Abstract
This study investigated the effect of silane-based silica (SiO2) Janus nanoparticles (JNPs) on stabilizing the foam generated by different types of gases. Two types of SiO2 JNPs were synthesized through surface modification using HMDS and APTS silane compounds. Static analyses were conducted to examine the impact of different concentrations of the synthesized nanoparticles in various atmospheres (air, CO2, and CH4) on surface tension, foamability, and foam stability. The results indicated that the synthesized SiO2 JNPs and bare SiO2 nanoparticles exhibited nearly the same ability to reduce surface tension at ambient temperature and pressure. Both of these nanoparticles reduced the surface tension from 71 to 58–59 mN m−1 at 15,000 ppm and 25 °C. While bare SiO2 nanoparticles exhibited no foamability, the synthesis of SiO2 JNPs significantly enhanced their ability to generate and stabilize gas foam. The foamability of HMDS-SiO2 JNPs started at a higher concentration than APTS-SiO2 JNPs (6000 ppm compared to 4000 ppm, respectively). The type of gas atmosphere played a crucial role in the efficiency of the synthesized JNPs. In a CH4 medium, the foamability of synthesized JNPs was superior to that in air and CO2. At a concentration of 1500 ppm in a CH4 medium, HMDS-SiO2 and APTS-SiO2 JNPs could stabilize the generated foam for 36 and 12 min, respectively. Due to the very low dissolution of CO2 gas in water at ambient pressure, the potential of synthesized JNPs decreased in this medium. Finally, it was found that HMDS-SiO2 JNPs exhibited better foamability and foam stability in all gas mediums compared to APTS-SiO2 JNPs for use in oil reservoirs. Also, the optimal performance of these JNPs was observed at a concentration of 15,000 ppm in a methane gas medium.
Similar content being viewed by others
Introduction
In today's context, the decrease in natural oil production from reservoirs has highlighted the necessity for implementing new methods to either increase or sustain production levels1. To date, various techniques collectively known as enhanced oil recovery (EOR) methods have been employed2,3,4. Many of these methods involve the injection of water, gas, and various chemicals into oil reservoirs. Among these, gas injection stands out as a crucial and practical EOR approach5,6. Gas injection is economically advantageous due to its widespread availability and, unlike water injection, it avoids issues such as scaling, rising aquifer levels, bacterial growth, and similar concerns. Typically, associated gas is reinjected into the reservoir to maintain the pressure necessary for continuous oil production. However, the key challenge in gas injection lies in controlling the mobility of the injected gas, given its high mobility, which poses significant challenges for its injection and movement within the reservoir7,8.
When gas is injected into oil reservoirs, several issues may arise within the reservoir itself, including the fingering phenomenon, gravity override, and channelization (especially in fractured carbonate reservoirs). Due to its high mobility, gas rapidly reaches the production zone during injection, ultimately resulting in only the injected gas being recovered from the production well after some time9. To address these challenges, foam formation has emerged as a highly practical and cost-effective method for controlling mobility and enhancing apparent viscosity10. Foam can be generated using water, gas, and a surfactant as stabilizers. Injecting foam into the porous medium can improve the mobility ratio of the injected fluid, enabling better sweep efficiency and increased oil recovery from a larger reservoir surface area11,12.
While foam is widely accepted for EOR, certain issues hinder its practicality. Foam is typically generated using surfactants, and as a result, it can become unstable in oil reservoirs when exposed to high pressure and temperature. Therefore, modification techniques, such as the use of nanoparticles and polymers, become necessary to stabilize foam under the actual conditions of oil reservoirs10. In such circumstances, nanoparticles play a crucial role in preventing foam collapse and altering the wettability of reservoir rock from oil-wet to strongly water-wet13.
However, the strong van der Waals forces interactions among nanoparticles present a technical challenge for their colloidal stability in the base fluid14,15. One of the most commonly employed nanoparticles in the EOR process is SiO2 nanoparticles, extensively used to enhance foam stability16,17,18,19,20. SiO2 nanoparticles are favored in EOR experiments due to their availability and unique physio-chemical properties21. They are cost-effective and highly compatible with oil reservoirs, minimizing formation damage and destructive interactions with reservoir rock. SiO2 nanoparticles exhibit remarkable mechanical and chemical resistance, even under harsh oil reservoir conditions, including high temperature, pressure, and salinity22. They enhance foam stability by reducing the rate of liquid drainage and increasing maximum capillary pressure. Being inherently hydrophilic and highly reactive, SiO2 nanoparticles can establish strong bonds with various substances, particularly water molecules23. By fine-tuning the hydrophilicity of the SiO2 nanoparticles' surface, these particles can reduce interfacial surface tension (IFT) and extend the stability of generated foam for several days by residing at the interface between the water and gas phases24,25. To enhance the hydrophobicity of SiO2 nanoparticles, silane materials can be employed as coating agents26. This coating layer results in particle accumulation in the water phase, leading to increased viscosity and, consequently, a significant improvement in foam stability27. Previous studies have demonstrated that SiO2-stabilized foams in different gases can recover more oil from porous media compared to surfactant-stabilized foam28,29.
With the recent advancements in nanoparticle surface modification techniques, the use of JNPs as a novel strategy to enhance foam efficiency in oil reservoirs has garnered attention30. JNPs have gained widespread use in recent years due to their distinctive properties for stabilizing gas–water emulsions31,32,33. Unlike common nanoparticles, JNPs exhibit two or more distinct characteristics. For example, the simplest JNPs feature both a hydrophobic and a hydrophilic part34,35. These JNPs possess significantly higher adsorption free energy compared to conventional nanoparticles, resulting in greater interfacial activity and improved adsorption stability. These attributes enhance the stability of gas foam, particularly under high-temperature and high-pressure conditions30. JNPs can be synthesized using methods such as masking, bottom-up assemblies, and controlled phase separations, with the masking method being the simplest and quickest36. These nanoparticles have the capability to stabilize gas–water emulsions for extended periods37,38.
Operational and reservoir conditions also play a pivotal role in the effectiveness of an EOR process39. Temperature is a primary factor influencing foam efficiency. Research has shown that at elevated temperatures, the surface tension between gas and water increases, leading to enhanced particle mobility and separation of surface-active ingredients40. Pressure is another significant factor; an increase in pressure reduces the surface tension between gas and water, consequently improving foamability. However, higher pressure also results in increased gas solubility in the water phase, decreased water viscosity, and reduced foam stability41. It is also anticipated that foam stability will improve with an increase in the concentration of JNPs up to an optimal level. Additionally, the type of gas used appears to have an impact under real conditions. Previous findings have indicated that the performance of N2 gas foam surpasses that of CO2, highlighting the importance of gas selection40,42.
Despite the numerous studies conducted, the utilization of these nanoparticles presents various challenges that researchers must address. There is a need for further investigation into surface modification methods and the optimal synthesis of JNPs. Additionally, the potential of methane (CH4) gas, readily available but not yet explored, as a suitable medium for foam production with the assistance of JNPs should be examined. In general, the impact of gas type on foam properties, encompassing foamability and foam stability of JNPs, remains unexplored.
Therefore, this study comprises two main components. The first part aims to synthesize SiO2 JNPs using two different silane agents, namely HMDS and APTS. Characterization of the synthesized JNPs involves various analyses, including FTIR, DLS, and SEM. To assess the colloidal stability of nanoparticles and their resistance to aggregation, Zeta potential measurements were conducted. In the context of gas injection processes in oil reservoirs, various gases are employed, and reducing the surface tension between the injected fluid and oil constitutes one of the primary mechanisms for increasing oil production. Consequently, the second part of the study investigates the effectiveness of synthesized nanoparticles in enhancing foamability, foam stability, and reducing surface tension for their application in EOR processes, representing a novel exploration in this area. Static tests were employed to determine the initial concentration of JNPs required to generate foam and to measure the height of the resulting foam in a visible foam generator column. Finally, three different gases, including CO2, CH4, and air, were utilized to examine the impact of gas type on the performance of synthesized JNPs at varying concentrations.
Materials and methods
Materials
Due to the favorable characteristics and significant potential of SiO2 nanoparticles in EOR processes, SiO2 nanoparticles served as the foundation for synthesizing JNPs. (3-Aminopropyl)triethoxysilane (APTS, Molar mass = 161.395 g/mol, Density = 0.77 g/cm3), Hexamethyldisilazane (HMDS, Molar mass = 221.372 g/mol, Density = 0.946 g/cm3), and commercial SiO2 nanoparticles with an average diameter of 30 nm were procured from Sigma Aldrich Company. Various materials were utilized in the synthesis process, including solid paraffin wax (CAS number = 8002-74-2, Melting point = 58–62 °C, Density = 0.82 g/cm3 at 20 °C) obtained from Tetra-Chem Company. Methanol (CH3OH) and Chloroform (CHCl3) were provided by Merck Company. All chemicals used were of analytical grade and employed without further modification. Additionally, deionized (DI) water was sourced from Zolal Company.
Synthesis of JNPs
In this study, the Pickering emulsion method was employed to prepare asymmetric SiO2 JNPs. This method is widely recognized for its utility in synthesizing a diverse range of JNPs characterized by small size, controllable morphology, and distinct shapes and compositions43. To facilitate the Pickering emulsion, paraffin wax was utilized as the oil medium. Additionally, two different silane agents, namely APTS and HMDS, were employed to confer hydrophobic properties to the surface of SiO2 nanoparticles. In the process of creating an oil-in-water Pickering emulsion, bare SiO2 nanoparticles were adsorbed onto the paraffin wax/water interface under high temperature conditions. Subsequently, the solution was rapidly cooled to solidify the paraffin wax phase, effectively immobilizing the SiO2 nanoparticles at the interface. Subsequent modification of the SiO2 nanoparticles was achieved through the application of silane agents. Notably, due to the protective layer of wax, only the exposed surface of the SiO2 nanoparticles could be modified44. One noteworthy advantage of this method is its versatility, allowing for the synthesis of various JNPs by simply altering the surface modifier agent. The following provides a detailed description of the JNP synthesis method employed in this study.
Initially, 100,000 ppm of SiO2 nanoparticles were subjected to sonication in 100 mL of DI-water for 1 h at maximum power. Subsequently, the SiO2 nanoparticles were required to adhere to the paraffin wax. To achieve this, solid paraffin wax (weighing 10 times that of the SiO2 nanoparticles) was slowly introduced into the prepared solution. The resulting mixture was stirred on a heated stirrer at 110 °C and 400 rpm for a duration of 3 h. Following this, the product was rapidly cooled in an ice bath to prevent the separation of nanoparticles from the paraffin wax, as depicted in Fig. 1. During this stage, the nanoparticles became distributed on the surface of the paraffin wax, with one side of them being coated with paraffin wax.
In the subsequent step, two super hydrophobic silanes were employed to coat the exposed surface of SiO2 nanoparticles, those not encapsulated within the paraffin. This was carried out to determine the most suitable silane for synthesizing SiO2 JNPs. To this end, a 100 mL suspension of paraffin-coated nanoparticles was subjected to centrifugation at 10,000 rpm for 30 min to remove excess liquid from the coated SiO2 nanoparticles. Subsequently, the resulting product was dissolved in a 20 mL methanol solvent. Next, 2 mL of either APTS or HMDS silane was added dropwise to the solution, and the mixture was stirred for 12 h in the case of APTS and 2 h for HMDS. It's important to note that the HMDS coating process generated NH3 gas as a byproduct. The sample was then centrifuged for 20 min to separate the methanol from the coated nanoparticles. Following this, 5 mL of chloroform was introduced to dissolve the paraffin wax produced during the process. This step was repeated to ensure the complete removal of paraffin wax from the surface of the nanoparticles. Finally, the precipitated products were dried in an oven at 100 °C for 12 h. The schematic of the SiO2 JNPs synthesis procedure is illustrated in Fig. 2.
Characterization
The synthesized products underwent several analyses for characterization. Fourier-transform infrared spectroscopy (FTIR, PerkinElmer Spectrum Version 10.03.06) was utilized to examine the bonding status and functional groups of the coating materials on the surface of the Synthesis JNPs. This analysis was conducted at room temperature within the 4000–400 cm−1 range. Dynamic light scattering (DLS, Brookhaven) analysis was employed to determine the hydrodynamic size distribution of the produced particles. In this method, the hydrodynamic diameter of the particles is measured. It's worth noting that due to partial agglomeration of the particles, the obtained size may be larger than their actual size. To reduce agglomeration, synthesized nanoparticles were dispersed in 10 mL of DI-water (at a concentration of 1 ppm) through sonication for 30 min. scanning electron microscopy (SEM) was used to examine the shape and morphology of the produced nanoparticles. To avoid the presence of any agglomerated particles in the captured images, the solution containing synthesized nanoparticles was appropriately diluted. A Zeta potential analyzer device (Zetasizer Nanoseries, Malvern) was employed to measure the surface charge of nanoparticles at room temperature. Zeta potential analysis assesses the repulsion/attraction forces between particles and provides insights into their colloidal stability in a solution.
Foam stability analysis
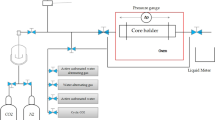
The analysis of foam stability assessed the suitability of the synthesized JNPs for use in EOR operations45. To this end, various solutions of these nanoparticles at different concentrations were prepared. Figure 3 provides a schematic representation of the experimental setup used to evaluate foam stability. In this procedure, a specific quantity of synthesized nanoparticles was dissolved in 100 mL of DI-water using sonication. Subsequently, these prepared solutions were introduced into the visualization cell, into which three different gases, namely CH4, CO2, and air, were injected using a pump. The stability of the resulting foam was gauged by monitoring the percentage changes in the height of the generated foam within the foam-generating cell over time. In these analyses, alterations in the foam column height and the extent of stability were measured comprehensively, starting from the moment gas injection ceased and the chamber was sealed, continuing until the foam generated by the nanoparticles had dissipated. All foam stability analyses were conducted under ambient temperature and pressure conditions.
Surface tension measurement
Surface tension is a crucial parameter for observing the behavior of a fluid at the interface of two phases. In this context, surface tension tests were conducted to investigate the interactions at the gas/water interface in the presence of nanoparticles. Initially, samples were prepared at various nanoparticle concentrations in DI-water. Subsequently, the density of each heterogeneous solution was measured using a pycnometer, and the surface tension of the solution was determined using the pendant drop method. These experiments were conducted under different gas atmospheres, specifically air, CO2, and CH4, while maintaining ambient pressure and temperature conditions.
Results and discussion
Characterization of synthesized JNPs
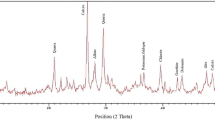
FTIR analysis was conducted to confirm the presence of HMDS and APTS functional groups on the surface of the synthesized JNPs. Figure 4 illustrates the spectra of both bare and SiO2 JNPs. In the IR spectra presented in Fig. 4a–c, several key peaks were observed. The peaks at 471–474 cm−1, 808–813 cm−1, and 1103 cm−1 correspond to the Si–O, Si–OH, and Si–O–Si bands in SiO2 nanoparticles, and these are consistent across all curves. The broad peaks in the 2800–3400 cm−1 range are associated with the stretching vibration of the O–H band. Peaks at 2856 and 2928 cm−1 are linked to the C–H stretching bonding of Si–CH3 resulting from the reaction with HMDS. Additionally, the absorption peak around 419 cm−1 confirms the presence of an HMDS agent on the surface of the synthesized nanoparticles46. Following the reaction of SiO2 nanoparticles with APTS, peaks around 1489 cm−1, 1570 cm−1, and 2928 cm−1 are observed, which can be attributed to the N–O, N–H, and C–H bands, respectively. These peaks provide evidence for the presence of APTS on the surface of the SiO2 nanoparticles47.
Figure 5 displays the DLS results of SiO2 nanoparticles before and after modifications. In Fig. 5a, it can be observed that the average hydrodynamic diameter of bare SiO2 nanoparticles was approximately 30–50 nm. The laser light diffraction spectrum in this figure encompassed a range of nanoparticles spanning from 15 to 100 nm. Following the surface modification with HMDS, as depicted in Fig. 5b, the average hydrodynamic diameter of SiO2 JNPs measured between 70 and 80 nm. In this graph, the smaller peak corresponds to bare SiO2 nanoparticles that did not undergo the reaction with HMDS. Conversely, the larger peak is attributed to nanoparticles that were either strongly agglomerated or not entirely separated from the wax paraffin. This observation suggests that a significant proportion of the bare SiO2 nanoparticles had indeed reacted with the HMDS silane agent. Figure 5c illustrates the DLS analysis for APTS-SiO2 JNPs. It is evident that the particle sizes are very consistent with each other, with the average hydrodynamic diameter of these nanoparticles falling within the range of approximately 100 nm. This also indicates that the reactivity of bare SiO2 nanoparticles with APTS silane and their separation from the wax paraffin using a chloroform solution were achieved more effectively.
Figure 6 presents a representative FESEM image of the synthesized SiO2 JNPs. This image illustrates that all the synthesized SiO2 JNPs exhibited an approximately spherical shape. Consequently, it can be deduced that the use of silane agents did not have a notable impact on the morphology of the bare SiO2 nanoparticles. Additionally, the average size of these nanoparticles was estimated to be less than 100 nm. Notably, the HMDS-SiO2 JNPs displayed a more favorable distribution, and they exhibited less agglomeration in comparison to the APTS-SiO2 JNPs. While the surface coverage of nanoparticles with a layer of silane agents did lead to an increase in their size, it also enhanced the resistance of these particles for use in acidic and alkaline environments48.
It's worth noting that the stability of JNP-stabilized gas foam is significantly influenced by the size of the synthesized JNPs. Reducing nanoparticle size is a critical parameter that can enhance foam stability, increase apparent foam viscosity, improve nanoparticle catalytic performance, and reduce the likelihood of sedimentation within the pores of the reservoir rock. Smaller nanoparticles tend to be readily absorbed at the gas–liquid interface due to their greater diffusion and higher surface-to-volume ratio49. Conversely, larger nanoparticles can contribute to foam stability by delaying the liquid drainage process when they are positioned within the lamella and at the plateau border50. Therefore, it is reasonable to assume that there exists an optimal size for JNPs that maximizes foam stability. This aspect should be investigated in future research.
Silane compounds consist of relatively long-chain molecules. While these compounds can effectively modify the surface of nanoparticles and enhance their colloidal stability, they generally lead to an increase in the size of nanoparticles compared to materials that employ an electrostatic stabilization mechanism. Consequently, it is recommended that, to maintain the small size of JNPs and establish an appropriate balance between hydrophilicity and hydrophobicity on the surface of SiO2 nanoparticles, acid agents such as citric acid or ascorbic acid be considered for the synthesis of SiO2 JNPs.
Zeta potential analysis
Zeta potential is a crucial parameter for assessing the stability of nanoparticles within a colloidal solution. The magnitude of the Zeta potential value reflects the ability of electrostatic repulsion forces between neighboring particles to disperse them within a solution. As such, Zeta potential is directly correlated with the stability of particles in a colloidal solution51. Zeta potential analysis was conducted and the results, measured in millivolts (mV), are presented in Table 1 to assess the colloidal dispersion stability of both synthesized coated and uncoated SiO2 nanoparticles.
The Zeta potential of bare SiO2 nanoparticles was measured at − 15.3 mV, which aligns with their apparent stability. After coating the SiO2 nanoparticles with HMDS, the Zeta potential of the coated nanoparticles increased to − 28.5 mV. During the silanization process, the surface of the nanoparticles became densely populated with amino group molecules, including the –NH2 functional group, leading to a notable increase in the negative charge on the nanoparticle's surface. This enhancement in surface charge contributes to improved nanoparticle dispersibility and stability in aqueous solutions, primarily through a steric stabilization mechanism. Consequently, repulsive forces between the particles are heightened, resulting in enhanced particle stability and reduced agglomeration in the solution52.
Conversely, the Zeta potential of coated SiO2 nanoparticles with APTS was measured at − 11.5 mV, signifying a slight reduction in particle stability. This decrease can be attributed to the presence of –NH2 groups within the structure of SiO2 JNPs, which leads to an increase in their hydrodynamic diameter. As a result, the size of SiO2 particles experiences a minor increase after being coated with APTS. This phenomenon necessitates a greater force to induce particle sedimentation and deposition in the solution, thereby reducing nanoparticle stability.
Effect of JNPs dispersed in different gas on the surface tension
Nanoparticles play a crucial role in enhancing oil recovery from reservoirs by lowering the surface tension between the injection fluid and the oil. The importance of surface tension stems from its direct impact on the stability of the interface between two phases53. Given that the choice of gas in an EOR operation varies depending on the type of oil reservoir and can influence the surface tension of the two phases54, we employed three different gases: air, CO2, and CH4. Figure 7 illustrates the influence of nanoparticle concentration on surface tension in the presence of these different gases.
The surface tension of water without nanoparticles measured between 71 and 72 mN m−1. Upon introducing bare SiO2 nanoparticles into the liquid phase, surface tension remained relatively unchanged at low nanoparticle concentrations. Typically, nanoparticles tend to establish an interface between water and gas, reducing the interfacial tension between the two phases. However, due to the strong hydrophilic nature of SiO2 nanoparticles' surfaces, they are present at the gas–liquid interface in small quantities and do not strongly adhere to it. Consequently, their impact on reducing interfacial tension is limited. As nanoparticle concentration in DI-water increased, the likelihood of nanoparticles being present at the interface also increased. Consequently, more particles could participate at the gas/liquid interface, hindering bubble coalescence and gradually affecting the interfacial surface, leading to reduced surface tension55. Consequently, the surface tension of the nanofluid fluctuated between 60 and 65 mN m−1 from a concentration of 1500 ppm onwards.
In contrast to bare SiO2 nanoparticles, JNPs had hydrophobic surfaces, causing them to predominantly reside at the interface of the two phases. The results demonstrated that HMDS-SiO2 and APTS-SiO2 JNPs had a similar effect on reducing surface tension between the two phases in the presence of air. This similarity arises from the strong hydrophobic properties of both synthesized HMDS-SiO2 and APTS-SiO2 JNPs, with only a small portion of the nanoparticle surface exhibiting hydrophilic characteristics. This amphiphilic structure enables these JNPs to occupy the interface between the two phases to a greater extent than bare SiO2 nanoparticles, resulting in a modest reduction in interfacial surface tension (approximately 1–2 mN m−1 compared to bare SiO2 nanoparticles). All three types of nanoparticles were able to reduce surface tension from 71 to 58–59 mN m−1 at 25 °C. Indeed, the balance between hydrophobic and hydrophilic characteristics (amphiphilic structure) plays a crucial role in the ability of JNPs to reduce surface tension. APTS and HMDS compounds, serving as appropriate silane coupling agents, are widely utilized grafting agents to enhance the interfacial behavior of inorganic oxides like SiO2. Silanization is a rapid process in its early stages but requires a substantial amount of time to complete the reaction. Hence, controlling the kinetics of silanization is crucial for regulating the surface modification process during JNP synthesis48,56.
While different gases had varying effects on surface tension, a consistent trend was observed in both CO2 and CH4 atmospheres. This similarity can be attributed to the low solubility of these gases in water at atmospheric pressure, as well as the organic nature of CH4 gas42. In this research, surface tension measurements conducted at atmospheric pressure revealed that CO2 gas only dissolves in water to a limited extent, whereas CH4, being an organic gas with a fundamentally different nature, does not dissolve in water. Consequently, the interaction between CO2 gas and the water droplet's surface resulted in a slight reduction in surface tension compared to the conditions under air42.
Effect of optimal concentration of nanoparticles on foam stability in the presence of different gases
Surfactants alone are insufficient to stabilize foams under challenging reservoir conditions, such as adsorption on rocks, mixing with oil, emulsion formation, and foam instability at high temperatures. Nanoparticles are expected to have the capability to stabilize foam under ambient conditions. The mechanism of nanoparticle placement is quite similar to that of surfactants in the formation of a thin layer at the water–gas interface57. Nanoparticles, being smaller than surfactants and benefiting from the Gypsum–Marangoni effect, possess more surface area and experience enhanced electrostatic repulsion forces between similarly charged particles within the thin water layer. These factors prevent lamellae collapse and promote foam stability58. Consequently, JNPs, sharing similarities with surfactants in nature59, have been employed in this study. Foam stability analyses were conducted with nanoparticles at various concentrations. Bare SiO2 nanoparticles are incapable of stabilizing lamellae due to their high hydrophilic properties and lack of a hydrophobic hydrocarbon chain necessary for forming weak bonds with the gas phase. Therefore, foam stability tests were exclusively conducted with the synthesized JNPs. It was also anticipated that the type of gas could potentially influence foam stability60. To investigate this hypothesis, nanoparticles were used at different concentrations under various gas atmospheres to determine the optimal nanoparticle amounts for dispersion in different gases. Figure 8 presents the results of foam stability analyses of synthesized JNPs in the presence of air.
As observed, HMDS-SiO2 JNPs did not significantly generate or stabilize foam in an air atmosphere. There was no noticeable change in foam height or production until the concentration reached 4000 ppm. However, as the concentration increased from 6000 to 15,000 ppm, the stability time improved from 2 to 28 min. Although the best results were achieved at 15,000 ppm, the optimal concentration for economic considerations was determined to be 10,000 ppm. In contrast to HMDS-SiO2 JNPs, APTS-SiO2 JNPs exhibited foam stability at a lower concentration, specifically 4000 ppm, with a stability time of 2 min. The balance between the hydrophobic and hydrophilic properties and the presence of amino groups on the surface of these nanoparticles facilitate their dissolution in water, imparting a slightly alkaline property to the solution. This slight alkalinity can have a minor impact on foam stabilization by reducing the interfacial tension61. The maximum foam stability, approximately 8 min, was observed at 15,000 ppm. Consequently, it can be inferred that APTS-SiO2 JNPs exhibited higher foamability than HMDS-SiO2 JNPs, while HMDS-SiO2 JNPs demonstrated approximately threefold higher foam stability. The results of foam stability analysis for synthesized JNPs in the presence of CO2 gas are presented in Fig. 9.
In contrast to the air atmosphere, the presence of CO2 negatively impacted the foamability achieved using the synthesized JNPs. As depicted, the HMDS-SiO2 JNPs exhibited limited ability to generate and stabilize foam in the CO2 atmosphere due to their pronounced hydrophobic nature. Only at two concentrations, 10,000 and 15,000 ppm, was some foam produced, and it displayed low stability, with a maximum of 8 min. Conversely, in the case of APTS-SiO2 JNPs, there was a modest improvement in foamability, with partial foam formation starting at a concentration of 4000 ppm. However, the foamability and foam stability of these JNPs were lower compared to HMDS-SiO2 JNPs. At a concentration of 15,000 ppm, the final stability was only 6 min. The results of foam stability analysis for synthesized JNPs in the presence of a CH4 atmosphere are illustrated in Fig. 10.
Figure 10 illustrates that the CH4 gas atmosphere was favorable for foamability when using the synthesized JNPs. Due to the hydrophobic nature of HMDS-SiO2 JNPs and the high resemblance of CH4 gas to an oil phase, a significant portion of the nanoparticles interfaced with the fluids, resulting in relatively good foam stability. However, the foam height did not increase substantially. This nanoparticle does not exhibit surfactant-like properties, and foam formation is primarily attributed to the creation of a nanoparticle layer at the fluid interface. Increasing the nanoparticle concentration from 4000 to 15,000 ppm led to an increase in foam stability from 4 to 36 min. The stability mechanism of APTS-SiO2 JNPs differed. This type of nanoparticle behaves somewhat similarly to surfactants due to the presence of a hydrocarbon chain. However, it generally has limited ability to stabilize the water–gas interface. The extent of foam generation and foam stability with these JNPs was lower compared to HMDS-SiO2 JNPs. Partial foamability was observed with this nanoparticle starting at a concentration of 2000 ppm. The final stability of the produced foam using this nanoparticle was achieved at a concentration of 15,000 ppm, with stability lasting for 10 min.
The summary of foamability and foam stability analysis results for the synthesized JNPs in this research is presented in Table 2. The amphiphilic structure of the synthesized SiO2 JNPs enables these particles to be highly effective at the water/gas interface. The presence of JNPs results in thicker lamellae, indicating slower liquid drainage and prevention of bubble coalescence. The greater the balance between the hydrophilic and hydrophobic heads in the amphiphilic structure of SiO2 JNPs, the stronger the tendency for these particles to be located at the water/gas interface. In this scenario, a robust layer with high adhesion energy is formed, leading to the production of long-term stable foam. This balance is more pronounced in the amphiphilic structure of HMDS-SiO2. Consequently, HMDS-SiO2 JNPs exhibit a greater ability to stabilize foam in the presence of various gases compared to APTS-SiO2 JNPs.
Conclusions
The primary objective of this study was to assess the effectiveness of JNPs in stabilizing foam for EOR processes. To achieve this goal, two types of JNPs were synthesized by coating SiO2 nanoparticles with two different silanes: HMDS and APTS. The synthesized JNPs were characterized through various analyses, including FTIR, DLS, and SEM. Foam production was conducted using three different gases: air, CO2, and CH4. The key findings of this study are as follows:
-
The average hydrodynamic diameter of HMDS-SiO2 and APTS-SiO2 JNPs was measured to be between 70–80 nm and 100 nm, respectively.
-
HMDS-SiO2 JNPs exhibited greater colloidal stability compared to APTS-SiO2 JNPs.
-
Incorporating bare SiO2 nanoparticles into the liquid phase did not significantly alter surface tension at low nanoparticle concentrations. Both HMDS-SiO2 and APTS-SiO2 JNPs had a similar effect on reducing surface tension in the presence of different gases. All three types of nanoparticles were able to reduce surface tension from 71 to 58–59 mN m−1 at 25 °C.
-
Bare SiO2 nanoparticles lacked the ability to stabilize the lamella. However, covering their surface with silane agents and synthesizing JNPs improved their performance in foam stabilization.
-
APTS-SiO2 JNPs exhibited better foamability than HMDS-SiO2 JNPs, while HMDS-SiO2 JNPs demonstrated approximately threefold higher foam stability.
-
In a CO2 medium, APTS-SiO2 JNPs showed superior foamability compared to HMDS-SiO2 JNPs. HMDS-SiO2 JNPs could only stabilize foam at concentrations of 10,000 and 15,000 ppm, with a maximum stability duration of 8 min. On the other hand, APTS-SiO2 JNPs could maintain foam stability for up to 6 min under optimal conditions.
-
In the CH4 atmosphere, increasing the concentration of HMDS-SiO2 JNPs from 4000 to 15,000 ppm resulted in increased foam stability, ranging from 4 to 36 min. In contrast, APTS-SiO2 JNPs exhibited a maximum foam stability time of only 10 min at a concentration of 15,000 ppm.
Overall, the study demonstrates the potential of JNPs, particularly HMDS-SiO2 JNPs, for enhancing foam stability in various gas environments relevant to EOR processes.
Data availability
All data generated or analysed during this study are included in this article. Email for contact: asaeedi@modares.ac.ir.
References
Gharibshahi, R., Omidkhah, M., Jafari, A. & Mehrooz, N. Parametric optimization of in-situ heavy oil upgrading using simultaneous microwave radiation and magnetic nanohybrids via Taguchi approach. Fuel 325, 124717 (2022).
Jafari, A., Hasani, M., Hosseini, M. & Gharibshahi, R. Application of CFD technique to simulate enhanced oil recovery processes: Current status and future opportunities. Pet. Sci. 17, 434–456 (2020).
Saeedi Dehaghani, A. H. & Ghalamizade Elyaderani, S. M. Application of ion-engineered Persian Gulf seawater in EOR: Effects of different ions on interfacial tension, contact angle, zeta potential, and oil recovery. Pet. Sci. 18, 895–908 (2021).
Karami, S., Dehaghani, A. H. S. & Haghighi, M. Investigation of smart water imbibition assisted with microwave radiation as a novel hybrid method of enhanced oil recovery. J. Mol. Liq. 335, 116101 (2021).
Lake, L. W., Johns, R. T., Rossen, W. R. & Pope, G. A. Fundamentals of Enhanced Oil Recovery (Society of Petroleum Engineers, 2014).
Saeedi Dehaghani, A. H., Sefti, M. V. & Amerighasrodashti, A. The application of a new association equation of state (AEOS) for prediction of asphaltenes and resins deposition during CO2 gas injection. Pet. Sci. Technol. 30, 1548–1561 (2012).
Sheng, J. J. Enhanced oil recovery in shale reservoirs by gas injection. J. Nat. Gas Sci. Eng. 22, 252–259 (2015).
Mogensen, K. & Masalmeh, S. A review of EOR techniques for carbonate reservoirs in challenging geological settings. J. Pet. Sci. Eng. 195, 107889 (2020).
Du, F. & Nojabaei, B. A review of gas injection in shale reservoirs: Enhanced oil/gas recovery approaches and greenhouse gas control. Energies 12, 2355 (2019).
Afifi, H. R. et al. A comprehensive review on critical affecting parameters on foam stability and recent advancements for foam-based EOR scenario. J. Mol. Liq. https://doi.org/10.1016/j.molliq.2021.116808 (2021).
Skauge, A., Solbakken, J., Ormehaug, P. A. & Aarra, M. G. Foam generation, propagation and stability in porous medium. Transp. Porous Media 131, 5–21 (2020).
Ahmadi, A., Saeedi Dehaghani, A. H. & Saviz, S. Experimental study of SDS foam stability in the presence of silica nanoparticle. J. Chem. Pet. Eng. 56, 203–213 (2022).
Zhang, Y. et al. Nanoparticles as foam stabilizer: Mechanism, control parameters and application in foam flooding for enhanced oil recovery. J. Pet. Sci. Eng. 202, 108561 (2021).
Lashari, N. & Ganat, T. Synthesized graphene oxide and fumed aerosil 380 dispersion stability and characterization with partially hydrolyzed polyacrylamide. Chin. J. Chem. Eng. 34, 307–322 (2021).
Aliabadian, E. et al. Application of graphene oxide nanosheets and HPAM aqueous dispersion for improving heavy oil recovery: Effect of localized functionalization. Fuel 265, 116918 (2020).
Alcorn, Z. P. et al. Pore-and core-scale insights of nanoparticle-stabilized foam for CO2-enhanced oil recovery. Nanomaterials 10, 1917 (2020).
Zhao, J., Torabi, F. & Yang, J. The synergistic role of silica nanoparticle and anionic surfactant on the static and dynamic CO2 foam stability for enhanced heavy oil recovery: An experimental study. Fuel 287, 119443 (2021).
Harati, S., Bayat, A. E. & Sarvestani, M. T. Assessing the effects of different gas types on stability of SiO2 nanoparticle foam for enhanced oil recovery purpose. J. Mol. Liq. 313, 113521 (2020).
Rattanaudom, P., Shiau, B.-J., Suriyapraphadilok, U. & Charoensaeng, A. Effect of pH on silica nanoparticle-stabilized foam for enhanced oil recovery using carboxylate-based extended surfactants. J. Pet. Sci. Eng. 196, 107729 (2021).
Rezaei, A., Derikvand, Z., Parsaei, R. & Imanivarnosfaderani, M. Surfactant-silica nanoparticle stabilized N2-foam flooding: A mechanistic study on the effect of surfactant type and temperature. J. Mol. Liq. 325, 115091 (2021).
Lashari, N. & Ganat, T. Emerging applications of nanomaterials in chemical enhanced oil recovery: Progress and perspective. Chin. J. Chem. Eng. 28, 1995–2009 (2020).
Bello, A., Ivanova, A. & Cheremisin, A. Enhancing N2 and CO2 foam stability by surfactants and nanoparticles at high temperature and various salinities. J. Pet. Sci. Eng. 215, 110720 (2022).
Gharibshahi, R., Jafari, A. & Asadzadeh, N. Influence of different nanoparticles on the gas injection performance in EOR operation: Parametric and CFD simulation study. Can. J. Chem. Eng. 1, 1–14 (2023).
Bhatt, S., Saraf, S. & Bera, A. Perspectives of foam generation techniques and future directions of nanoparticle-stabilized CO2 foam for enhanced oil recovery. Energy Fuels 37, 1472–1494 (2023).
Rahman, A., Torabi, F. & Shirif, E. Surfactant and nanoparticle synergy: towards improved foam stability. Petroleum 9, 255–264 (2023).
Horozov, T. S. Foams and foam films stabilised by solid particles. Curr. Opin. Colloid Interface Sci. 13, 134–140 (2008).
Binks, B. P., Kirkland, M. & Rodrigues, J. A. Origin of stabilisation of aqueous foams in nanoparticle–surfactant mixtures. Soft Matter 4, 2373–2382 (2008).
Risal, A. R. et al. Experimental investigation of enhancement of carbon dioxide foam stability, pore plugging, and oil recovery in the presence of silica nanoparticles. Pet. Sci. 16, 344–356 (2019).
Hurtado, Y., Franco, C. A., Riazi, M. & Cortés, F. B. Improving the stability of nitrogen foams using silica nanoparticles coated with polyethylene glycol. J. Mol. Liq. 300, 112256 (2020).
Sun, N. et al. Janus nanographene oxide with aerophilic/hydrophilic characteristics for enhancing foam stability in high-temperature reservoirs. J. Mol. Liq. 371, 121087 (2023).
Tohidi, Z., Teimouri, A., Jafari, A., Gharibshahi, R. & Omidkhah, M. R. Application of Janus nanoparticles in enhanced oil recovery processes: Current status and future opportunities. J. Pet. Sci. Eng. 208, 109602 (2022).
Wu, H. et al. Silica-based amphiphilic Janus nanofluid with improved interfacial properties for enhanced oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 586, 124162 (2020).
Jia, H. et al. Potential application of novel amphiphilic Janus-SiO2 nanoparticles stabilized O/W/O emulsion for enhanced oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 622, 126658 (2021).
Khezrian, S., Khoee, S. & Caceres, M. Synthesis of combinatorial Janus nanoparticles based on EpCAM-PEG/PCL for targeted therapy of human colorectal adenocarcinoma. J. Biomed. Mater. Res. Part A 108, 2291–2304 (2020).
Khoee, S. & Jalaeian Bashirzadeh, M. Preparation of Janus-type superparamagnetic iron oxide nanoparticles modified with functionalized PCL/PHEMA via photopolymerization for dual drug delivery. J. Appl. Polym. Sci. 138, 49627 (2021).
Ghanbarinia Firozjah, R., Sadeghi, A. & Khoee, S. Ultrasonic de-cross-linking of the pH-and magneto-responsive PHEMA/PMMA microgel to janus nanoparticles: A new synthesis based on “grafting from”/“grafting to” polymerization. ACS Omega 5, 27119–27132 (2020).
Schröder, J. H. et al. Interfacial stabilization by soft Janus nanoparticles. Polymer (Guildf) 106, 208–217 (2016).
Fujii, S. et al. Gas bubbles stabilized by Janus particles with varying hydrophilic–hydrophobic surface characteristics. Langmuir 34, 933–942 (2018).
Lyu, Y. et al. Thermal-resistant, shear-stable and salt-tolerant polyacrylamide/surface-modified graphene oxide composite. J. Mater. Sci. 54, 14752–14762 (2019).
Wang, Y. et al. The stability study of CO2 foams at high pressure and high temperature. J. Pet. Sci. Eng. 154, 234–243 (2017).
Yu, J., Liu, N., Li, L. & Lee, R. L. Generation of nanoparticle-stabilized supercritical CO2 foams. In Carbon Management Technology Conference (Carbon Management Technology Conference, 2012).
Aarra, M. G., Skauge, A., Solbakken, J. & Ormehaug, P. A. Properties of N2-and CO2-foams as a function of pressure. J. Pet. Sci. Eng. 116, 72–80 (2014).
Li, X. et al. Preparation and application of Janus nanoparticles: Recent development and prospects. Coord. Chem. Rev. 454, 214318 (2022).
Fu, J. et al. Janus nanoparticles for cellular delivery chemotherapy: Recent advances and challenges. Coord. Chem. Rev. 422, 213467 (2020).
AlYousef, Z., Almobarky, M. & Schechter, D. Enhancing the stability of foam by the use of nanoparticles. Energy Fuels 31, 10620–10627 (2017).
Xu, B. & Zhang, Q. Preparation and properties of hydrophobically modified nano-SiO2 with hexadecyltrimethoxysilane. ACS Omega 6, 9764–9770 (2021).
Pourjavadi, A., Kohestanian, M. & Keshavarzi, N. Immobilization of Au nanoparticles on poly (glycidyl methacrylate)-functionalized magnetic nanoparticles for enhanced catalytic application in the reduction of nitroarenes and Suzuki reaction. Appl. Organomet. Chem. 34, e5828 (2020).
Gharibshahi, R., Omidkhah, M., Jafari, A. & Fakhroueian, Z. Hybridization of superparamagnetic Fe3O4 nanoparticles with MWCNTs and effect of surface modification on electromagnetic heating process efficiency: A microfluidics enhanced oil recovery study. Fuel 282, 118603 (2020).
Ab Rasid, S. A., Mahmood, S. M., Kechut, N. I. & Akbari, S. A review on parameters affecting nanoparticles stabilized foam performance based on recent analyses. J. Pet. Sci. Eng. 208, 109475 (2022).
Fameau, A.-L. & Salonen, A. Effect of particles and aggregated structures on the foam stability and aging. C. R. Phys. 15, 748–760 (2014).
Gharibshahi, R., Omidkhah, M., Jafari, A. & Fakhroueian, Z. Experimental investigation of nanofluid injection assisted microwave radiation for enhanced heavy oil recovery in a micromodel system. Korean J. Chem. Eng. 39, 562–575 (2022).
Bini, R. A., Marques, R. F. C., Santos, F. J., Chaker, J. A. & Jafelicci, M. Jr. Synthesis and functionalization of magnetite nanoparticles with different amino-functional alkoxysilanes. J. Magn. Magn. Mater. 324, 534–539 (2012).
Hadler, K. & Cilliers, J. J. The effect of particles on surface tension and flotation froth stability. Min. Metall. Explor. 36, 63–69 (2019).
Talebian, S. H., Tan, I. M., Sagir, M. & Muhammad, M. Static and dynamic foam/oil interactions: Potential of CO2-philic surfactants as mobility control agents. J. Pet. Sci. Eng. 135, 118–126 (2015).
Cheraghian, G. & Hendraningrat, L. A review on applications of nanotechnology in the enhanced oil recovery part A: Effects of nanoparticles on interfacial tension. Int. Nano Lett. 6, 129–138 (2016).
Liu, Y., Li, Y., Li, X.-M. & He, T. Kinetics of (3-aminopropyl) triethoxylsilane (APTES) silanization of superparamagnetic iron oxide nanoparticles. Langmuir 29, 15275–15282 (2013).
Fu, C., Yu, J. & Liu, N. Nanoparticle-stabilized CO2 foam for waterflooded residual oil recovery. Fuel 234, 809–813 (2018).
Lin, X. et al. Marangoni effect-driven transfer and compression at three-phase interfaces for highly reproducible nanoparticle monolayers. J. Phys. Chem. Lett. 11, 3573–3581 (2020).
Perro, A., Reculusa, S., Ravaine, S., Bourgeat-Lami, E. & Duguet, E. Design and synthesis of Janus micro-and nanoparticles. J. Mater. Chem. 15, 3745–3760 (2005).
Alooghareh, M. H., Kabipour, A., Sisakht, S. M. M. & Razavifar, M. Effects of different gases on the performance of foams stabilized by Cocamidopropyl betaine surfactant and silica nanoparticles: A comparative experimental study. Petroleum 8, 546–551 (2021).
Phukan, R., Gogoi, S. B. & Tiwari, P. Effects of CO2-foam stability, interfacial tension and surfactant adsorption on oil recovery by alkaline-surfactant-alternated-gas/CO2 flooding. Colloids Surf. A Physicochem. Eng. Asp. 597, 124799 (2020).
Acknowledgements
The authors would like to thank Tarbiat Modares University for supporting this research.
Author information
Authors and Affiliations
Contributions
A.H.S.D.: Supervision, project administration. R.G.: writing and editing, formal analysis, data curation. M.M.: Conceptualization, methodology, investigation, validation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saeedi Dehaghani, A.H., Gharibshahi, R. & Mohammadi, M. Utilization of synthesized silane-based silica Janus nanoparticles to improve foam stability applicable in oil production: static study. Sci Rep 13, 18652 (2023). https://doi.org/10.1038/s41598-023-46030-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-46030-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.