Abstract
Hydroquinone, Mercury (Hg), and Arsenic (As) are hazardous to health upon long-term exposure. Hydroquinone, Hg, and As were analysed in skin-lightening cosmetics randomly purchased from different cosmetic outlets within the Ilorin metropolis, Nigeria. The amount of hydroquinone in the samples was determined using a UV-spectrophotometry method at 290 nm. Hg and As were quantified using atomic absorption spectrophotometry (AAS). UV-spectrophotometry method validation showed excellent linearity (r2 = 0.9993), with limits of detection (0.75 µg/mL), limits of quantification (2.28 µg/mL), relative standard deviation (0.01–0.35%), and recovery (95.85–103.56%) in the concentration range of 5–50 µg/mL. Similarly, r2, LOD, and LOQ for Hg and As were 0.9983 and 0.9991, (0.5 and 1.0 µg/L) and 1.65 and 3.3 µg/L) respectively. All the samples contained hydroquinone, Hg and As in varying amounts. The amounts of hydroquinone, Hg and As present were in the ranges of 1.9–3.3%, 0.08–2.52 µg/g and 0.07–5.30 µg/g respectively. Only three of the analysed samples contained hydroquinone within the permissible limit of 2.0% w/w in cosmetic products. All the samples analysed contained mercury and arsenic in varying amounts. The need to periodically monitor the levels of hydroquinone, mercury, and arsenic in skin-lightening cosmetics marketed in Nigeria is recommended.
Similar content being viewed by others
Introduction
Globally, women, young girls, and some men frequently lighten their skin tones1,2,3. The skin-lightening industry is experiencing rapid growth on a global scale, driven by a substantial demand for cosmetics aimed at achieving lighter skin tones2. The use of skin-lightening cosmetics transcends sociodemographic boundaries4,5, including religious (Christianity, Islam, traditional beliefs), marital status (single, married, divorced), economic status (affluent and underprivileged), educational attainment (literate or illiterate), and social class (low, middle, and upper)3,4,5.The overwhelming desire for skin-lightening cosmetics in society is mostly due to the idea that having light skin is a sign of beauty, success, and power5. Some other reasons given for using skin-lightening cosmetics include improving personal appearance, following current fashion trends, treating skin imperfections like acne or melasma, refining facial and bodily complexion, and satisfying the preferences of one’s spouse4,5.
Nigeria, other African countries, and Asians, demonstrate a significant consumer base for skin-lightening cosmetics. This trend is corroborated by the WHO survey that reported 77% of Nigerian women frequently use skin-lightening cosmetics, as opposed to 25, 27, 35, and 59% for Mali, Senegal, South Africa, and Togo, respectively6,7. Many of these skin-lightening cosmetics, which are regrettably smuggled into Nigeria, contain banned substances, including hydroquinone and heavy metals. Many other countries around the world also use skin-lightening cosmetics8,9. Nevertheless, a significant concern arises from the inclusion of hazardous substances such as hydroquinone, mercury (Hg), and arsenic (As) in many of these cosmetics10,11,12,13,14,15,16,17,18,19. Hydroquinone, Hg, and As have all been identified as potentially dangerous chemicals in skin-lightening cosmetics10,11,12,13,14,15,16,17,18,19. While these chemicals are hazardous and cause major health problems, they can be sold illegally or under alternative brand names and are frequently not disclosed on the label4.
Hydroquinone is an aromatic organic compound and a potent inhibitor of melanin production used in the treatment of melasma9,20. Melasma, pigmented acne scars, post-inflammatory hyperpigmentation, and skin discoloration are major disorders of hyperpigmentation21, and the topical preparations for treating this disorder include hydroquinone, kojic acid, azelaic acid, glycolic acid, retinoids, and salicylic acid21. Specifically, hydroquinone remains the most effective lightening agent for treating common hyperpigmentation disorders globally21. Tyrosinase, a rate-limiting enzyme in the conversion of tyrosine to melanin in human skin, is inhibited by hydroquinone21,22,23. This inhibition decreases the production of melanin in the melanocytes, which explains its ability to lighten the skin21,22,23. Although it is a widely used skin-lightening agent, it is characterised by several adverse effects, which include carcinoma4,9,24, irritative dermatitis, melanocyte destruction, contact dermatitis, and ochronosis21,25, that leave the skin discoloured when consistently applied for a long period of time4,26,27,28. The hydroquinone metabolites p-benzoquinone and glutathione conjugates are frequently linked to the development of cancer18,24,25. When hydroquinone-containing cosmetics are used topically over an extended period, p-benzoquinone and hydroquinone conjugates with glutathione may accumulate and cause DNA damage and mutations21.
Hydroquinone-induced ochronosis adverse effects have been reported to be more prevalent among Africans who use skin-lightening cosmetics. For instance, 756 of the 789 cases of exogenous ochronosis reported in a study were of African extraction10,25. Concerns over the safety issues surrounding the use of hydroquinone have led many countries around the world, including the UK, EU, US, Australia, Asia, Africa, and others9,10,29,30 to prohibit the use of hydroquinone in cosmetics28,29. For instance, in 1980, the government of South Africa set a 2% permissible limit on hydroquinone in cosmetics4. Similarly, the US, UK, and EU instituted regulations with a 2% limit for cosmetic products and a 4% limit for topical preparations intended for dermatological use5,8,9,23,25. In Nigeria, the National Agency for Food and Drug Administration and Control (NAFDAC) initially authorised a limit of 2% hydroquinone in skin-lightening cosmetics4. The adverse effects of hydroquinone in cosmetics persist despite the limit. Consequently, the use of hydroquinone in cosmetics was eventually banned. Nonetheless, cosmetics containing hydroquinone continue to be used freely, with many lacking ingredient labelling, manufacturer addresses, and NAFDAC registrations4. Generally, ochronosis was noted even with cosmetic formulations containing 2% hydroquinone when used for an extended period4. In addition to the pharmaceutical uses of hydroquinone, it has been extensively mishandled by the cosmetics industry in unregulated skin-lightening cosmetics, causing safety issues with significant public health relevance. Most often, these ingredients are intentionally added by the manufacturers in amounts exceeding the permissible limit, primarily to increase the sales of their cosmetic products20,31. Although many countries globally have banned the use of hydroquinone in skin-lightening cosmetics, where its use is permitted, it should not exceed 2%28.
Prolonged and excessive exposure to mercury (Hg) and arsenic (As) has been shown to result in their accumulation in the body, disrupting normal body functions. Specifically, toxicants in cosmetics cause skin damage such as irritation, rashes, discoloration, and scarring17. Additionally, they compromise the skin’s immunity against bacterial and fungal infections, hinder Purkinje cell dendrite growth, disrupt DNA and RNA functions, and contribute to renal and neurological dysfunctions, cataracts, glaucoma, and Cushing syndrome10,11,12,14,32. Similarly, addition of heavy metals to cosmetics has been reported to impair fetal development in pregnant women24,33. Prolonged skin exposure to mercury may damage vital body organs11,34. Mercury exists not only in its elemental form but also as organic and inorganic compounds35. Mercury salts are routinely added to cosmetic products for skin-lightening purposes, despite their adverse effects. Mercury salts have been reported to irreversibly inhibit tyrosinase activity (a key catalyst in the production of melanin), which results in a decrease in pigmentation11,12,36. Mercury salts have a significant risk of causing gastrointestinal problems, cancer, hepatotoxicity, nephrotoxicity, and damage to the central nervous system (CNS)11,13,36. Notably, a study by Weldon et al. (2000)37found that 87% of 119 Mexican women using mercury containing cosmetics had elevated mercury concentrations in their urine, associated with mercury poisoning at concentrations exceeding 20 µg/L. Also, Al-Saleh et al. (2005)38 found elevated levels of Hg in cosmetics from many countries, with samples from Thailand, Lebanon, and England having the highest amounts ranging from 1281 to 5650 ppm. Uram et al. (2010)39 observed irregular labels and descriptions on the cosmetic products made in Mexico. The maximum permissible limit for Hg set by the US FDA and WHO in cosmetics is1 µg/g13,40.
Further, chronic exposure to arsenic, a prevalent contaminant in beauty products, causes skin problems, nervous system abnormalities, and various cancers11,41. Arsenic interferes with cell functions by inhibiting enzymes containing sulfhydryl groups19. For example, it inhibits pyruvate dehydrogenase, an important enzyme in oxidative phosphorylation, resulting in cell damage11,19. Its ability to induce oxidative stress and disrupt mitochondrial function contributes to neurotoxicity11,14. Arsenic crosses the placenta and causes spontaneous abortion during early gestation, stillbirth, preterm birth, and low birth weight33.
The previous studies conducted in Nigeria reported varying amounts of harmful chemicals in cosmetic products4,5,7,42,43,44,45. While some studies in Nigeria assessed individual components like hydroquinone or heavy metals separately (Table 1), our study examines both hydroquinone and two significant heavy metals commonly found in skin-lightening cosmetics. Additionally, to the best of our knowledge, no study has previously examined hydroquinone, mercury, and arsenic levels in skin-lightening cosmetics in Ilorin, Nigeria. To mitigate health hazards associated with prolonged exposure to these chemicals, regular assessment of their presence in skin-lightening cosmetics sold in Nigeria is imperative. Our study aims to evaluate the quantities of hydroquinone, Hg, and As in cosmetic products, shedding light on the extent of this issue in Ilorin, Nigeria. The findings will contribute relevant, and current information that can guide the implementation of policies and regulations to address this concern in Nigeria. Furthermore, it will raise awareness about the risks associated with the unrestricted use of skin-lightening cosmetics in Nigeria.
Materials and methods
Materials
Reference hydroquinone powder (Batch No. L186301702) was purchased from Loba Chemie PVT. Ltd., India. Ten skin-lightening cosmetics were randomly purchased within the Ilorin metropolis. All other chemicals and reagents were of analytical grade.
Equipment
Automatic micropipette (1 mL), TLC plates, electronic analytical balance (PA214, Ohus, USA), eelectric oven (OV/50/DIG-R38, Genlab), electric water bath-420 (1111-40-DFSKW-1000DB, Lab Science, England), UV lamp (254 nm), melting point apparatus (AVI, China), whatman filter paper 1 (10 mm), nanodrop photometer (C40 version: NPOS 4.2 build 14,900 serial No: S40727), buck scientific atomic absorption spectrophotometer (210/211 VGP).
Collection of skin-lightening cosmetic samples
Ten (10) skin-lightening cosmetics were randomly purchased from different cosmetics outlets across three local government areas in Ilorin (Ilorin South, Ilorin Central, and Ilorin North), Nigeria. Virtual inspection and registration verification of all samples were conducted in compliance with WHO recommendations46. The samples were identified by their names, the name of the company, batch number, National Agency for Food and Drug Administration and Control (NAFDAC) registration number, manufacturing, and expiration dates, as well as the manufacturer’s address. The samples were labelled A to K and kept in a cool, dry place away from sunlight until analysis.
Identification of reference hydroquinone powder
The reference hydroquinone powder was further identified by preparing a 100 µg/mL test solution of the powder in methanol and scanning it in a UV spectrophotometer (Nanodrop) between 200 and 400 nm to determine its maximum absorbance wavelength (λmax). The melting point of the reference hydroquinone was also determined using the AVI digital melting point apparatus.
Identification of hydroquinone in skin-lightening cosmetic samples
Sample preparation
From each sample, 0.5 g was weighed using an electronic analytical balance and transferred into a 250 mL beaker. Absolute methanol (25 mL) was then added. The mixture was homogenised for 5 min in a water bath at 60 °C and allowed to cool until the fat content separated. Whatman filter paper 1 (10 mm) was used to separate the dissolved hydroquinone from the residues. Thereafter, the filtrate was concentrated in the oven at 60 °C, and the concentrate was used for the TLC screening of the samples for the presence of hydroquinone.
Preparation of solutions of reference hydroquinone powder
0.1 g of hydroquinone was weighed with an electronic analytical balance, transferred into a 25 mL beaker, and dissolved in 5 mL of methanol. The solution was used within 24 h to prevent possible degradation.
TLC analysis for hydroquinone in skin-lightening cosmetic samples
The TLC plates were placed in the oven at 60 °C for 1 h. The activated TLC plates were spotted with the reference hydroquinone solution, and the concentrate was obtained from each sample using capillary tubes and labelled appropriately. The spotting was done at a baseline of 1 cm from the lower end of the TLC plate, and the plate was allowed to dry. The solvent system (n-hexane/acetone, 60:40) was prepared and poured into the chromatographic tank to a depth of 0.5 cm. The tank was covered with a lid for an hour to allow saturation of the tank with the solvent. The spotted TLC plate was carefully inserted into the saturated chromatographic tank using a pair of forceps and then covered to allow for the development of the plate via an ascending technique of the solvent.
The TLC plate was removed from the tank when the solvent font reached 10 cm away from the spotted samples, and the solvent font was marked. The developed chromatogram was allowed to dry. After drying, the chromatogram was examined for possible spots using a UV lamp (254 nm), and the identified spots on the chromatogram were carefully marked. The Rf values were calculated for each spot of the sample using the equation below and compared with the Rf for reference hydroquinone. Samples with a similar Rf value to hydroquinone were identified and reported to contain hydroquinone.
Rf value was calculated using the formula:
Rf = \(\frac{\mathrm{Distance\: moved\: by\: solute}}{\mathrm{Distance\: moved\: by\: solvent\: font}}\)
Preparation of standard stock and working standard solutions
0.1 g of the reference hydroquinone standard was accurately weighed using an electronic analytical balance and transferred into 100-mL volumetric flasks. It was then dissolved in 10 mL of methanol to obtain a standard stock solution of 1000 µg/mL (the primary standard solution). From the primary standard solution, a 0.5 mL aliquot was pipetted out and made up to 5 mL to obtain a secondary or working standard solution of 100 µg/mL.
Generation of the standard calibration curve
From the secondary standard solution, aliquots of 0.25, 0.5, 0.6, 0.75, 1, 1.25, 1.5, 1.75, 2, 2.25, and 2.5 mL were pipetted and transferred to a series of pre-labelled test tubes and made up to the 5 mL mark with methanol to obtain a 5, 10, 12, 15, 20, 25, 30, 35, 40, 45, and 50 µg/mL calibration standard. Each of the concentration levels was prepared in triplicate, and the absorbances were read at 290 nm on a Nanodrop UV Spectrophotometer and recorded accordingly. The standard calibration curve was plotted on Excel using the average absorbances of the triplicate measures (n = 3) against corresponding concentrations.
Sensitivity
The limit of detection (LOD) and the limit of quantification (LOQ) were determined according to the International Council for Harmonization (ICH) guidelines47. LOD and LOQ for hydroquinone were calculated using 3.3*σ/S and 10*σ/S respectively, where σ is the standard deviation of the y-intercept and S is the slope of the calibration curve.
Method validation
The method was validated according to ICH guidelines for validation of analytical procedures (ICHAS)47. Typical validation characteristics evaluated include linearity, range, precision, and accuracy.
Intra-day and inter-day precision
Intra- and inter-day precision was performed by analysing three different batches of the test solutions, each prepared in triplicate, within 24 h (8 a.m., noon, and 4 p.m.) and on three consecutive days, respectively. Observed mean concentrations, standard deviations, % relative standard deviation (%RSD), and % recovery were calculated.
Assay of hydroquinone in skin-lightening cosmetic samples
This study adopted and validated the previously reported assay method for hydroquinone in cosmetic samples42,45. 0.5 g of each was weighed with an electronic analytical balance and transferred to a 250-mL beaker, to which 25 mL of methanol was added. The mixture was homogenised for 5 min in a water bath at 60 °C and allowed to cool until fat separation occurred. The solution was then filtered using Whatman Paper 1 (10 nm). 0.1 mL of the filtrate was pipetted out using an automatic micropipette and transferred into the well-labeled test tubes. This was then made up to a 10 mL mark with absolute methanol, properly mixed, and the absorbance read at 290 nm on a nanodrop UV spectrophotometer. This procedure was done in triplicate (n = 3) for each sample. The mean absorbance reading for each sample was extrapolated from a freshly prepared calibration curve to obtain the concentration of hydroquinone present in each sample.
Determination of mercury (Hg) and arsenic (As) in skin-lightening cosmetic samples
Sample preparation, digestion, and analysis
First, 0.5 g of each cosmetic sample was weighed in duplicate and transferred into a cleaned, dried 25-mL beaker. A 10 mL solvent mixture of concentrated nitric acid and perchloric acid (2:1) was added to each sample and used for digestion. The beaker was covered with a coverslip and placed on a hot plate at a regulated temperature of 105 °C under a fume cupboard, and then heated until there was a colour change from black or brown to colourless to complete the digestion process. The digested sample was allowed to cool, and the clear digest was filtered through Whatman filter paper into a cleaned, dried, 25-mL volumetric flask, and 5 mL of concentrated HCl was added to stabilise the metals. The resultant solution was made up to the 25-mL mark with de-ionized water. The same procedure was repeated for blank reagents without samples. This final solution was kept for the assay of mercury using a cold vapour atomic absorption spectrophotometer model at 253.7 nm with deuterium background correction to determine the amount of mercury in the sample.
Second, arsenic was analysed using Hydride Generation Atomic Absorption Spectrophotometry (HGAAS), which involves the conversion of all arsenic to As3+ species. 5 mL each of a 5% KI and a 1 M HCl solution was added to a 5 mL aliquot of the digested sample in a cleaned, dried, 25 mL volumetric flask, and the volume was then made up to the 25 mL mark with de-ionized water. The solution was allowed to stand overnight at room temperature for complete conversion of As5+ to As3+, which was then analysed using HGAAS for total arsenic content at 193.7 nm with deuterium background correction. The procedure was repeated with blank reagents without a sample. The amounts of mercury and arsenic present in each sample were calculated in mg/g using the formula presented below:
Quality control for the assays
Quality assurance and quality control procedures were used to ensure the high quality of the analytical data. The solutions of reference standards for mercury and arsenic (1000 mg/L) were diluted in the range of 1 to 4 mg/L. These solutions were used to prepare the AAS calibration curves for mercury and arsenic used to determine the concentrations of Hg and As in the samples. The concentrations of Hg and As in each sample were determined using a cold vapour atomic absorption spectrophotometer and Hydride Generation Atomic Absorption Spectrophotometry (HGAAS), respectively. The sensitivity (LOD and LOQ) of the methods for Hg and As estimation was checked by spiking the blank matrix at 0.5 (50%), 1.0 (100%), and 1.5 (150%) times the set limit of 1 ppm. A blank reagent was run in between samples to minimise contamination. The preparation of solutions and analyses were done in a spotless laboratory setting. The glassware that was used throughout the analyses was first carefully washed with detergent, soaked in 10% nitric acid for 24 h, rinsed with de-ionized water, and dried in an oven. All the reagents used were of analytical grade.
Statistical analysis
The statistical analysis of the results was performed on Microsoft Excel 2016 (Microsoft Inc., Office 2016). The data obtained were presented as mean, standard deviation (SD), relative standard deviation (RSD), and regression coefficient (r2).
Result and discussion
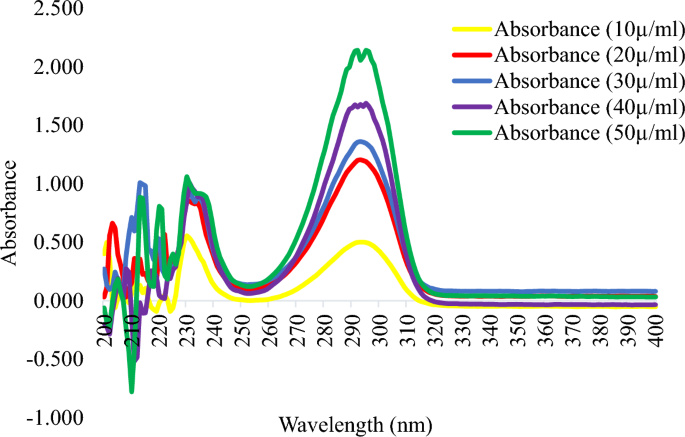
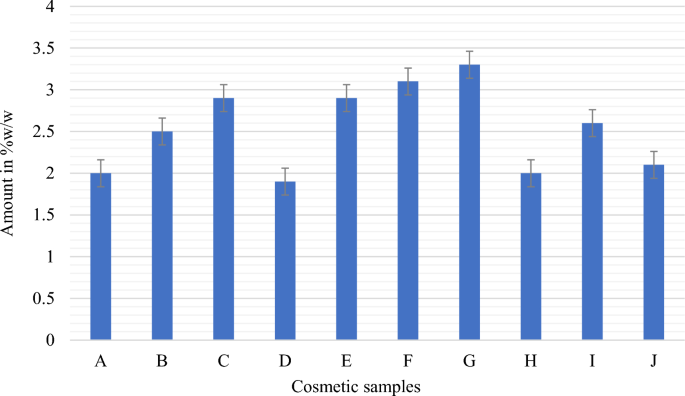
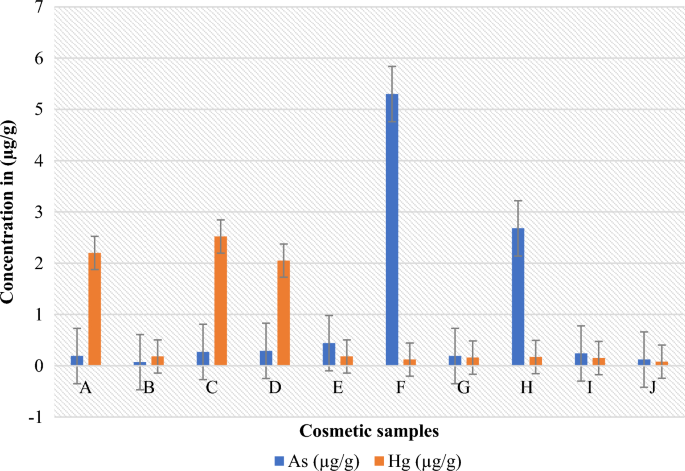
This study demonstrated that the cosmetic products that were randomly selected for evaluation had significant amounts of hydroquinone, mercury, and arsenic. Table 2 lists the specific characteristics of the skin-lightening cosmetics that were the subject of this investigation, whereas Table 3 lists the optical characteristics of the calibration curve for the UV spectrophotometer. The spectra patterns for the reference hydroquinone powder at different concentrations, ranging from 10 to 50 µg/mL, are shown in Figure 1. Figure 2 displays the samples with hydroquinone concentrations that are above the permissible limit for cosmetic products. The precision for the analysis of hydroquinone is shown in Table 4. Table 5 shows the optical characteristics of mercury and arsenic as well as the AAS instrumentation parameters. While the levels of arsenic in samples F and H were extremely high, samples A, C, and D showed significant amounts of mercury (Hg) in the cosmetic samples, which are over the permitted limit of 1 µg/g for cosmetic products (Figure 3).
Descriptive characteristics of the skin-lightening cosmetic samples
All the products were within the labelled expiration date as of the time of the analysis, while only six of the ten products were registered with the National Agency for Food and Drug Administration and Control (NAFDAC). Most of the products evaluated were manufactured in Cote d'Ivoire (54.6%) and the Republic of Togo (27.3%). Two of the products investigated had no information about the country of origin. Three of the products explicitly indicated the presence of 2% hydroquinone on their product labels. Similarly, one of the cosmetics indicated the presence of hydroquinone on its label but omitted the amount.
In contrast, the remaining six products neither acknowledged the presence of hydroquinone nor disclosed any related amount on their skin-lightening cosmetic labels. Also, none of the ten skin-lightening cosmetics purchased provided any information about the presence of mercury or arsenic on their product labels. Furthermore, one sample out of the ten failed to include a batch number on its label. Surprisingly, it was observed that none of the ten skin-lightening cosmetics analysed were manufactured in Nigeria. This suggests that a significant portion of these skin-lightening products were imported into the country, potentially without adequate consideration for the possible harmful effects of their constituents.
Identification of reference hydroquinone powder using UV scans and melting points
From Figure 1, the lowest and highest absorbance readings were obtained at concentrations of 10 and 50 µg/mL, respectively. The peak absorbance was obtained at 290 nm, which corresponds to the British Pharmacopoeia (BP) specification for hydroquinone (BP 2016). The melting point obtained was 172 °C, which also corresponds to the BP specification. Thus, the identity of our reference hydroquinone was confirmed with a UV scan and melting point determination.
Identification of hydroquinone in the skin-lightening cosmetic samples
The result obtained shows that all ten skin-lightening cosmetic samples had comparable Rf values with the reference hydroquinone powder, indicating the presence of hydroquinone in the samples.
Quantitative determination of hydroquinone in skin-lightening cosmetic samples
The concentration of hydroquinone in each cosmetic sample was determined using a validated UV-spectrophotometric method at a wavelength of 290 nm. The method showed good linearity over a concentration range of 5 to 50 µg/mL (Table 3). The linearity equation was y = 0.0307x + 0.0192, and the mean regression coefficient (r2) was 0.9993 (Table 3). The method showed good precision and accuracy, with percentage relative standard deviation (%RSD) and percentage (%) recovery ranges of 0.01–0.35% and 95.85–103.56%, respectively (Table 3). The results showed that all the %RSD was below 2%, thereby attesting to the sensitivity, reliability, and accuracy of the analytical method. The limits of detection (LOD) and limits of quantification (LOQ) obtained were 0.7524 and 2.2801 µg/mL, respectively (Table 3). Intra-day validation gave the lowest and highest percentage recovery of 98.23% and 104.82%, respectively. The inter-day validation gave 98.23% and 99.85% as the lowest and highest percentage recovery, respectively (Table 4).
Quantitative analysis to determine the content of hydroquinone in the ten cosmetic samples showed varying amounts, ranging from 1.9 to 3.3% w/w. Samples D and G contained the lowest and highest amounts of hydroquinone, respectively. Further, samples A, D, and H contained 2%, 1.9%, and 2% w/w hydroquinone, respectively (Fig. 2), which were within the 2% w/w maximum permissible limit set by the WHO for cosmetics48. The remaining seven samples (B, C, E, F, G, I, and J) were found to contain hydroquinone in amounts ranging from 2.1 to 3.3% w/w, surpassing the allowable limit of 2% for cosmetic products48. In this study, most of the cosmetic packages did not provide information about the presence of hydroquinone, while only four samples explicitly stated the inclusion of hydroquinone on their product labels. Among these, only sample D contained the permitted amount of hydroquinone (1.9% w/w). Conversely, samples E and I contained 2.9 and 2.6% w/w of hydroquinone, respectively, exceeding the permitted threshold for cosmetics. Despite claims on their labels that they contained 2% w/w hydroquinone, samples D, E, and I were found to contain higher levels. Similarly, sample F was assayed to contain 3.1% w/w hydroquinone, surpassing the allowable limit. Notably, although sample F indicated the presence of hydroquinone on its label, it failed to specify the amount present.
These findings unveil a concerning reality: many skin-lightening cosmetics marketed in Ilorin, Nigeria contain varying and often excessive amounts of hydroquinone. What is particularly troubling is that these hydroquinone concentrations surpass the permissible limits for cosmetics. Consistent with other studies are the facts that most skin-lightening products contain chemicals such as hydroquinone and heavy metals that make them unsafe for use13,28. Despite this knowledge, these products continue to be widely distributed and used without strict regulatory control. Our findings also revealed that some of these cosmetic products marketed in Ilorin, Nigeria lack NAFDAC approval. Regrettably, many of those with NAFDAC approval failed quality assessments, surpassing the permissible limits for hydroquinone in cosmetics28,49. Prolonged and consistent use of hydroquinone-containing cosmetics elevates the risk of developing disorders attributed to hydroquinone4,25,26,27.
Long-term use of hydroquinone in cosmetics can lead to severe and significant adverse health effects, including conditions such as carcinoma4,9,24 and ochronosis21,25. These are diseases of public health importance, underscoring the necessity to proactively prevent their occurrence. Therefore, implementing preventive measures becomes paramount, and a crucial aspect of this involves eliminating potential risk factors such as harmful substances in cosmetic products. Moreover, the ramifications of long-term hydroquinone usage extend beyond these serious disorders. Prolonged exposure to hydroquinone has been associated with a range of adverse effects, including irritative dermatitis, melanocyte destruction, contact dermatitis, neuronal damage, and mutations4,21,26,27,28.
Mercury and arsenic contents in skin-lightening cosmetic samples
Skin-lightening cosmetics used by women, young girls, and certain men for cosmetic purposes often contain heavy metals like mercury and arsenic50. The present study evaluated the mercury content of ten samples of skin-lightening cosmetics. The observed mercury concentrations ranged from 0.08 to 2.52 μg/g, with the highest and lowest concentrations being detected in samples C and J, respectively (Fig. 3). In an effort to ensure consumer safety, both the FDA and WHO have set a limit of 1.0 μg/g for mercury content in skin-lightening cosmetics13,40,49,51. Significantly, the findings of this study revealed that each of the ten examined skin-lightening cosmetics contained varying amounts, and three of these samples exceeded the permissible limit set for cosmetic products, indicating an unsettling presence of elevated mercury levels.
Similarly, none of the cosmetic products displayed product labels indicating the presence of mercury on their packaging, corroborating the previously reported findings4. The exceedingly high Hg levels in some of these cosmetics raise serious health concerns40. The identification of excessive mercury levels in three of the examined cosmetics serves as a crucial warning of potential health risks for regular users of skin-lightening cosmetics. Additionally, it is important to note that even relatively small quantities of mercury in skin-lightening cosmetics have been shown to have harmful effects. For instance, it was discovered that mice exposed to skin-lightening cosmetics containing 0.319 μg/g of mercury had histological abnormalities in their brain, liver, and kidney tissue38.
Also, regular use of mercury-containing cosmetics during pregnancy and lactation was reported to cause growth abnormalities in newborns11,52. Further, long-term exposure to high concentrations of Hg has been associated with adverse health effects, including hepatic damage53, renal impairment54,55,56, and neurological damage11, such as ataxia, muscle weakness, increased tendon reflex, numb limbs, speech distortion, and difficulty chewing and swallowing11. Many other studies have also reported high mercury concentrations in skin-lightening cosmetics38,39. Prolonged exposure to mercury causes significant health risks of utmost public health importance. Such exposures have contributed to a heightened prevalence of cancers, neuronal disorders, and hepatic and renal failure11,13,36.
The severe health problems caused by exposure to Hg have prompted many countries, like the EU29, the US57, Canada58, and some African countries39(Ghana, Uganda, and Nigeria), to impose bans on the use of Hg in cosmetic products40,57. Nevertheless, the effects of the ban on mercury in cosmetics remain uncertain in Nigeria, given that cosmetics containing mercury are still widely consumed by users5. The intentional use of mercury in skin-lightening cosmetics is prohibited by the Drugs and Cosmetics Act59. The prevalent occurrence of unregulated hazardous chemicals and heavy metals in skin-lightening cosmetics is a well-documented issue in many developing countries. Nonetheless, this concern transcends national boundaries and is indeed a global problem. For example, Sahu et al. (2014)59 reported mercury in 17 of 32 skin-lightening cosmetics investigated. In a similar study by Hammann et al. (2014)60, a total of 549 skin-lightening cosmetics selected from 32 countries were confirmed to contain mercury. Addressing this menace requires the implementation of stringent regulations and effective compliance strategies to reduce the prevalence of cosmetics containing mercury. From a global perspective, it becomes imperative to establish legislation and rigorously enforce measures that prohibit the production, importation, and export of cosmetics containing mercury. Such measures would undoubtedly prove advantageous in mitigating the public health hazards associated with the routine use of mercury-containing cosmetic products.
Conversely, samples B and F contained 0.07 and 5.3 µg/g of arsenic, respectively, representing the lowest and highest measured amounts (Fig. 3). When compared to the previous studies, these values stand out as significantly elevated. For instance, the amount of arsenic found in cosmetics by Adepoju-Bello et al. (2012)61 ranged from 0.006 to 0.013 µg/g, and a similar study reported a maximum of 0.2 μg/g arsenic in body lotions62. However, the amounts of arsenic in samples F and H of our study were 5.3 μg/g and 2.68 μg/g, respectively (Fig. 3). These amounts were too high in cosmetic products, although some previous studies also reported high amounts of arsenic in skin-lightening cosmetics. For example, Mohammed et al. (2017)18 reported a range of 1.016–6.612 μg/g of arsenic in skin-lightening cosmetics, while Alquadami et al. (2013)63 reported arsenic levels between 0.34 and 14.76 μg/g in 34 examined skin-lightening cosmetics.
It is noteworthy that despite our findings of elevated arsenic levels in samples F and H (Fig. 3), the product labels on the cosmetic packaging did not mention arsenic as one of the ingredients. Furthermore, our study identified arsenic in all ten skin-lightening cosmetics studied. This raises questions regarding the source of arsenic in cosmetics, which could stem from intentional inclusion or inadvertent contamination during the manufacturing process. The introduction of arsenic into cosmetics could potentially occur through the tear and wear of metallic equipment and machinery used during the manufacturing processes64. However, it is important to note that significant arsenic contamination through these processes is not typically anticipated to be substantial.
Chronic exposure to arsenic has been reported as a significant risk factor for developing various types of cancer11,65,66,67. Long-term exposure to arsenic has been associated with a range of health problems. These encompass palmar and solar keratosis, gastrointestinal symptoms such as vomiting and diarrhea, dermatological abnormalities, neuropathy, ischemic heart disease, cognitive impairment including confusion and memory loss, respiratory disorders, hormonal imbalance, compromised immune system function, and an increased risk of diabetes64,66,67. The ability of arsenic to induce oxidative stress and mitochondrial dysfunction is the main cause of the observed neurological diseases11. Oxidative stress damages the DNA, which results in neuronal cell death14. Despite the harmful effects of arsenic, it has been found to be a component of eye shadow, lotions, cosmetics, and lipsticks68. Regrettably, no strict regulations prohibit the use of arsenic in cosmetic preparations except in the European Union69. Hence, there is currently no established limit to the amounts of arsenic that are permitted in cosmetics. This regulatory gap underscores the urgent need for comprehensive guidelines to restrict and monitor the presence of arsenic in cosmetic preparations to mitigate potential health hazards associated with its usage.
The health hazards and public health relevance of undue exposure to hydroquinone, mercury, and arsenic require that policymakers and regulatory authorities put strict restrictions on the production and importation of skin-lightening cosmetics containing these dangerous chemicals. Such measures are crucial in curbing the proliferation of unregulated cosmetic products in the market, thereby ensuring the well-being of Nigerian citizens, and mitigating the economic hardship brought about by the burden of associated health problems.
Conclusion
The ten cosmetic products examined in this study contained hydroquinone, mercury, and arsenic in varying amounts. Based on our findings and the previously reported studies, it is obvious that skin-lightening cosmetics containing these dangerous chemicals remain readily accessible in Nigeria. This persists despite the associated health risks and the explicit prohibition of their production, importation, and distribution. As a critical measure, it becomes imperative to declare the use of these products illegal, subjecting them to stringent regulatory oversight guided by the stakeholders entrusted with ensuring the safety of goods and services in Nigeria. Therefore, the establishment and enforcement of comprehensive guidelines governing the manufacturing and importation of cosmetic products in the country are imperative. This framework should ensure a thorough safety evaluation of both the raw materials and the final products prior to their introduction to the market. To protect the health of Nigerians, the findings in our study recommend the introduction and consistent enforcement of regular quality control assessments, overseen by relevant regulatory bodies. Such assessments should encompass skin-lightening cosmetics and all other cosmetic products available to consumers. The government has the duty to ensure that manufacturers of skin-lightening cosmetics comply with the established standards and specifications governing cosmetic production in Nigeria. Furthermore, regulatory authorities should actively cultivate public awareness and diligently oversee the strict regulatory control of skin-lightening cosmetics being marketed in the country. This unified effort is instrumental in mitigating the prevailing threat and ensuring the protection of the health and well-being of Nigerians.
Data availability
All data used in this study are available from the corresponding author upon reasonable request.
References
Peltzer, K., Pengpid, S. & James, C. The globalization of whitening: Prevalence of skin lighteners (or bleachers) use and its social correlates among university students in 25 countries. Int. J. Dermatol. 55, 165–172. https://doi.org/10.1111/ijd.12860 (2016).
Shroff, H., Diedrichs, P. C. & Craddock, N. Skin color, culture capital, and beauty products: An investigation of the use of skin fairness products in Mumbai, India. Front. Public Health 5, 1–9. https://doi.org/10.3389/fpubh.2017.00365 (2018).
Pramanik, S., Kumar, M. & Qureshi, A. Mercury in skin-care products in India and consumer exposure risks. Regul. Toxicol. Pharmacol. RTP 121, 104870. https://doi.org/10.1016/j.yrtph.2021.104870 (2021).
Olumide, Y. M. et al. Complications of chronic use of skin lightening cosmetics. Int. J. Dermatol. 47, 344–353. https://doi.org/10.1111/j.1365-4632.2008.02719.x (2008).
Adebajo, S. B. An epidemiological survey of the use of cosmetic skin lightening cosmetics among traders in Lagos, Nigeria. West Afr. J. Med. 21, 51–55 (2002).
Fihlani, A. P. Where black is not really beautiful. Johannesburg: BBC News; 2013. Retrieved from. (https://www.bbc.com/news/world-africa-20444798)-accessed 05/08/2023, 2013).
Egbi, O. G. & Kasia, B. Prevalence, determinants and perception of use of skin lightening products among female medical undergraduates in Nigeria. Skin Health Dis. 1, e46. https://doi.org/10.1002/ski2.46 (2021).
Dadzie, O. E. & Petit, A. Skin bleaching: Highlighting the misuse of cutaneous depigmenting agents. J. Eur. Acad. Dermatol. Venereol. 23, 741–750. https://doi.org/10.1111/j.1468-3083.2009.03150.x (2009).
Dlova, N. C., Hamed, S. H., Tsoka-Gwegweni, J. & Grobler, A. Skin lightening practices: An epidemiological study of South African women of African and Indian ancestries. Br. J. Dermatol. 173, 2–9. https://doi.org/10.1111/bjd.13556 (2015).
Matsumoto, M. et al. Risk assessment of skin lightening cosmetics containing hydroquinone. Regul. Toxicol. Pharmacol. RTP 81, 128–135. https://doi.org/10.1016/j.yrtph.2016.08.005 (2016).
Balali-Mood, M., Naseri, K., Tahergorabi, Z., Khazdair, M. R. & Sadeghi, M. Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 12, 643972. https://doi.org/10.3389/fphar.2021.643972 (2021).
Chen, J. et al. Inhibition of tyrosinase by mercury chloride: Spectroscopic and docking studies. Front. Pharmacol. 11, 81. https://doi.org/10.3389/fphar.2020.00081 (2020).
WHO. Mercury in Skin Lightening Products. Preventing Disease through Healthy Environments.Geneva, World Health Organization (https://www.who.int/health-topics/chemical-safety)-accessed 05/08/2023, 2019).
Mochizuki, H. Arsenic neurotoxicity in humans. Int. J. Mol. Sci. https://doi.org/10.3390/ijms20143418 (2019).
Alam, M. F. et al. Assessment of some heavy metals in selected cosmetics commonly used in Bangladesh and human health risk. J. Anal. Sci. Technol. 10, 2. https://doi.org/10.1186/s40543-018-0162-0 (2019).
Jose, A. & Ray, J. G. Toxic content of certain commercially available fairness creams in Indian market. Cogent Med. 5, 1433104. https://doi.org/10.1080/2331205X.2018.1433104 (2018).
Dwijayanti, E. & Susanti,. Analysis of mercury (Hg) in whitening cream distributed in Palu City by atomic absorption spectroscopy (AAS). J. Appl. Chem. Sci. 5, 430–433. https://doi.org/10.22341/jacs.on.00501p430 (2018).
Mohammed, T., Mohammed, E. & Bascombe, S. The evaluation of total mercury and arsenic in skin bleaching creams commonly used in Trinidad and Tobago and their potential risk to the people of the Caribbean. J. Public Health Res. 6, 184–189. https://doi.org/10.4081/jphr.2017.1097 (2017).
Shen, S., Li, X. F., Cullen, W. R., Weinfeld, M. & Le, X. C. Arsenic binding to proteins. Chem. Rev. 113, 7769–7792. https://doi.org/10.1021/cr300015c (2013).
Siti Zulaikha, R., Sharifah Norkhadijah, S. I. & Praveena, S. M. Hazardous Ingredients in cosmetics and personal care products and health concern: A review. Public Health Res. 5, 7–15. https://doi.org/10.5923/j.phr.20150501.02 (2015).
Nordlund, J. J., Grimes, P. E. & Ortonne, J. P. The safety of hydroquinone. J. Eur. Acad. Dermatol. Venereol. 20, 781–787. https://doi.org/10.1111/j.1468-3083.2006.01670.x (2006).
Chang, T. S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 10, 2440–2475. https://doi.org/10.3390/ijms10062440 (2009).
Mastore, M., Kohler, L. & Nappi, A. J. Production and utilization of hydrogen peroxide associated with melanogenesis and tyrosinase-mediated oxidations of DOPA and dopamine. FEBS J. 272, 2407–2415. https://doi.org/10.1111/j.1742-4658.2005.04661.x (2005).
Street, J. C., Gaska, K., Lewis, K. M. & Wilson, M. L. Skin bleaching. A neglected form of injury and threat to global skin. Afr. Saf. Promot. J. 12, 52–71 (2014).
Levitt, J. The safety of hydroquinone: A dermatologist’s response to the 2006 Federal Register. J. Am. Acad. Dermatol. 57, 854–872. https://doi.org/10.1016/j.jaad.2007.02.020 (2007).
Al-Saleh, I. Potential health consequences of applying mercury-containing skin-lightening creams during pregnancy and lactation periods. Int. J. Hyg. Environ. Health 219, 468–474. https://doi.org/10.1016/j.ijheh.2016.03.002 (2016).
McGregor, D. Hydroquinone: An evaluation of the human risks from its carcinogenic and mutagenic properties. Crit. Rev. Toxicol. 37, 887–914. https://doi.org/10.1080/10408440701638970 (2007).
Westerhof, W. & Kooyers, T. J. Hydroquinone and its analogues in dermatology—A potential health risk. J. Cosmet. Dermatol. 4, 55–59. https://doi.org/10.1111/j.1473-2165.2005.40202.x (2005).
EU. Regulation (EC) No 1223/2009 of the European Parliament and of the Council. Off. J. Eur. Union 342, 59–209 (https://www.who.int/publications/i/item/WHO-CED-PHE-EPE-219.213)-accessed-205/208/2023, 2009).
FDA. Hydroquinone studies under the National Toxicology Program (NTP). Available online: https://ntp.niehs.nih.gov/ntp/noms/support_docs/hydroquinone_may2009.pdf-accessed-05/08/2022, (2009).
Iwegbue, C. M. et al. Safety evaluation of metal exposure from commonly used moisturizing and skin-lightening creams in Nigeria. Regul. Toxicol. Pharmacol. RTP 71, 484–490. https://doi.org/10.1016/j.yrtph.2015.01.015 (2015).
Ladizinski, B., Mistry, N. & Kundu, R. V. Widespread use of toxic skin lightening compounds: Medical and psychosocial aspects. Dermatol. Clin. 29, 111–123. https://doi.org/10.1016/j.det.2010.08.010 (2011).
Rahman, A. et al. Arsenic exposure and risk of spontaneous abortion, stillbirth, and infant mortality. Epidemiology 21, 797–804. https://doi.org/10.1097/EDE.0b013e3181f56a0d (2010).
Huang, Y. K. et al. Changes in urinary arsenic methylation profiles in a 15-year interval after cessation of arsenic ingestion in southwest Taiwan. Environ. Health Perspect. 117, 1860–1866. https://doi.org/10.1289/ehp.0900560 (2009).
Michalek, I. M. et al. A systematic review of global legal regulations on the permissible level of heavy metals in cosmetics with particular emphasis on skin lightening products. Environ. Res. 170, 187–193. https://doi.org/10.1016/j.envres.2018.12.029 (2019).
IPCS. Elemental mercury and inorganic mercury compounds: Human health aspects. Geneva, World Health Organization, International Programme on Chemical Safety (Concise International Chemical Assessment Document 50). Available online: https://apps.who.int/iris/handle/10665/42607, (2003).
Weldon, M. M. et al. Mercury poisoning associated with a Mexican beauty cream. West J. Med. 173, 15–18; discussion 19 (2000). https://doi.org/10.1136/ewjm.173.1.15
Al-Saleh, I. et al. Does low mercury containing skin-lightening cream (fair & lovely) affect the kidney, liver, and brain of female mice? Cutan. Ocul. Toxicol. 24, 11–29. https://doi.org/10.1081/cus-200046179 (2005).
Uram, E., Bischofer, B. P. & Hagemann, S. Market analysis of some mercury-containing products and their mercury-free alternatives in selected regions. Gesellschaft für Anlagenund Reaktorsicherheit (GRS) mbH. Available online:https://www.osti.gov/etdeweb/biblio/21327903, (2010).
USFDA. FDA’s testing of cosmetics for arsenic, cadmium, chromium, cobalt, lead, mercury, and nickel content. Silver Spring, United States Food and Drug Administration (https://www.fda.gov/cosmetics/potential-contaminants-cosmetics/fdas-testing-cosmetics-arsenic-cadmium-chromium-cobalt-lead-mercury-and-nickel-content) (2019).
Jaishankar, M., Tseten, T., Anbalagan, N., Mathew, B. B. & Beeregowda, K. N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 7, 60–72. https://doi.org/10.2478/intox-2014-0009 (2014).
Odumosu, P. O. & Ekwe, T. O. Identification and spectrophometric determination of hydroquinone levels in some cosmetic creams. Afr. J. Pharm. Pharmacol. 4, 231–234 (2010).
Adepoju-Bello, A. A. et al. Evaluation of the concentration of toxic metals in cosmetic products in Nigeria. Afr. J. Biotechnol. 11, 16360–16364. https://doi.org/10.5897/AJB12.1411 (2012).
Popoola, O. E., Bisi-johnson, M. A., Abiodun, A. & OS, I. Heavy metal content and antimicrobial activities of some naturally occurring facial cosmetics in Nigeria. Ife J. Sci. 15, 637–644 (2013).
Nduka, J. K., Odiba, I. O., Aghoghome, E. I., Umedum, N. L. & Nwosu, M. J. Evaluation of harmful substances and health risk assessment of mercury and arsenic in cosmetic brands in Nigeria. Int. J. Chem. 8, 178–187. https://doi.org/10.5539/ijc.v8n1p178 (2016).
WHO. WHO guidelines for sampling of pharmaceutical products and related materials. WHO Technical Report Series, No. 929, 2005 (2005).
ICH-Q2(R1). Validation of Analytical Procedures: Methodology Text and Methodology. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. Available online:https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (2005).
Nourmoradi, H., Foroghi, M., Farhadkhani, M. & Vahid Dastjerdi, M. Assessment of lead and cadmium levels in frequently used cosmetic products in Iran. J. Environ. Public Health 2013, 962727. https://doi.org/10.1155/2013/962727 (2013).
Sukender, K., Jaspreet, S., Sneha, D. & Munish, G. AAS estimation of heavy metalsand trace elements in Indian herbal cosmetic preparations. Res. J. Chem. Sci. 2, 46–51 (2012).
Ramakant, S., Poornima, S., Sapina, J., Mathur, H. B. & Agarwal, H. C. Heavy metal in cosmetics-Centre for Science and Environment. Available online:https://cdn.cseindia.org/userfiles/Heavy_Metals_in_Cosmetics_Report.pdf. Cen. Sci. Environ. 45 (2014).
UNEP. Minamata Convention Initial Assessments (MIAs). Nairobi: United Nations Environment Programme. (http://www.mercuryconvention.org/Convention/Text/tabid/3426/language/en-US/Default.aspx) (2019).
Dórea, J. G. Additional comments to “Potential health consequences of applying mercury-containing skin-lightening creams during pregnancy and lactation periods”. Int. J. Hyg. Environ. Health 219, 920–921. https://doi.org/10.1016/j.ijheh.2016.07.010 (2016).
Lee, M. R., Lim, Y. H., Lee, B. E. & Hong, Y. C. Blood mercury concentrations are associated with decline in liver function in an elderly population: A panel study. Environ. Health 16, 17. https://doi.org/10.1186/s12940-017-0228-2 (2017).
Li, Y., Zhang, B., Yang, L. & Li, H. Blood mercury concentration among residents of a historic mercury mine and possible effects on renal function: A cross-sectional study in southwestern China. Environ. Monit. Assess 185, 3049–3055. https://doi.org/10.1007/s10661-012-2772-0 (2013).
Li, P., Du, B., Chan, H. M. & Feng, X. Human inorganic mercury exposure, renal effects and possible pathways in Wanshan mercury mining area, China. Environ. Res. 140, 198–204. https://doi.org/10.1016/j.envres.2015.03.033 (2015).
Zhang, C. et al. Maternal inorganic mercury exposure and renal effects in the Wanshan mercury mining area, southwest China. Ecotoxicol. Environ. Saf. 189, 109987. https://doi.org/10.1016/j.ecoenv.2019.109987 (2020).
USFDA. CFR – Code of Federal Regulations Title 21. Chapter I: Food and Drug Administration. Subchapter G: Cosmetics. Silver Spring, United States Department Food and Drug Administration (http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=700.13&SearchTerm=mercury) (2019).
Health-Canada. Guidance on heavy metal impurities in cosmetics. Ottawa, Health Canada (http://www.hc-sc.gc.ca/cps-spc/pubs/indust/heavy_metals-metaux_lourds/index-eng.php)-accessed 05/08/2023 (2012).
Sahu, R., Saxena, P. & Johnson, S. Heavy metals in Cosmetics-Centre for Science and Environment (2014).
Hamann, C. R. et al. Spectrometric analysis of mercury content in 549 skin-lightening products: Is mercury toxicity a hidden global health hazard? J. Am. Acad. Dermatol. 70, 281-287.e283. https://doi.org/10.1016/j.jaad.2013.09.050 (2014).
Adepoju-Bello, A. A. et al. Evaluation of the concentration of toxic metals in cosmetic products in Nigeria. Afr. J. Biotechnol. 11, 16360–16364. https://doi.org/10.5897/AJB12.1411 (2012).
Hepp, N. M., Mindak, W. R., Gasper, J. W., Thompson, C. B. & Barrows, J. N. Survey of cosmetics for arsenic, cadmium, chromium, cobalt, lead, mercury, and nickel content. J. Cosmet. Sci. 65, 125–145 (2014).
Alqadami, A. A., Abdalla, M. A., AlOthman, Z. A. & Omer, K. Application of solid phase extraction on multiwalled carbon nanotubes of some heavy metal ions to analysis of skin whitening cosmetics using ICP-AES. Int. J. Environ. Res. Public Health 10, 361–374. https://doi.org/10.3390/ijerph10010361 (2013).
Naujokas, M. F. et al. The broad scope of health effects from chronic arsenic exposure: Update on a worldwide public health problem. Environ. Health Perspect. 121, 295–302. https://doi.org/10.1289/ehp.1205875 (2013).
Smit, N., Vicanova, J. & Pavel, S. The hunt for natural skin whitening agents. Int. J. Mol. Sci. 10, 5326–5349. https://doi.org/10.3390/ijms10125326 (2009).
Smith, A. H., Lopipero, P. A., Bates, M. N. & Steinmaus, C. M. Public health. Arsenic epidemiology and drinking water standards. Science (New York, N. Y.) 296, 2145–2146. https://doi.org/10.1126/science.1072896 (2002).
Li, L. & Chen, F. Oxidative stress, epigenetics, and cancer stem cells in arsenic carcinogenesis and prevention. Curr. Pharmacol. Rep. 2, 57–63. https://doi.org/10.1007/s40495-016-0049-y (2016).
Sainio, E. L., Jolanki, R., Hakala, E. & Kanerva, L. Metals and arsenic in eye shadows. Contact Dermat. 42, 5–10. https://doi.org/10.1034/j.1600-0536.2000.042001005.x (2000).
Ullah, A. K. M. A., Maksud, M. A., Khan, S. R., Lutfa, L. N. & Quraishi, S. B. Dietary intake of heavy metals from eight highly consumed species of cultured fish and possible human health risk implications in Bangladesh. Toxicol. Rep. 4, 574–579. https://doi.org/10.1016/j.toxrep.2017.10.002 (2017).
Acknowledgements
The authors thank Dr. Emeka of the Central Laboratory, Tanke. Ilorin, for making his laboratory facilities available for the analysis. We are thankful to Mrs. F.F. Abdulmojeed for her technical support.
Author information
Authors and Affiliations
Contributions
O.D.B. conceived, designed, carried out data analysis, and wrote the first draft of the manuscript. B.A.K. performed the experiments and participated in the data analysis. O.I.E. participated in the study’s conception and contributed to the critical revision of the manuscript. S.T.A. contributed to the study design, gave advice on data analysis, and contributed to the critical revision of the manuscript. A.J.A. R.S.O., N.S.N., and M.T.B. contributed to the critical revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bamidele, O.D., Kayode, B.A., Eniayewu, O.I. et al. Quality assessment of hydroquinone, mercury, and arsenic in skin-lightening cosmetics marketed in Ilorin, Nigeria. Sci Rep 13, 20992 (2023). https://doi.org/10.1038/s41598-023-47160-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-47160-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.