Abstract
Ripening as a physico-chemical change is part of a continuous developmental process and hormones play a major role in this processes. The present study was carried out to investigate the effect of external melatonin (0 and 10 μM) injection at the light green stage on the ripening of strawberry fruit. The fruit was sampled for morphological, biochemical, and gene expression analysis during (0, 5, 10, and 15 days after treatment). The results showed a lower accumulation of anthocyanin content was observed in fruits treated with 10 μM. The injection of 10 μM melatonin caused a lower total soluble solid content and fruit color, and higher titratable acidity and softening. The total phenol content was higher in fruit treated with 10 µM melatonin, accompanied by increased PAL enzyme activity and gene expression, increased DPPH scavenging capacity, and higher content of quercetin, gallic, caffeic, and chlorogenic acids. The delay in fruit ripening was associated with suppression of H2O2 level and endogenous ABA accumulation caused by lower expression of NCEDs genes. In general, it is concluded that activating the melatonin ROS scavenging cascade might be responsible for the delayed ripening and development of strawberry fruit. Therefore, our study demonstrates that the exogenous application of 10 μM melatonin can slow the ripening of strawberry fruit.
Similar content being viewed by others
Introduction
Fruit ripening is a coordinated process influenced by genetic, physiological, and biochemical factors. These processes lead to changes in color, texture, aroma, taste, odor, and nutritional quality1. The mechanism that regulates ripening is not fully understood in non-climacteric fruit but may be related to changes in hormonal concentrations2 . Studies have shown that ABA plays a crucial role in the ripening of non-climacteric fruits while, in climacteric fruits, the role of ethylene in the ripening is widely known3. Recent studies showed ABA and melatonin have positive or negative interaction and influenced several physiological process including stress tolerance and fruit ripening4,4,6.
Melatonin (N-acetyl-5-methoxytryptamine) is an indoleamine synthesized through tryptophan metabolism from serotonin5. Melatonin, as an antioxidant and signal molecule, prevents adverse effects of stress in plants by (1) increasing plant resistance to biological and non-biological stresses such as salinity, drought, cold, and microorganisms7 and by (2) inhibiting ROS activity8. Due to its hydrophilic and lipophilic structure, melatonin quickly penetrates cells and has higher antioxidant activity than some vitamins9 . Furthermore, Wei et al.10 reported that melatonin, as a phytohormone, plays a receptor-dependent signal role, and its uptake through G protein-coupled (PMTR1 or CAND2) receptors in plasma membranes are essential for closing pores by controlling H2O2 and Ca2+ signaling.
The role of exogenous melatonin in delaying banana fruit ripening is through reduced starch degradation and decreasing the expression of genes involved in ethylene biosynthesis (ACO and ACS)11. Tijero et al.5 reported that 100 µM melatonin treatment delayed the ripening of the unripe cherry fruit by increasing internal melatonin and internal cytokinin and preventing anthocyanin accumulation in the fruit. Liu et al.12 also suggested that treating pear fruit with 100 µM melatonin increased fruit firmness after harvest by reducing the expression of ACO and ACS genes, reduced ethylene biosynthesis, and prevented PG expression and Cel genes. In contrast with above studies, the effect of melatonin in non-climacteric fruit has shown that 1000 µM melatonin treatment accelerates the ripening of strawberry fruit by increasing anthocyanins and endogenous ABA4. Xu et al.6 reported that melatonin treatment (100 μM) promotes grape berry ripening by increasing anthocyanin levels than control samples. They found that ripening accelerated by increasing endogenous anthocyanin content. Similar to conflict effect of melatonin on ripening, methyl jasmonate also had different effect on ripening depending to its concentration13. Therefore, more study is needed to reveal melatonin impact on non-climacteric fruits.
Strawberry (Fragaria × ananassa Duch.) is a globally popular fruit with an attractive appearance and a rich source of anthocyanins, flavonoids, phenolic compounds, and folate. Strawberries, which are non-climacteric, should be harvested at full maturity to achieve maximum edibility14. This fruit is also highly perishable due to low mechanical resistance, high respiration rate, and high susceptibility to pathogen attack. Some undesirable changes observed during postharvest include weight loss, accelerated softening, mechanical injury, and Botrytis cinerea-induced decay15. Aghdam and Fard16 reported that the reduction of decay in strawberry fruit treated with melatonin during storage might be due to the accumulation of H2O2 signaling to stimulate the GABA shunt pathway, providing sufficient intracellular ATP along with the accumulation of more phenols and anthocyanins and as a result increasing the DPPH inhibition capacity. Liu et al. (2018) reported that the reduction of decay of strawberry fruit treated with melatonin during storage may be due to endogenous melatonin accumulation. In addition, Pang et al.17 reported that strawberry fruit treated with melatonin showed better overall quality, represented by lower weight loss, higher firmness, higher DPPH scavenging capacity, higher phenols, anthocyanins and ascorbic acid accumulation.
Adequate knowledge about physiological, biochemical, and molecular regulatory mechanisms associated with fruit ripening will help improve strategies to delay ripening and enhance marketability. This experiment aimed to study the effect of melatonin on strawberry fruit ripening and the expression of genes involved in the regulation of development and ripening. In addition, the effects of exogenous melatonin treatment on melatonin biosynthesis-related genes and antioxidant systems were evaluated.
Materials and methods
Plant materials and melatonin treatment
Strawberry (Fragaria × ananassa cv. Sabrina) plants were purchased from a commercial unit affiliated with the Agricultural Research Center and Natural Resources of Kurdistan province, then were grown in a greenhouse under controlled conditions (20–25 °C, relative humidity 70–85%) in University of Kurdistan. Melatonin concentrations (10 μM) and distilled water (used as a control) were injected into the fruit: 200 μL of the treatment solutions were injected by sterile hypodermic syringe into the fruit core through the pedicel, at 10 days after flowering (DAF) that fruit are in light green (the fruit begins to expand rapidly and becomes much larger) stage4, 18. Thirty uniformly sized fruit per replicate were sampled at 0 (before treatment), 5, 10, and 15 days after treatment. Fifteen fruit per replicate of each treatment were chosen to evaluate color, firmness, total soluble solid (TSS), and titratable acid (TA). For biochemical analysis and gene expression, fifteen more fruit in each replicate of each treatment were frozen in liquid nitrogen, powdered, and then quickly stored at − 80 °C.
Endogenous anthocyanin content
Anthocyanin extraction was carried out according to Bodelon et al.19. Briefly, 2 g frozen fruit powder was homogenized with 18 mL 0.5% (v/v) HCl in methanol at 4 °C for 1 h to extract the pigments. Then filtered by a single layer of Whatman No 1. The absorbance of the resulting clear liquid was measured at 520 nm (maximum absorbance for anthocyanins). Anthocyanin content was calculated using the following formula: Abs520 × dilution factor × (molecular weight of pelargonidin × molar extinction coefficient) and expressed as g pelargonidin equivalent (PE)/kg fresh weight (FW).
Fruit firmness, TSS, TA, and color
Fruit firmness was measured using a penetrometer with an 8-mm tip diameter (FHT200, Chine) and expressed in Newton (N). A refractometer (Atago, Tokyo, Japan) was used to determine the TSS of fruit juice at 20 °C. TA was determined by diluting the remaining juice (1/10, v/v) and titrating with NaOH to pH 8.2, and expressed as TA % citric acid. Using a colorimeter (TES 135 A, Taiwan), the color was measured on two opposite sides (equatorial region), and a* parameter was recorded to represent the redness. The color was measured at 10 and 15 days after treatments when fruit color changed.
Endogenous melatonin accumulation
Melatonin extraction was done at 4 °C, according to Arnao and Hernandez20. Briefly, 2 g frozen fruit powder was homogenized in vials containing 3 mL chloroform. The samples were shacked at 4 °C in darkness for 15 h and then filtered by Whatman No. 1. The extract were evaporated to dryness in freeze dryer (RV06-ML, Germany), and the residue was dissolved in acetonitrile (0.5 mL). The endogenous accumulation of melatonin was analyzed with HPLC equipment (Knuer Scientific Instruments, Berlin, Germany) using a C18 column (250 mm × 4.6 mm length, 5 μm particle size) with a fluorescence detector, and the results were expressed as μg/kg FW. The isocratic mobile phase consisted of water: acetonitrile (50:50) at a flow rate of 0.2 mL/min.
Endogenous ABA accumulation
Frozen fruit powder (2 g) was homogenized with 70% (v/v) methanol and stirred overnight at 4 °C according to the methods of Kelen et al.21. The extract was filtered by Whatman filter (No.1), and the methanol evaporated under vacuum. Residue was dissolved in 0.1 M phosphate buffer, and same amount of ethyl acetate was added to the obtained solution to dissolve impurities. After removing the ethyl acetate phase, by 1 N HCl, the pH of the aqueous phase was adjusted to 2.5. The solution partitioned by diethyl ether and then passed through anhydrous sodium sulfate. Later, under vacuum, the diethyl ether phase was evaporated. Then, the dry residue containing ABA was dissolved in 2.0 mL of methanol, and used for ABA analysis. The ABA was analyzed with HPLC equipment (Knuer Scientifc Instruments, Berlin, Germany) using a C18 column (250 mm × 4.6 mm length, 5 μm particle size) at a 0.8 mL/min flow rate. An injection volume of 20 µL was used for each analysis. The endogenous content of ABA was monitored at 265 nm and expressed as mg/kg FW.
Endogenous H2O2 accumulation
Endogenous H2O2 accumulation was expressed as the reaction of H2O2 with potassium iodide as Alexieva et al.22 stated. Briefly, frozen fruit powder (1 g) was homogenized in a mortar containing 10 mL of 0.1% (w/v) trichloroacetic acid. The homogenate was centrifuged at 12,000 g for 15 min. Then, 500 μL of the supernatant solution was added to 500 μL of potassium phosphate buffer 10 mM (pH = 7) and 2 mL of potassium iodide. The resulting mixture was placed in the dark for 1 h at 25 °C. The absorbance of the samples was measured at 390 nm using spectrophotometer (Carry 100, Varian, USA). The endogenous H2O2 accumulation was expressed as mmol/kg FW.
Activity of PAL enzyme
The PAL enzyme extracted according to Yao et al.23. Frozen fruit powder (0.5 g) was ground with 5 mL borate buffer (pH 8.8) contained 15 mM β-mercaptoethanol and 0.15% w/v PVP. The homogenate was centrifuged at 10,000 g for 20 min at 4 °C. For assaying PAL activity, the supernatant was used as a source of crude enzyme. The reaction mixture contained 0.5 mL of the supernatant, 1 mL of 0.02 mol/L l-phenylalanine and borate buffer (0.05 mol/L, pH 8.7). Incubation was performed at 30 °C for 1 h, and the reaction was stopped by adding 1 mL of 2 M HCl. The absorbance was measured at 290 nm. The activity of the PAL enzyme was expressed as mmol cinnamic acid/h kg FW.
Endogenous accumulation of total phenols (TP) and DPPH scavenging capacity
Based on the method of Dewanto et al.24 and using the Folin-Ciocalteu reagent, TPC content was measured. The absorbance was calculated and measured as g gallic acid equivalent (GAE) (PE)/kg FW. DPPH scavenging capacity was measured using a spectrophotometer (Carry 100, Varian, USA) based on the method represented by Brand-Williams et al.25. The following equation was used to calculate the extracts' DPPH free radical scavenging activity. DPPH scavenging effects (%) = [(A0−A1/A0) × 100], where A0 absorbance in the absence of extract, A1 absorbance in the presence of solution.
RNA extraction, synthesis of cDNA and quantitative real-time PCR
The RNA was extracted by a Kit (Takara, Japan) according to the manufacturer’s protocol and the list of the gene-specific primers’ sequences was shown in Table 1. RT-qPCR was conducted using SYBR Premix Ex Taq (Takara, Japan) conforming to the producer’s manual. RNA was reverse-transcribed into cDNA using a PrimeScript RT reagent kit (Takara, Japan) conforming to the producer’s manual. The final volume containing 1 μL of cDNA, 100 nM primers, and 5 μL of 2 × SYBR GREEN I Master Mix (Takara, Japan) was prepared conforming to the producer’s manual. The comparative threshold cycle (Ct) value method calculated the relative expression level. The expression level was normalized by using the control gene actin and calculating the relative expression of the genes by the 2−ΔΔCτ method.
Endogenous accumulation of phenolic compounds
Half gram (0.5 g) of strawberry powder was extracted with 1 mL methanol (HPLC grade) for 30 min in ultrasonic bath. The extract was filtered by Whatman filter (No. 4) and then filtered by 0.45 µm Millipore filter. A gradient solvent system was employed with solvent A: acetonitrile and solvent B: water-acetic acid (97.5:2.5, v/v). The total run time was 35 min. The elution program was: 10 A/90 B (0 min), 40 A/60 B (15 min), 70 A/30 B (24 min) and 10 A/90 B (35 min) with flow rate of 0.5 mL/min. The phenolic compositions were recognized by comparing their retention times with those of the standards. Standards tests were performed at a concentration of 6.25–800 ppm. HPLC analysis was carried out using Knauer liquid chromatography apparatus consisting of a 1050 Smartline Pump, a 2600 Smartline Photodiode Array Detector, DGU-14A degasser. The separation was achieved on a 150 mm × 4.6 mm with a pre-column, Thermo Hypersil-Keystone C18 reversed-phase column provided by Knauer (Berlin, Germany). The peaks were monitored at 280 nm. Injection volume was 15 μL, and the temperature was maintained at 25 °C. The EZchrom Elite software were used to collect and integrate data.
Statistical analysis
A completely randomized design with five replications was used to conduct this study. Statistical data analysis used SAS (Version 9.4; SAS Institute, 2003) software. The data were subjected to two-way analysis of variance (ANOVA) and presented as means ± standard error. To identify the difference between the means, the least differences (LSD) were calculated at the 0.05 level.
All local, national or international guidelines and legislation were adhered to in the production of this study.
Results
Endogenous anthocyanin content
The anthocyanin content increased during sampling in both control and melatonin treatment (Fig. 1). Anthocyanin of control and 10 μM melatonin showed no significant differences at 0 and 5 days after treatment. While, it was lower in 10 μM melatonin treatments than control at 10 and 15 days after treatment.
Fruit firmness, TSS, TA, and color
The fruit firmness decreased during fruit ripening. The firmness of 10 µM melatonin was higher than control at 5 and 10 days after treatment (Table 2). TSS content continually increased during 15 days (Table 2). However, the TSS content was lower in 10 μM melatonin than control at 10 days (5% vs. 5.4%) and 15 days (6.4% vs. 7%). The TA content decreased in the fruit. The lower TA (0.6%) was observed in the control at 15 day after treatment, while it was 0.9% in 10 µM melatonin treated fruit at same time. The first redness signs (a* index) was observed at 10 days after treatment and a* increased thereafter. The a* in 10 µM melatonin treatment was lower than control at 10 and 15 days after treatment (Table 2).
Accumulation of endogenous melatonin
Endogenous melatonin accumulated during fruit ripening by 10 days after treatment and declined thereafter at 15 days after treatment (Fig. 2A). Endogenous melatonin in 10 µM melatonin treatment was higher than control at 10 days after treatment (33.7 μg/g FW vs. 28.6 μg/g FW).
Effect of melatonin treatment on endogenous melatonin accumulation (A) and relative expression of FaASMT (B), FaSNAT (C), FaT5H (D), and FaTDC (E) genes of strawberry fruit at different time after treatment. Data represent the means of five replicates and their standard errors. Vertical bars without the same letter on top indicate significant differences at p < 0.05.
The expression level of genes (TDC, T5H, and ASMT) involved in melatonin biosynthesis increased during fruit ripening while their expressions in 10 µM melatonin were lower than control at all days after treatment (Fig. 2B, D and E). The expression of SNAT increased during fruit ripening by 10 days after treatment but declined thereafter at 15 days after treatment (Fig. 2C).
Expression of the GAMYB and SnRK2.6 genes during fruit development
As shown in Fig. 3A, the expression of the GAMYB gene increased during the ripening process. Fruit treated with 10 μM melatonin showed 0.65-fold less expression (1.9) at 10 days compare to control fruit (2.9). Expression of the SnRK2.6 gene (Fig. 3B) was higher at the early stages than at the ripening stages. The fruit treated with 10 μM melatonin displayed 1.25-fold higher expression of the SnRK2.6 gene than the control samples. Our results showed that the relative expression of the CHS gene in 10 μM melatonin decreased by 0.52-fold and 0.72-fold than control at 10 and 15 days (Fig. 3C).
Effect of melatonin treatment on the relative expression of FaGAMYB (A), FaSnRk2.6 (B) and FaCHS (C) of strawberry fruit at different time after treatment. Data represent the means of five replicates and their standard errors. Vertical bars without the same letter on top indicate significant differences at p < 0.05.
Accumulation of H2O2 and endogenous ABA
As shown in Fig. 4A, the endogenous accumulation of H2O2 increased during ripening. Fruit treated with 10 µM melatonin exhibited a significantly (p < 0.05) lower H2O2 accumulation at 5 days (0.85 mmol/kg vs. 1.86 mmol/kg) and 10 days (2.85 mmol/kg vs. 3.79 mmol/kg) compared to control samples. Also, the relative expression of the FaRBOH gene as a signaling H2O2 accumulation molecule in fruit treated with 10 µM melatonin decreased by 0.82-fold and 0.68-fold than control samples at 10 and 15 days (Fig. 4C). As shown in Fig. 4B, ABA content in 10 μM melatonin treatment and control increased continuously until 10 days after treatment, then gradually decreased at 15 days. Fruit treated with 10 µM melatonin exhibited a significantly (p < 0.05) lower ABA accumulation at 5 days (0.11 mg/kg vs. 0.14 mg/kg) and 10 days (0.10 mg/kg vs. 0.15 mg/kg) compared to control samples. The delayed ripening of strawberry fruits in response to the injection of 10 μM melatonin was accompanied by a lower endogenous content of ABA, resulting from a lower expression of NCED1, 2, 3, and 4 genes (Fig. 4D–G).
The effects of melatonin injection on H2O2 accumulation (A), ABA concentration (B), relative expression of FaRBOH (C) FaNCED1 (D), FaNCED2 (E), FaNCED3 (F), and FaNCED4 (G) gens of strawberry fruit at different time after treatment. Data represent the means of five replicates and their standard errors. Vertical bars without the same letter on top indicate significant differences at p < 0.05.
The activity of PAL, TPC and DPPH scavenging capacity
Strawberries treated with 10 µM melatonin exhibited higher PAL enzyme activity than control samples at 15 days (Fig. 5A), ascribed to 1.22-fold higher PAL gene expression (Fig. 5B) but there was no significant different in the PAL activity between fruit treatment and control samples at 5 and 10 days after treatment. As shown in Fig. 5C, fruit treated with 10 µM melatonin exhibited higher phenol accumulation at 10 days (40 g/kg vs. 25 g/kg) and 15 days (76 g/kg vs. 56 g/kg) during fruit development. DPPH scavenging capacity was higher in melatonin treated fruit at 5 (47% vs. 33%) and 10 days (66% vs. 55%), but at 15 day, the control samples had a higher DPPH scavenging capacity (Fig. 5D).
Effect of melatonin treatment on the PAL activity (A), FaPAL (B), total phenolics content (C), and DPPH scavenging capacity (D) of strawberry fruit at different time after treatment. Data represent the means of five replicates and their standard errors. Vertical bars without the same letter on top indicate significant differences at p < 0.05.
Phenolic compounds
The HPLC results showed that the phenolic constituent changed during different days after treatment. The content of quercetin, gallic acid, caffeic acid, and chlorogenic acid increased during ripening, but rosmarinic acid increased until 5 days, and decreased slightly. The fruit treated with 10 µM melatonin showed higher content of quercetin than control at 10 days (0.05 g/kg vs. 0.04 g/kg) and 15 days (0.07 g/kg vs. 0.05 g/kg) (Table 3). The higher concentration of gallic acid was observed in melatonin treatment than control samples (0.29 g/kg vs. 0.24 g/kg) at 15 days after treatment. The total rosmarinic acid and cafeic acid concentrations in 10 µM melatonin treatment were higher than control at 5 and 10 days while no significant difference was observed at 15 days. There was no significant differences in chlorogenic acid of melatonin treatment and control at 5 and 10 days while it was higher in melatonin treatment than control at 15 days (0.13 g/kg vs. 0.10 g/kg) (Table 3).
Discussion
In recent years, melatonin as a signal and antioxidant molecule has been shown to play a role in the ripening of fruit26,25,28. Fruit treated with 10 μM melatonin showed lower anthocyanin content than control. There are various reports on melatonin’s different roles in regulating crops’ ripening and its effect on the reduction of anthocyanins in the fruit ripening process29. The decrease of anthocyanin can be caused by the increase of cytokines due to exogenous melatonin, because there is a positive correlation between melatonin and cytokinin30. Consistent with our results, Tijero et al.5 reported that treatments with melatonin (10 and 100 μM) reduced anthocyanin content and delayed ripening of cherry fruit due to an increase in the endogenous cytokinin. This findings were against previous report that 1000 μM melatonin enhanced anthocyanin content in strawberry4.
Since tissue softness is one of the most important ripening indicators, the samples treated with 10 µM melatonin had less softness than the control. Our results showed that samples treated with 10 μM melatonin had lower TSS content and color (a*) than the control. Sucrose, fructose and glucose are the main soluble sugars in strawberries31. Melatonin can delay ripening by reducing respiration and ethylene production, thus delaying fruit softening and color change and causing changes in TSS and TA content32. Zhai et al.33 reported that the use of melatonin (100 μM) in pear fruit maintained the firmness of the treated fruits, and this effect of melatonin was through reducing the expression of poly-galactronase and cellulase genes, which are effective in reducing the firmness of the fruit. Melatonin is an amphiphilic molecule, which allows it to enter almost all intracellular compartments by crossing membranes34. Zhou et al.35 reported that melatonin acts as a mediator and is able to inhibit the reduction process of organic acids in fruit and increase the storage life. In addition, the effect of melatonin on delay ripening has been reported in other fruits such as mango32, and sweet cherry5.
The results demonstrated that application of melatonin (10 μM) markedly decreased H2O2 accumulation. Melatonin has direct antioxidant activity7 and can decrease the accumulation of OH, O-1, H2O2, and NO free radicals and regulate antioxidant enzymes36,35,38. Decreasing the endogenous level of H2O2 can reduce anthocyanin, and plays a vital role in preventing tomato fruit ripening39. Fruit ripening induces an oxidative condition and may accompanied by an increase in H2O26. We found that 10 μM melatonin treatment delayed the ripening by suppressing H2O2 signaling, and decreasing in anthocyanin compared to the control. Similar findings have been reported about the effectiveness of melatonin in reducing H2O2 content in strawberries 40.
Our findings showed that treatment with 10 μM melatonin reduced the endogenous accumulation of ABA in strawberry fruit compared to control samples. Transmission and signaling of ABA is a model for non-climacteric fruit ripening such as strawberries18. The ripening of non-climacteric fruits has been classically considered to be mainly regulated by ABA, which is involved in the accumulation of anthocyanins and sugars5. Moreover, ABA is associated with fruit softening and sugar accumulation in strawberry fruit3. The most effect of ABA on fruit ripening is the positive regulation of anthocyanin accumulation in grapes6 and strawberries18. Thus, ABA as a ripening promoter in non-climacteric fruit involved in sugar accumulation and changing the fruit’s color by regulating the biosynthesis of anthocyanin and phenylpropanoid pathway3. Sandhu et al.41 found that ABA treatment stimulates anthocyanins and total phenolic production, enhancing the antioxidant capacities in ‘Muscadine’ grapes. Our previous results showed that a higher endogenous accumulation of ABA and a higher accumulation of anthocyanin (due to higher expressions of PAL and CHS genes) attributed to a higher expression of the GAMYB gene and a lower expression of SnRK2.6 gene, resulting from the endogenous accumulation of melatonin, involved in the ripening of strawberry fruits4. Based on our results, strawberry fruit ripening is dependent on melatonin concentration. Similar to our results, another study showed that different concentrations of methyl jasmonate (MeJA) had different effects on grape fruit ripening. García-Pastor et al.13 showed that MeJA at 5 and 10 mM delayed berry ripening and decreased berry weight and volume as well as vine yield, however, treatments with MeJA at 1, 0.1 and 0.01 mM accelerated ripening and increased total phenolics and individual anthocyanin concentrations. Regarding the mechanisms of action of melatonin during fruit ripening and its relationship with ABA, Fu et al.42 reported that ABA may act as a downstream signal of melatonin in response to stress. Consistent with our results, Liu et al.12 reported that pear fruit treatment with melatonin delayed fruit ripening by reducing the endogenous accumulation of ABA and inhibiting ethylene synthesis.
According to our results, the effectiveness of melatonin in decreasing CHS and SnRK2.6 gene expression might be the reasons for the decrease of anthocyanins during ripening. According to the literature, increasing the expression of CHS and CHI genes in fruit is effective in their coloring43. Zhang et al.8 reported that the accumulation of anthocyanins in cabbages in response to melatonin treatment was due to higher phenylpropanoid biosynthesis-related genes (PAL, C4H, CHS, CHI, and F3H). SnRK2.6 as a negative regulator of fruit ripening, rapidly decreased during strawberry fruit development, and silencing the expression level of FaSnRK2.6 inhibited the ripening of this fruit 2. Han et al. (2015)2 found that ABA affects the expression of SnRK2.6, and the expression of this gene decreases ABA concentration, fruit color and aroma metabolism genes such as CHS and CHI, which generally slow down fruit ripening. Thus, inhibition of GAMYB gene expression and increased SnRK2.6 gene expression could be why 10 μM exogenous melatonin reduced endogenous ABA accumulation and delayed ripening stages of strawberry fruit. The higher GAMYB gene expression in the white stage of strawberry fruit may act as an essential regulator of ripening by increasing biosynthesis and ABA signaling, and activate the phenylpropanoid pathway and pigment accumulation in the fruit44.
In fruit treated with 10 μM melatonin, PAL enzyme activity and the TPC increased by increasing PAL gene expression during the ripening process. Besides, strawberries treated with 10 µM melatonin exhibited higher DPPH scavenging capacity. It has been reported that the free radical scavenging capacity is enhanced by melatonin, which makes it very effective in protecting organisms against oxidative stress, even at low concentrations38,45. Therefore, triggering the endogenous accumulation of melatonin in strawberries treated with 10 µM melatonin might be responsible for promoting the activity of the phenylpropanoid pathway, represented by higher PAL, and increased DPPH scavenging capacity. Consistent with our results, Sharafi et al.46 reported that melatonin treatment in tomato fruit increased PAL enzyme activity, phenol accumulation, and increased ATP and carbon skeleton in the fruit. Moreover, Aghdam et al.47 reported that applying 100 μM melatonin on pomegranate fruit increased phenylpropanoid pathway activity, PAL enzyme activity, phenol content and DPPH antioxidant capacity.
According to our results, the phenolic constituent in strawberry fruit treated with 10 µM melatonin increased. Similar to our findings, melatonin treatment in grapes increased the accumulation of chlorogenic, gallic, caffeic, cinnamic, and coumaric acid phenols and flavonoids, catechin, epicatechin, and pelargonidin by increasing the expression of CHS and PAL genes48. In addition, the effectiveness of melatonin in increasing phenolic compounds in plums has also been reported49.
Conclusion
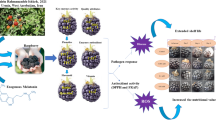
Our results showed that the fruits treated with 10 μM melatonin had less TSS, red color and more firmness and TA than the control samples at ripening stage, so were more unripe based on ripening indicators. Melatonin could regulate the expression of some genes involved in fruit ripening, such as CHS, GAMYB, SnRK2.6, and PAL through the decrease of endogenous ABA. Furthermore, melatonin as an antioxidant, plays an essential role in accumulating antioxidants and phenolic compounds during fruit development by reducing the oxidative stress of endogenous H2O2 (Fig. 6). This article examined the role of melatonin on ABA and H2O2 in regulating the ripening process through the phenylpropanoid pathway, such as fruit coloring, and highlighting a deeper understanding of the factors that control non-climacteric fruit ripening. Thus, it can provide a tool to delay strawberry fruit ripening. As melatonin is a non-harmful compound, it will help producers and growers to adjust the harvest time based on market needs.
Schematic diagram for the effects of melatonin on some physiological and biochemical aspects of fruit ripening in strawberry. Melatonin treatment delayed the ripening by suppressing H2O2 and ABA signaling attributed to a higher expression of GAMYB gene and a lower expression of SnRK2.6 gene caused decreased in anthocyanin.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ABA:
-
Abscisic acid
- ASMT:
-
N-Acetylserotonin methyltransferase
- CHS:
-
Chalkone synthase
- NCED:
-
9-Cisepoxycarotenoid dioxygenase
- PAL:
-
Phenylalanine ammonia-lyase
- RBOH:
-
NADPH oxidase-dependent H2O2 production
- ROS:
-
Reactive oxygen species
- TPC:
-
Total phenol content
- SNAT:
-
Serotonin N-acetyltransferase
- TDC:
-
Tryptophan decarboxylase
- T5H:
-
Tryptophan 5-hydroxylase
- SnRK2.6:
-
Sucrose nonfermenting1-related protein kinase2 gene
References
Giovannoni, J., Nguyen, C., Ampofo, B., Zhong, S. & Fei, Z. The epigenome and transcriptional dynamics of fruit ripening. Annu. Rev. Plant Biol. 68, 61–84. https://doi.org/10.1146/annurev-arplant-042916-040906 (2017).
Han, Y., Dang, R., Li, J., Jiang, J. & Zhang, N. SUCROSE NONFERMENTING1-RELATED PROTEIN KINASE2. 6, an ortholog of OPEN STOMATA1, is a negative regulator of strawberry fruit development and ripening. Plant Physiol. 167, 915-930. https://doi.org/10.1104/pp.114.251314 (2015).
Jia, H. et al. Abscisic acid and sucrose regulate tomato and strawberry fruit ripening through the abscisic acid-stress-ripening transcription factor. Plant Biol. J. 14, 2045–2065. https://doi.org/10.1111/pbi.12563 (2016).
Mansouri, S., Sarikhani, H., Sayyari, M. & Aghdam, M. S. Melatonin accelerates strawberry fruit ripening by triggering GAMYB gene expression and promoting ABA accumulation. Sci. Hortic. 281, 901–919. https://doi.org/10.1016/j.scienta.2021.109919 (2021).
Tijero, V., Muñoz, P. & Munné-Bosch, S. Melatonin as an inhibitor of sweet cherries ripening in orchard trees. Plant Physiol. Biochem. 140, 88–95. https://doi.org/10.1016/j.plaphy.2019.05.007 (2019).
Xu, L., Yue, Q., Xiang, G., Bian, F. E. & Yao, Y. Melatonin promotes ripening of grape berry via increasing the levels of ABA, H2O2, and particularly ethylene. Hortic. Res. 5, 41–51. https://doi.org/10.1038/s41438-018-0045-y (2018).
Arnao, M. B. & Hernández-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 59(2), 133–150. https://doi.org/10.1111/jpi.12253 (2015).
Zhang, N. et al. Melatonin improved anthocyanin accumulation by regulating gene expressions and resulted in high reactive oxygen species scavenging capacity in cabbage. Front. Plant Sci. 7, 197. https://doi.org/10.3389/fpls.2016.00197 (2016).
Pérez-Llorca, M., Muñoz, P., Müller, M. & Munné-Bosch, S. Biosynthesis, metabolism and function of auxin, salicylic acid and melatonin in climacteric and non-climacteric fruits. Front. Plant Sci. 10, 136. https://doi.org/10.3389/fpls.2019.00136 (2019).
Wei, Y. et al. Melatonin biosynthesis enzymes recruit WRKY transcription factors to regulate melatonin accumulation and transcriptional activity on W-box in cassava. J. Pineal Res. 65(1), e12487. https://doi.org/10.1111/jpi.12487 (2018).
Hu, W. et al. Natural variation in banana varieties highlights the role of melatonin in postharvest ripening and quality. J. Agric. Food Chem. 65(46), 9987–9994. https://doi.org/10.1021/acs.jafc.7b03354 (2017).
Liu, J. et al. Melatonin inhibits ethylene synthesis via nitric oxide regulation to delay postharvest senescence in pears. J. Agric. Food Chem. 67, 2279–2288. https://doi.org/10.1021/acs.jafc.8b06580 (2019).
García-Pastor, M. E. et al. Methyl jasmonate effects on table grape ripening, vine yield, berry quality and bioactive compounds depend on applied concentration. Sci. Hortic. 247, 380–389. https://doi.org/10.1016/j.scienta.2018.12.043 (2019).
Yan, J. W. et al. The effect of the layer-by-layer (LBL) edible coating on strawberry quality and metabolites during storage. Postharvest. Biol. Technol. 147, 29–38. https://doi.org/10.1016/j.postharvbio.2018.09.002 (2019).
Javanmardi, Z., Koushesh-Saba, M., Nourbakhsh, H. & Amini, J. Efficiency of nanoemulsion of essential oils to control Botrytis cinerea on strawberry surface and prolong fruit shelf life. Int. J. Food Microbiol. 384, 109979. https://doi.org/10.1016/j.ijfoodmicro.2022.109979 (2023).
Aghdam, M. S. & Fard, J. R. Melatonin treatment attenuates postharvest decay and maintains nutritional quality of strawberry fruits (Fragaria×anannasa cv. Selva) by enhancing GABA shunt activity. Food Chem. 221, 1650–1657. https://doi.org/10.1016/j.foodchem.2016.10.123 (2017).
Pang, L. et al. Insights into exogenous melatonin associated with phenylalanine metabolism in postharvest strawberry. Postharvest. Biol. Technol. 168, 111244. https://doi.org/10.1016/j.postharvbio.2020.111244 (2020).
Jia, H. et al. Sucrose functions as a signal involved in the regulation of strawberry fruit development and ripening. New Phytol. 198, 453–465. https://doi.org/10.1111/nph.12176 (2013).
Bodelon, O. G., Blanch, M., Sanchez Ballesta, M. T., Escribano, M. I. & Merodio, C. The effects of high CO2 levels on anthocyanin composition, antioxidant activity and solublesugar content of strawberries stored at low non-freezing temperature. Food Chem. 122, 673–678. https://doi.org/10.1016/j.foodchem.2010.03.029 (2010).
Arnao, M. B. & Hernández-Ruiz, J. Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J. Pineal Res. 46(1), 58–63. https://doi.org/10.1111/j.1600-079X.2008.00625.x (2009).
Kelen, M., Demiralay, E. C., Şen, S. & Alsanckak, G. O. Separation of abscisic acid, indole-3-acetic acid, gibberellic acid in 99 R (Vitis berlandieri x Vitis rupestris) and rose oil (Rosa damascena Mill.) by reversed phase liquid chromatography. Turk. J. Chem. 28(5), 603–610 (2004).
Alexieva, V., Sergiev, I., Mapelli, S. & Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Env. 24(12), 1337–1344. https://doi.org/10.1046/j.1365-3040.2001.00778.x (2001).
Yao, X. et al. The changes in quality ingredients of Qi chrysanthemum flowers treated with elevated UV-B radiation at different growth stages. J. Photochem. Photobiol. 146, 18–23. https://doi.org/10.1016/j.jphotobiol.2015.02.023 (2015).
Dewanto, V., Wu, X., Adom, K. K. & Liu, R. H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 50(10), 301–310. https://doi.org/10.1021/jf0115589 (2002).
Brand-Williams, W., Cuvelier, M. E. & Berset, C. L. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 28(1), 25–30. https://doi.org/10.1016/S0023-6438(95)80008-5 (1995).
Mukherjee, S. Novel perspectives on the molecular crosstalk mechanisms of serotonin and melatonin in plants. Plant Physiol. Biochem. 132, 33–45. https://doi.org/10.1016/j.plaphy.2018.08.031 (2018).
Mukherjee, S. Recent advancements in the mechanism of nitric oxide signaling associated with hydrogen sulfide and melatonin crosstalk during ethylene-induced fruit ripening in plants. Nitric Oxide 82, 25–34. https://doi.org/10.1016/j.niox.2018.11.003 (2019).
Shan, S. et al. DNA methylation mediated by melatonin was involved in ethylene signal transmission and ripening of tomato fruit. Sci. Hortic. 291, 110566. https://doi.org/10.1016/j.scienta.2021.110566 (2022).
Verde, A., Míguez, J. M. & Gallardo, M. Role of melatonin in apple fruit during growth and ripening: Possible interaction with ethylene. Plants 11(5), 688. https://doi.org/10.3390/plants11050688 (2022).
Ma, X., Zhang, J., Burgess, P., Rossi, S. & Huang, B. Interactive effects of melatonin and cytokinin on alleviating drought-induced leaf senescence in creeping bentgrass (Agrostis stolonifera). Env. Exp. Bot. 145, 1–11. https://doi.org/10.1016/j.envexpbot.2017.10.010 (2018).
Giampieri, F. et al. Strawberry as a health promoter: An evidence based review. Food Funct. 6(5), 1386–1398. https://doi.org/10.1039/C5FO00147A (2015).
Liu, S. et al. Delay of ripening and softening in ‘Guifei’mango fruit by postharvest application of melatonin. Postharvest Biol. Technol. 163, 111–136. https://doi.org/10.1016/j.postharvbio.2020.111136 (2020).
Zhai, R. et al. Melatonin limited ethylene production, softening and reduced physiology disorder in pear (Pyrus communis L.) fruit during senescence. Postharvest. Biol. Technol. 139, 38–46. https://doi.org/10.1016/j.postharvbio.2018.01.017 (2018).
Allegra, M. et al. The chemistry of melatonin’s interaction with reactive species. J. Pineal Res. 34(1), 1–10. https://doi.org/10.1034/j.1600-079X.2003.02112.x (2003).
Zhou, Y. et al. Exogenous nitric oxide-induced postharvest disease resistance in citrus fruit to Colletotrichum gloeosporioides. J. Sci. Food Agric. 96(2), 505–512. https://doi.org/10.1002/jsfa.7117 (2016).
Ba, L. J. et al. Effects of melatonin treatment on maintenance of the quality of fresh-cut pitaya fruit. Int. Food Res. J. 29(4), 796–805. https://doi.org/10.47836/ifrj.29.4.07 (2022).
Magri, A. & Petriccione, M. Melatonin treatment reduces qualitative decay and improves antioxidant system in high bush blueberry fruit during cold storage. J. Sci. Food Agric. https://doi.org/10.1002/jsfa.11774 (2022).
Shekari, A., Hassani, R. N., Aghdam, M. S., Rezaee, M. & Jannatizadeh, A. The effects of melatonin treatment on cap browning and biochemical attributes of Agaricus bisporus during low temperature storage. Food Chem. 348, 129074. https://doi.org/10.1016/j.foodchem.2021.129074 (2021).
Torun, H. & Uluisik, S. Postharvest application of hydrogen peroxide affects physicochemical characteristics of tomato fruits during storage. Hortic. Env. Biotech. 2022, 1–11. https://doi.org/10.1007/s13580-021-00403-5 (2022).
Manafi, H., Baninasab, B., Gholami, M., Talebi, M. & Khanizadeh, S. Exogenous melatonin alleviates heat-induced oxidative damage in strawberry (Fragaria × ananassa Duch. cv. Ventana) plant. J. Plant G. Reg. 2022, 1–13. https://doi.org/10.1007/s00344-020-10279-x (2022).
Sandhu, A. K. et al. Effects of exogenous abscisic acid on antioxidant capacities, anthocyanins, and flavonol contents of muscadine grape (Vitis rotundifolia) skins. Food Chem. 126(3), 982–988. https://doi.org/10.1016/j.foodchem.2010.11.105 (2011).
Fu, J. et al. Improved cold tolerance in Elymus nutans by exogenous application of melatonin may involve ABA-dependent and ABA-independent pathways. Sci. Rep. 7(1), 1–11. https://doi.org/10.1038/srep39865 (2017).
Han, Y., Chen, C., Yan, Z., Li, J. & Wang, Y. The methyl jasmonate accelerates the strawberry fruits ripening process. Sci. Hortic. 249, 250–256. https://doi.org/10.1016/j.scienta.2019.01.061 (2019).
Vallarino, J . G. et al. Central role of Fa GAMYB in the transition of the strawberry receptacle from development to ripening. New Phytol. 208, 482-496. https://doi.org/10.1111/nph.13463 (2015).
Galano, A. & Reiter, R. J. Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J. Pineal Res. 65(1), e12514. https://doi.org/10.1111/jpi.12514 (2018).
Sharafi, Y. et al. Melatonin treatment promotes endogenous melatonin accumulation and triggers GABA shunt pathway activity in tomato fruits during cold storage. Sci. Hortic. 254, 222–227. https://doi.org/10.1016/j.scienta.2019.04.056 (2019).
Aghdam, M. S. et al. Melatonin treatment maintains nutraceutical properties of pomegranate fruits during cold storage. Food Chem. 303, 125385. https://doi.org/10.1016/j.foodchem.2019.125385 (2020).
Xu, L. et al. Melatonin enhances phenolics accumulation partially via ethylene signaling and resulted in high antioxidant capacity in grape berries. Front. Plant Sci. 8, 14–26. https://doi.org/10.3389/fpls.2017.01426 (2017).
Xu, R., Wang, L., Li, K., Cao, J. & Zhao, Z. Integrative transcriptomic and metabolomic alterations unravel the effect of melatonin on mitigating postharvest chilling injury upon plum (cv. Friar) fruit. Postharvest. Biol. Technol. 186, 111819. https://doi.org/10.1016/j.postharvbio.2021.111819 (2022).
Acknowledgements
We thank the University of Kurdistan for providing facilities and financial support.
Author information
Authors and Affiliations
Contributions
M.K.S., H.S. and S.M.: conceptualization and designing the study, methodology. M.K.S. and H.S.: supervision of the research, financial preparation. S.M.: performing the experiment, collection of data. M.K.S. and S.M.: contribution to literature research and original draft preparation. S.M., H.S. and M.K.S.: analyzing and interpretation of data and visualization. M.K.S. and H.S.: guiding all aspects of the research project and revising the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mansouri, S., Koushesh Saba, M. & Sarikhani, H. Exogenous melatonin delays strawberry fruit ripening by suppressing endogenous ABA signaling. Sci Rep 13, 14209 (2023). https://doi.org/10.1038/s41598-023-41311-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-41311-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.