, volatile fatty acid concentrations and the immune status of the weaned pig. Animal 2, 1465–1473 (2008).
Leonard, S. G., Sweeney, T., Bahar, B., Lynch, B. P. & O’Doherty, J. V. Effects of dietary seaweed extract supplementation in sows and post-weaned pigs on performance, intestinal morphology, intestinal microflora and immune status. Br. J. Nutr. 106, 688–699 (2011).
Ryan, M. T. et al. Effects of nutrient supplementation with laminarin derived from Laminaria hyperborea and Laminaria digitata on mucin gene expression in the porcine ileum. Livest. Sci. 133, 236–238 (2010).
Leung, J. M., Graham, A. L. & Knowles, S. C. L. Parasite-Microbiota interactions with the vertebrate gut: Synthesis through an ecological lens. Front. Cell. Infect. Microbiol. 9, 843 (2018).
Williams, A. R. et al. Emerging interactions between diet, gastrointestinal helminth infection, and the gut microbiota in livestock. BMC Vet. Res. 17, 62 (2021).
Kaplan, R. M. & Vidyashankar, A. N. An inconvenient truth: global worming and anthelmintic resistance. Vet. Parasitol. 186, 70–78 (2012).
Roepstorff, A., Bjørn, H. & Nansen, P. Resistance of Oesophagostomum spp. in pigs to pyrantel citrate. Vet. Parasitol. 24, 229–239 (1987).
Gerwert, S., Failing, K. & Bauer, C. Prevalence of levamisole and benzimidazole resistance in Oesophagostomum populations of pig-breeding farms in North Rhine-Westphalia, Germany. Parasitol. Res. 88, 63–68 (2002).
Macrelli, M. et al. First detection of ivermectin resistance in Oesophagostomum dentatum in pigs. Vet. Parasitol. 270, 1–6 (2019).
Dangolla, A., Bjørn, H., Willeberg, P. & Barnes, E. H. Faecal egg count reduction percentage calculations to detect anthelmintic resistance in Oesophagostomum spp. in pigs. Vet. Parasitol. 68, 127–142 (1997).
Pettersson, E. et al. First report on reduced efficacy of ivermectin on Oesophagostomum spp. on Swedish pig farms. Vet. Parasitol. Reg. Stud. Rep. 25, 100598 (2021).
Petkevicius, S. et al. The effect of two types of diet on populations of Ascaris suum and Oesophagostomum dentatum in experimentally infected pigs. Parasitology 111, 395–401 (1995).
Jensen, A. N. et al. The effect of a diet with fructan-rich chicory roots on intestinal helminths and microbiota with special focus on Bifidobacteria and Campylobacter in piglets around weaning. Animal 5, 851–860 (2011).
Hoste, H. et al. Tannin containing legumes as a model for nutraceuticals against digestive parasites in livestock. Vet. Parasitol. 212, 5–17 (2015).
Peña-Espinoza, M., Thamsborg, S. M., Desrues, O., Hansen, T. V. & Enemark, H. L. Anthelmintic effects of forage chicory (Cichorium intybus) against gastrointestinal nematode parasites in experimentally infected cattle. Parasitology 143, 1279–1293 (2016).
Hussain, A. et al. Fermentation, a feasible strategy for enhancing bioactivity of herbal medicines. Food Res. Int. 81, 1–16 (2016).
Puupponen-Pimiä, R. et al. Fermentation and dry fractionation increase bioactivity of cloudberry (Rubus chamaemorus). Food Chem. 197, 950–958 (2016).
Jørgensen, H., Sholly, D., Pedersen, A. Ø., Canibe, N. & Knudsen, K. E. B. Fermentation of cereals—Influence on digestibility of nutrients in growing pigs. Livest. Sci. 134, 56–58 (2010).
Lyberg, K., Lundh, T., Pedersen, C. & Lindberg, J. E. Influence of soaking, fermentation and phytase supplementation on nutrient digestibility in pigs offered a grower diet based on wheat and barley. Anim. Sci. 82, 853–858 (2006).
Bonde, C. S. et al. Bio-guided fractionation and molecular networking reveal fatty acids to be principal Anti-Parasitic compounds in Nordic seaweeds. Front. Pharmacol. 12, 674520 (2021).
Vils, E. Håndbog i svinehold (Landsbrugforlaget 2006).
Roepstorff, A. & Nansen, P. Epidemiology, diagnosis and control of helminth parasites of swine. FAO Anim. Health Man. 3, 171 (1998).
Roepstorff, A., Eriksen, L., Slotved, H. C. & Nansen, P. Experimental Ascaris suum infection in the pig: Worm population kinetics following single inoculations with three doses of infective eggs. Parasitology 115(Pt 4), 443–452 (1997).
Andersen-Civil, A. I. S. et al. Dietary proanthocyanidins promote localized antioxidant responses in porcine pulmonary and gastrointestinal tissues during Ascaris suum-induced type 2 inflammation. FASEB J. 36, e22256 (2022).
Dawson, H. D. et al. Localized multigene expression patterns support an evolving Th1/Th2-like paradigm in response to infections with Toxoplasma gondii and Ascaris suum. Infect. Immun. 73, 1116–1128 (2005).
Masure, D. et al. the intestinal expulsion of the roundworm Ascaris suum is associated with eosinophils, intra-epithelial T cells and decreased intestinal transit time. PLoS Negl. Trop. Dis. 7, e2588 (2013).
Slotved, H. C. et al. Use of an agar-gel technique for large scale application to recover Ascaris suum larvae from intestinal contents of pigs. Acta Vet. Scand. 38, 207–212 (1997).
Slotved, H. C. et al. Recovery of Oesophagostomum dentatum from pigs by isolation of parasites migrating from large intestinal contents embedded in agar-gel. Vet. Parasitol. 63, 237–245 (1996).
Myhill, L. J. et al. Mucosal barrier and Th2 immune responses are enhanced by dietary inulin in pigs infected with Trichuris suis. Front. Immunol. 9, 2557 (2018).
Skovgaard, K. et al. Rapid and widely disseminated acute phase protein response after experimental bacterial infection of pigs. Vet. Res. 40, 23 (2009).
De Coster, W., D’Hert, S., Schultz, D. T., Cruts, M. & Van Broeckhoven, C. NanoPack: Visualizing and processing long-read sequencing data. Bioinformatics (Oxford, England) 34, 2666–2669 (2018).
Caporaso, J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336 (2010).
McDonald, D. et al. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J 6, 610–618 (2012).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857 (2019).
R: A language and environment for statistical computing [Internet]. (R Foundation for Statistical Computing, Vienna, Austria, 2020. https://www.r-project.org/.
R: a language and environment for statistical computing (R Foundation for Statistical Computing, 2020. https://www.r-project.org/.
McMurdie, P. J. & Holmes, S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8, e61217 (2013).
Wickham, H. Welcome to the Tidyverse. J. Open Source Softw. 4, 1686 (2019).
“ggplot2” Based Publication Ready Plots. 2020. https://cran.r-project.org/package=ggpubr.
Wickham, H. Reshaping data with the reshape package. J. Stat. Softw. 21, 1–20 (2007).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Pretty Heatmaps. 2019. https://cran.r-project.org/package=pheatmap.
ColorBrewer Palettes. 2014. https://cran.r-project.org/package=RColorBrewer.
RVAideMemoire: Testing and Plotting Procedures for Biostatistics. 2021. https://cran.r-project.org/package=RVAideMemoire.
Evans, F. D. & Critchley, A. T. Seaweeds for animal production use. J. Appl. Phycol. 26, 891–899 (2014).
Christensen, M. S., Høy, C. E., Becker, C. C. & Redgrave, T. G. Intestinal absorption and lymphatic transport of eicosapentaenoic (EPA), docosahexaenoic (DHA), and decanoic acids: Dependence on intramolecular triacylglycerol structure. Am. J. Clin. Nutr. 61, 56–61 (1995).
Williams, A. R. et al. A polyphenol-enriched diet and Ascaris suum infection modulate mucosal immune responses and gut microbiota composition in pigs. PLoS ONE 12, e0186546 (2017).
Niu, Q. et al. Dynamic distribution of the gut microbiota and the relationship with apparent crude fiber digestibility and growth stages in pigs. Sci. Rep. 5, 9938 (2015).
Gophna, U., Konikoff, T. & Nielsen, H. B. Oscillospira and related bacteria: From metagenomic species to metabolic features. Environ. Microbiol. 19, 835–841 (2017).
Levine, U. Y., Looft, T., Allen, H. K. & Stanton, T. B. Butyrate-producing bacteria, including mucin degraders, from the swine intestinal tract. Appl. Environ. Microbiol. 79, 3879–3881 (2013).
Selvarajan, R. et al. Distribution, interaction and functional profiles of epiphytic bacterial communities from the rocky intertidal seaweeds, South Africa. Sci. Rep. 9, 19835 (2019).
Rolhion, N. et al. A Listeria monocytogenes bacteriocin can target the commensal Prevotella copri and modulate intestinal infection. Cell Host Microbe 26, 691-701.e695 (2019).
Scher, J. U. et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2, e01202 (2013).
Amat, S., Lantz, H., Munyaka, P. M. & Willing, B. P. Prevotella in pigs: The positive and negative associations with production and health. Microorganisms 8, 1584 (2020).
Sweeney, T. et al. Effect of purified β-glucans derived from Laminaria digitata, Laminaria hyperborea and Saccharomyces cerevisiae on piglet performance, selected bacterial populations, volatile fatty acids and pro-inflammatory cytokines in the gastrointestinal tract of pigs. Br. J. Nutr. 108, 1226–1234 (2012).
Smith, A. G. et al. The effects of laminarin derived from Laminaria digitata on measurements of gut health: Selected bacterial populations, intestinal fermentation, mucin gene expression and cytokine gene expression in the pig. Br. J. Nutr. 105, 669–677 (2011).
Gupta, S. & Abu-Ghannam, N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci. Technol. 22, 315–326 (2011).
Cox, S., Abu-Ghannam, N. & Gupta, S. An assessment of the antioxidant and antimicrobial activity of six species of edible Irish seaweeds. Int. Food Res. J. 17, 205–220 (2010).
Andreasen, A. et al. Immune and inflammatory responses in pigs infected with Trichuris suis and Oesophagostomum dentatum. Vet. Parasitol. 207, 249–258 (2015).
Petersen, H. H., Andreasen, A., Kringel, H., Roepstorff, A. & Thamsborg, S. M. Parasite population dynamics in pigs infected with Trichuris suis and Oesophagostomum dentatum. Vet. Parasitol. 199, 73–80 (2014).
Myhill, L. J. et al. Parasite-probiotic interactions in the gut: Bacillus sp. and Enterococcus faecium regulate type-2 inflammatory responses and modify the gut microbiota of pigs during helminth infection. Front. Immunol. 12, 793260 (2022).
Schiener, P., Black, K. D., Stanley, M. S. & Green, D. H. The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. J. Appl. Phycol. 27, 363–373 (2015).
You, L. et al. Beneficial effects of three brown seaweed polysaccharides on gut microbiota and their structural characteristics: An overview. Int. J. Food Sci. Technol. 55, 1199–1206 (2020).
Bahar, B., O’Doherty, J. V., Smyth, T. J. & Sweeney, T. A comparison of the effects of an Ascophyllum nodosum ethanol extract and its molecular weight fractions on the inflammatory immune gene expression in-vitro and ex-vivo. Innov. Food Sci. Emerg. Technol. 37, 276–285 (2016).
Nabuurs, M. J., Hoogendoorn, A., van der Molen, E. J. & van Osta, A. L. Villus height and crypt depth in weaned and unweaned pigs, reared under various circumstances in The Netherlands. Res. Vet. Sci. 55, 78–84 (1993).
Acknowledgements
We would like to thank the company Nordic Seaweed for providing the seaweed material, Mette Schelde from University of Copenhagen for tissue RNA extractions, and Lise-Lotte Christiansen, Audrey Inge Schytz Andersen-Civil, Suyog Subedi, Marica Engstrom and Karen Jensen for their support and help during the animal study. Data was generated through accessing research infrastructure at University of Copenhagen, including FOODHAY (Food and Health Open Innovation Laboratory, Danish Roadmap for Research Infrastructure).
Funding
This work was funded by the Green Development and Demonstration Program (GUDP) (Project No. 34009–17–1220; SEAPAR).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: A.R.W., S.M.T., H.M., C.S.B. Performed the experiments: C.S.B., H.M., A.R.W., S.M.T., L.J.M., L.Z., P.J., K.S., N.B.G. Analysed the data: C.S.B., L.J.M., A.R.W., S.M.T., H.M., D.S.N., N.B.G., L.K. Wrote the paper: C.S.B. All authors approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bonde, C.S., Mejer, H., Myhill, L.J. et al. Dietary seaweed (Saccharina latissima) supplementation in pigs induces localized immunomodulatory effects and minor gut microbiota changes during intestinal helminth infection. Sci Rep 13, 21931 (2023). https://doi.org/10.1038/s41598-023-49082-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-49082-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.