Abstract
CRISPR/Cas9-based genome editing represents an unprecedented potential for plant breeding. Unlike animal cells, plant cells contain a rigid cell wall, genome editing tool delivery into plant cells is thus challenging. In particular, the delivery of the Cas9-gRNA ribonucleoprotein (RNP) into plant cells is desired since the transgene insertion into the genome should be avoided for industrial applications in plants. In this study, we present a novel RNP delivery approach in rice. We applied the sonication-assisted whisker method, conventionally developed for DNA delivery in plants, for RNP delivery in rice. Combined with marker gene delivery, we successfully isolated OsLCYβ genome-edited lines generated by RNPs. The calli and regenerated shoot of the OsLCYβ mutant showed abnormal carotenoid accumulation. In addition, we also detected, although at a low frequency, genome editing events in rice calli cells by RNP delivery using the sonication-assisted whisker method without any additional. Therefore, the sonication-assisted whisker method could be an attractive way to create RNP-based genome-edited lines in plants.
Similar content being viewed by others
Introduction
Plant breeding has entered a new era with the invention of the clustered regularly interspaced short palindromic repeats (CRISPR)-associated protein 9 (Cas9)-based genome editing system and its subsequent improvements1. Ribonucleoproteins (RNPs) of the Streptococcus pyogenes Cas9 (SpCas9) protein with a single guide RNA (gRNA) specifically bind to the DNA harboring gRNA target sequence followed by the protospacer adjacent motif (PAM) 5′-NGG-3′ sequence. RNPs induce double-strand breaks (DSB) at the DNA target site. The programmable feature of the CRISPR technology displays relevant potential in genome breeding. The DNA fragment that encodes the CRISPR-Cas9 components, Cas9 and gRNA, is usually large, over 7 kbp long including promoter or terminators. To lower the chances of this large-size DNA randomly integrating into the genome, it is important to directly deliver the RNP. Unlike DNA delivery by Agrobacterium, RNP cannot easily be delivered to the plant cells across the cell wall. Accordingly, RNP delivery technology development gathers attention recent years2.
The delivery method for RNA encoding CRISPR-Cas9 or RNP in plants have been developed intensively3,4,5,6. Currently, the PEG7, 8 and the particle bombardment9 methods are the two major ways of introducing RNPs into plant cells. However, the former requires the isolation of protoplasts, from which regenerating individuals is generally difficult6. The particle bombardment method allows for a relatively easier regeneration of plant individuals than the PEG method since it usually targets intact totipotent cells (e.g., those in the callus or shoot apical meristem). However, the RNP introduction efficiency is generally low9, 10, partially due to the small number of RNP molecules that could be introduced into the cells. Moreover, during microinjectile preparation, the RNPs in the buffer should be dried, potentially leading to the inactivation of the Cas9 endonuclease activity. Therefore, the development of new methods for large-scale RNP introduction into plant cells retains relevant research interest.
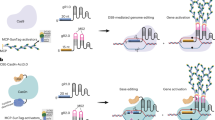
The whisker method is a gene delivery approach in plants using high aspect-ratio silicone-carbide or aluminum borate whiskers, which could penetrate the cell wall and membrane11, 12. Whiskers premixed with plasmids directly pierce the plant cell in solution without any dry-up process. This simple introduction using whiskers allows for less species-dependent gene delivery into plant cells. Several studies reported transgenic plant generation using the whisker method in rice, maize, cotton, and others13,14,15,16. We previously published a significantly improved version of the whisker method, the sonication-assisted whisker transformation, coined the whisker-supersonic method (WSS) in rice17, 18. The WSS approach uses sonication to introduce the whiskers into the plant cells. Using this method, transgenic soybeans have been efficiently developed18, 19. This method was also applicable to the tree species Cryptomeria japonica20. However, no report has demonstrated its applicability to deliver proteins into plant cells.
In this study, we demonstrate the development of the sonication-assisted whisker-based RNP delivery and genome editing in rice. We successfully identified the RNP-delivered calli with mutations at the target genes with the co-transfection of a selection marker-harboring plasmid. We disrupted the OsLCYβ gene in rice using this method and demonstrated that it led to abnormal carotenoid accumulations both in the genome-edited calli and regenerated individuals with practical efficiency. Taken together, the sonication-assisted whisker method could be a feasible and attractive tool for RNP-based genome editing in plants.
Results
RNP delivery using the whisker method and genome editing event identification
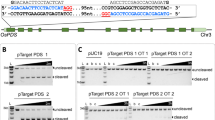
First, we attempted RNP delivery into rice embryonic cell suspensions using a selection marker (Fig. 1). The OsPDS locus was selected as a genome editing target site using a gRNA reportedly efficient for genome editing21. For the large-scale RNP preparation, we purified mg-scale recombinant SpCas9 fused with a nuclear localization signal (NLS) and performed in vitro gRNA synthesis (Supplementary Fig. 1a,b). The activity of the RNPs, consisting of SpCas9-NLS and the gRNA targeting OsPDS, was assessed using an in vitro cleavage assay (Supplementary Fig. 1c). We confirmed that nearly 90% of the substrate DNA could be digested by the prepared RNPs during a 30-min incubation at 37 °C. Next, we mixed the RNPs with the potassium titanate whiskers and the plasmid harboring both hygromycin phosphotransferase (HPT) and Chiridius poppei Yellowish-Green Fluorescent Protein (CpYGFP) expression cassettes22, and subjected them to sonication with rice cell suspensions according to the protocol we established previously17. For RNP delivery optimization, we tested several RNP concentrations (Table 1). We washed the whisker-treated calli with the R2 medium and incubated them without any antibiotics for the recovery culture. After the 6-day recovery culture, we conducted a hygromycin-based selection of the transformed calli for 1–2 weeks. The selected calli were split into fragments, one of which was subjected to DNA extraction and genome sequence analysis using Amplicon sequencing (Amplicon-seq). The rest of the selected calli, not used for genome DNA analyses, were incubated further and transferred into a regeneration medium to obtain a shoot.
Overview of the experimental procedure of CRISPR/Cas9 RNP delivery with the sonication-assisted whisker method using selection marker-harboring plasmids. The recombinant SpCas9-NLS and in vitro transcribed gRNA were mixed to prepare the RNPs. The RNPs were mixed with the whiskers, the selection marker-harboring, plasmids, and specific amounts of rice embryonic suspension cells (250 packed cell volume [PCV]). The calli mixed with whiskers and RNPs were ultrasonicated by a sonicator. Sonication-treated cells were washed with the R2 medium and incubated without antibiotics for the recovery culture. After the 6-day recovery culture, we conducted a hygromycin-based selection of the transformed calli for 1–2 weeks. Selected calli were split into fragments, one of which was subjected to DNA extraction and genome sequence analysis using Amplicon sequencing (Amplicon-seq). The rest of the selected calli, not used for genome DNA analyses, were incubated further and transferred into a regeneration medium to obtain the shoot. OsPDS exon12 was selected as the genome editing target (inlet). The target sequence of the gRNA and the PAM sequence were marked as red and blue, respectively. The restriction enzyme PstI recognition site is underlined. The in vitro transcribed gRNA sequence is also indicated in the inlet.
During the selection, we obtained several white-colored calli with phenotypes identical to that of the OsPDS mutant, and detected the mutations at the target sites in the genome of the calli (Fig. 2a). Several calli could be selected by the hygromycin harbor 1-bp insertion at the putative DSB site in the OsPDS, indicating successful genome editing events by the RNPs (Fig. 2b). Related to the RNP concentration, the highest investigated concentration, 100 pmol/250 packed cell volume (PCV), was proven the most efficient for genome editing (9 out of 22 selected calli, Table 1). The genome editing efficiency was similar to that of the conventional plasmid delivery approaches using the sonication-assisted whisker methods (10 out of 20 selected calli, Table 1). These results suggest that 100 pmol RNPs/250 PCV is a sufficient concentration to produce genome-edited lines in rice embryonic cell suspensions using the sonication-assisted whisker method. The low ratio of isolating mosaic mutant in 100 pmol RNPs/250 PCV suggests that genome editing events occurred just after the whisker treatments in the small number of whisker-inserted cells (Supplementary Table 1). Notably, the Amplicon-seq results showed that the 1-bp insertion is dominant in the patterns of the RNP-induced mutations, whereas > 10-bp deletions frequently occurred in the mutants produced by plasmids even though the gRNA sequence used in the case of the two conditions was identical (Supplementary Table 1, Supplementary Fig. 2). Although the difference was not statistically significant, this tendency implies that transient RNP delivery induces smaller-size mutations compared with the plasmid delivery, in which case continuous RNP production and resultant continuous mutation induction would occur. Consistently, the biallelic mutant ratio in the genome-edited lines was lower in the RNP-delivered lines than that in the plasmid-delivered lines (Table 1, Supplementary Table 1). The regeneration from the RNP-delivered calli occurred normally, resulting in albino individuals in certain cases (Fig. 2c). Although the OsPDS mutant exhibited growth termination, we could anticipate the induced mutation inheritance in the case of non-lethal genes.
Representative results of OsPDS locus genome editing in rice by RNP delivery using the sonication-assisted whisker method. (a) A representative image of white-colored calli after the incubation of the selection medium for 2 weeks; (b) genome sequences of the OsPDS with targeted mutations in the whisker-treated calli after the incubation of the selection medium for 2 weeks. DNA from independently selected calli were analyzed by Amplicon-seq. The gRNA target sequence and the PAM sequence are colored in red and blue, respectively. The insertion bases are highlighted in magenta. The numbering on the left corresponds to independent calli (e.g., #6 has two types of mutations in one callus). The genotypes are also summarized in Supplementary Table 1. The percentages in the right of the sequences indicate the percentage of the reads with mutation/the reads with no mutations; (c) a representative image of regenerated plants with green- (control, left), and white-colored shoots (genome-edited, right).
Targeted mutagenesis of lycopene cyclase genes in rice
Next, we attempted to assess the sonication-assisted whisker method with the optimized conditions for RNPs applicable for functional genetics, by challenging to isolate genome-edited lines of the genes with mutants not reported in rice. We selected LYCOPENE CYCLASE BETA (OsLCYβ, Os02g0190600) and LYCOPENE CYCLASE EPSILON (OsLCYε, Os01g0581300) as the target genes for genome editing (Fig. 3a,b). A previous study implies that the expression level reduction of these genes results in rice with high lycopene content23, although no single gene knockouts of these genes have been reported so far. We designed three gRNAs for OsLCYβ or OsLCYε. The activity of each gRNA was assessed by in vitro cleavage assays (Supplementary Fig. 3). The in vitro cleavage assay revealed that most RNPs with these gRNAs, LCYβ-1, LCYβ-2, LCYβ-3, and LCYε-3, exhibited higher activities compared to that with the gRNA targeting OsPDS (Supplementary Fig. 3), suggesting that highly efficient genome editing could be expected when using RNPs with the aforementioned gRNAs. We transfected RNPs together with the plasmid harboring HPT and CpYGFP to the rice calli under the 100 pmol/250 PCV condition (Table 2). The hygromycin-selected calli were subjected to Amplicon-seq analyses and certain selected lines exhibited mutations at the target sites (Fig. 3c, Tables 1, 2). However, the genome editing efficiency was not higher than that in the case of OsPDS disruption. Mutants with biallelic mutations in each gene were also isolated (two out of four selected calli in LCYβ-2, two out of three in LCYβ-3, one LCYε-2, and one LCYε-3). These results indicate that the sonication-assisted-whisker-based RNP delivery could be used for the isolation of the genome-edited lines of genes other than OsPDS. Unexpectedly, no genome editing events occurred under certain conditions using RNPs with the gRNA with higher or similar activity in vitro as the gRNA for OsPDS (Supplementary Fig. 3, Table 2, e.g., LCYβ-1, and LCYε-1). The discrepancy between the gRNA activity in vitro and in vivo is frequently reported24, and it is usually thought to derive from the cell selection, propagation, and DNA repair status complexity in the target cells. In addition, there is a possibility that gRNA in vitro cleavage efficiency is correlated with genome editing efficiency in calli at higher concentration condition > 100 pmol/250 PCV. This could also be the case for the sonication-assisted whisker method since obtaining the selected calli took 2–3 weeks.
Isolation of OsLCYβ and OsLCYε mutants using RNP delivery by the sonication-assisted whisker method. (a) gRNA design for OsLCYβ genome editing. OsLCYβ exon1 was selected as the genome editing target. The target sequences of each gRNA and the PAM sequence are marked in red and blue, respectively. The in vitro transcribed gRNA sequence is also indicated; (b) gRNA design for OsLCYε genome editing. OsLCYε exon1 was selected as the genome editing target. The coloration of the schematic image is the same as those of (a). (c) Detailed mutations detected in the selected calli of the OsLCYβ or OsLCY ε mutants. The name of gRNAs is indicated in each sequence alignment. The genome sequence with no mutation is described as “WT.” The insertions and the PAM sequence are highlighted in magenta and blue, respectively. The gRNA target sequence is colored with red. Dashed lines indicate the putative DSB sites.
Analyses of the genome-edited OsLCY β line
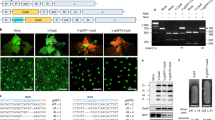
In the isolated genome-edited OsLCYβ lines, we observed that certain calli had red pigments (Fig. 4a,b). In the LCYβ-2 #2 mutant, we obtained a regenerated plant with abnormal pigmentation (Fig. 4c). Since LCYβ catalyzes δ-carotene transition to α-carotene as well as trans-lycopene to γ-carotene, its knock-out mutations possibly induce δ-carotene accumulation, resulting in yellow–red color and the presence of trans-lycopene25. To assess carotenoid accumulation in the mutant, we conducted liquid chromatography (LC) with a photodiode array (PDA) and electrospray mass spectrometer (MS), LC-PDA-MS analyses (Fig. 4d). In the callus of the LCYβ-2 #2 mutant, as expected, δ-carotene was markedly accumulated compared with the non-RNP-treated control calli, accumulating almost no δ-carotene (Fig. 4, Supplementary Fig. 4). In addition to δ-carotene, we also detected lycopene accumulation in the callus. Consistently, lycopene with red color is the upstream substrate of δ-carotene in the carotenoid biosynthesis pathway23. OsLCYβ disruption could induce not only δ-carotene accumulation but also that of upstream substrates. Callus pigmentation could be explained by both lycopene and δ-carotene accumulation. However, carotenoid accumulation in the regenerated shoot from the callus of the LCYβ-2 #2 mutant was not similar to that in the callus (Supplementary Fig. 5). Clear reductions of β-carotene, lutein, violaxanthin, and antheraxanthin in the LCYβ-2 #2 mutant plantlet could be detected. It was consistent that these carotenoids were downstream of OsLCYβ in the carotenoid biosynthesis pathway. Notably and unexpectedly, we identified lutein acetate accumulation during our MS/MS analyses in the LCYβ-2 #2 mutant plantlet. The LCYβ-2 mutants did not develop their flowers and displayed growth termination, suggesting that OsLCYβ is an essential gene in rice. In summary, these results demonstrated that RNP delivery by the sonication-assisted whisker method can be used for functional genetics in rice.
Phenotypic analysis of the OsLCYβ mutant generated by RNP delivery using the sonication-assisted whisker method. (a) Representative images of non-RNP-treated calli (left) and genome-edited lines of LCYβ-2 #2 after the 2-week selection (right); (b) the callus of LCYβ-2 #2 showed clear red pigmentation; (c) representative images of the shoot from the non-RNP-treated calli (left) and that from the genome-edited calli of LCYβ-2 #2; (d) quantification of the carotenoids in the LCYβ-2 #2 calli, **indicate statistical significance analyzed by the Welch Two Sample t-test between WT and LCYβ-2 #2 (p < 0.05).
Selection marker-free RNP delivery-based genome editing in rice
All the above-described genome editing experiments were conducted using RNPs with the plasmid harboring selection markers. Due to CRISPR-Cas9 RNP complex has affinity to double strand DNA1 and can bind with plasmid DNA, it is not obvious that RNPs can be delivered by whisker method without addition of any carrier DNAs. Accordingly, we tried OsPDS-target RNP delivery by the sonication-assisted whisker method without plasmid co-transfection and any selections (Supplementary Fig. 6). We did not obtain any callus with a white-color phenotype in the pilot experiment of this approach, indicating a significantly lower genome-edited than non-edited cell population. To detect the rare genome editing events, a cleaved amplified polymeric sequences assay was used (see “Methods” section in detail). We performed Amplicon-seq analyses of the PstI-digested PCR products, amplified from the genome DNA of the calli harvested 2 weeks after the RNP transfection. We observed six out of 36 samples harboring the 1-bp insertion or deletion mutations at the target site while the samples treated with no RNPs never displayed such mutations (Supplementary Fig. 6). This result suggests that the sonication-assisted whisker method could deliver RNPs to induce genome editing at the target site in rice embryonic cell suspensions without plasmid co-transfection.
Discussion
In this study, we determined that the condition of successful RNP delivery using the sonication-assisted whisker method in rice embryonic suspension cells is 100 pmol RNPs/250 PCV (Table 1). We isolated several biallelic mutants using 100 pmol RNPs/250 PCV conditions with hygromycin selection (Figs. 2, 3). In the conventional plasmid delivery using the sonication-assisted whisker method, 20 µg plasmids/125–250 PCVs were used for isolating 10–20 transgenic lines17. Therefore, 100 pmol RNPs per one condition should be highly sufficient in common usage for isolating genome-edited lines in rice. It is noteworthy that the RNP amount for the 100 pmol/condition using the sonication-assisted whisker method is comparable to that of the PEG or the particle bombardment methods9, 26, 27. This suggests that the sonication-assisted whisker method is a practical approach to delivering CRISPR RNPs to rice calli.
The insertion or deletion (indels) sizes induced by the RNP delivery mainly remained under 10 bp, whereas those induced by the plasmid delivery were beyond 10 bp in this study (Figs. 2, 3, Supplementary Table 1, Supplementary Fig. 2). Continuous Cas9 and gRNA expression by the plasmids could potentially induce DSBs multiple times even after the target site carried small indels since the CRISPR/Cas9 system could cleave the target DNA sequence with insertion or deletion and change its mutations repeatedly28. RNP delivery possibly induces smaller indels at the target site. This characteristic is preferable to induce small indels, which mimic naturally-occurring mutations.
Unlike T-DNA delivery by Agrobacterium, gene delivery methods achieved by physical principles, including particle bombardment and the whisker method, could induce the fragmentation of the DNAs to be delivered6. The larger the DNA fragment to be delivered is, the possibility of fragmented DNA random integration becomes higher. Therefore, the size of the plasmid to be delivered should be as small as possible for transgene elimination. In the case of CRISPR/Cas9-based genome editing, the large-size DNA fragments for expressing Cas9 and gRNA are usually problematic29. Delivery of both the small-size plasmid harboring the selection marker and CRISPR/Cas9 components as RNPs reduces the risk of random integration of the transgene into the genome. Unfortunately, the isolation of null segregant has not been achieved in this study since the target genes were lethal. Establishing procedures and assessment of the efficiency for isolating null segregants should be further challenges for the whisker mediated RNP delivery method. The feasible isolation of genome-edited lines by the sonication-assisted whisker method in this study would contribute to developing a platform of transgene-free genome-edited lines.
OsLCYβ genome-edited rice was first developed by the sonicated-assisted whisker method with RNPs (Figs. 3, 4, Supplementary Figs. 4, 5). Drastic reduction of β-carotene, violaxanthin, antheraxanthin were observed in the plantlet of the OsLCYβ gene-edit line. Chlorophylls may not be maintained without carotenoids since the adequate carotenoid:chlorophyll ratio is essential for the function and maintenance of the photosynthetic reaction center complex30, thereby leading to the albino phenotype31. In addition to lycopene and δ-carotene accumulation in the callus, lutein acetate was unexpectedly accumulated and other carotenoids were reduced in the regenerated shoot of the OsLCYβ mutant. Lutein acetate accumulation was also reported in rice leaves during senescence, possibly due to the degradation of light-harvesting complex II32. Different from lutein, lutein acetate cannot be part of the photosynthetic reaction center complex32. Adequate carotenoid shortage in the photosynthetic reaction center complex could explain why the shoot of the LCYβ-2 #2 mutant, which cannot synthesize the carotenoids for photosynthesis, displays no green color.
This study is the first to report that RNPs could be delivered into plant cells by the whisker method. In particular, RNPs were probably introduced without plasmid co-transfection (Supplementary Fig. 6). The results suggest that the sonication-assisted whisker method could be used not only to conduct RNP delivery but also to deliver macromolecules other than plasmids. Although the detailed underlying mechanism of how RNPs entered into plant cells has not been clarified in this study, the sonication-assisted whisker method could be potentially used to introduce proteins into plant cells in general. Unlike particle bombardment, RNPs were probably introduced into the plant cells in solution without any dry-up process, thereby protein delivery with less inactivation could be achieved. For example, as a future perspective, recombinant antibiotic resistance proteins co-transfected with CRISPR/Cas RNPs could make DNA-free genome-edited cell selection possible.
The sonication-assisted whisker method has already been used as a versatile method for gene delivery in plants14,15,16,17,18. The demonstration of RNP-based genome editing in rice in this study could easily be extrapolated to various plant species. The sonication-assisted whisker method would be an attractive tool for RNP-based gene delivery not only in the model crops but also in industrially important plant species.
Methods
Recombinant SpCas9 preparation
The C-terminally His6-tagged SpCas9 with SV40 NLS gene was cloned into pET28a (Addgene #4732733). For recombinant SpCas9-NLS overexpression, the plasmid was transformed into the E. coli strain BL21 (DE3) pLysRARE2. The bacteria containing the plasmid were pre-cultured with the supplementation of 25 μg/mL kanamycin and 34 μg/mL chloramphenicol. When the OD600 reached 0.6, 0.25 mM IPTG was added to induce protein expression at 18 °C overnight. Cell pellets were resuspended in buffer A [50 mM Tris–HCl, pH 8.0, 500 mM NaCl, 5 mM MgCl2, and 10% (vol/vol) glycerol] with 0.5 mg/mL lysozyme and 0.1 mg/mL DNase I, then disrupted by sonication. The supernatant was loaded onto a 1-mL Ni–NTA Superflow column pre-equilibrated with buffer A. The recombinant SpCas9 protein was eluted with 250 mM imidazole, then two-times diluted with buffer B [50 mM Tris–HCl, pH 8.0, 5 mM MgCl2, and 10% (vol/vol) glycerol]. The diluted sample was loaded onto a Heparin HP column. The protein fractions were pooled and finally purified by a HiLoad 16/600 Superdex 200 pg column in buffer C [20 mM Tris–HCl pH 7.5, 150 mM NaCl, 1 mM MgCl2, and 10% (vol/vol) glycerol]. Pooled fractions were concentrated by ultrafiltration to a final concentration of 100 μM and stored at − 80 °C until further use.
In vitro gRNA synthesis
The gRNA was transcribed using a lab-made T7 RNA polymerase. The double-strand DNA encoding the T7 promoter and gRNA sequence was amplified by PCR using four overlapping primers [F1: 5′-TAATACGACTCACTATANNNNNNNNNNNNNNNNNNNNNGTTTTAGAGCTAGAAATAGCAAGTTAAAATAAG-3′ (N means 20-bp target sequence dependent on the target gene, see Supplementary Table 1 for further details); F2: 5′-GGATCCTAATACGACTCACTATAG-3′; R1: 5′-GCACCGACTCGGTGCCACTTTTTCAAGTTGATAACGGACTAGCCTTATTTTAACTTGCTATTTCTAGCTC-3′; R2: 5′-AAAAAAGCACCGACTCGGTGCCAC-3′] and purified using a MinElute PCR Purification Kit (QIAGEN Inc.). The in vitro transcription was performed at 37 °C for 6 h. The reaction mixture was purified using 8% denaturing urea-polyacrylamide gel electrophoresis. RNAs were extracted from the gel slices and refolded simultaneously in H2O at 4 °C for 18 h. The extracted RNA samples were precipitated with ethanol, dissolved in H2O, and stored at − 80 °C.
In vitro gRNA activity assessment
Target site-containing substrate DNA fragments were PCR-amplified using rice genomic DNA as a template. The amplified DNA fragment (10 nM) was mixed with the recombinant SpCas9–sgRNA complex (10–500 nM, molar ratio, 1:2) in 10 μL of Cas9 reaction buffer (20 mM HEPES–NaOH, pH 7.5, 100 mM NaCl, 5 mM MgCl2, and 0.1 mM EDTA), then incubated at 37 °C for 30 min. The reaction mixture was quenched by adding 10 μL of EDTA (50 mM final concentration)-containing quench buffer, then incubated at 60 °C for 5 min. The reaction products were analyzed using a MultiNA microchip electrophoresis system (SHIMADZU Inc.). For the time course experiment of the in vitro cleavage assay, the amplified DNA fragment (10 nM) was incubated at 25 °C or 37 °C with the recombinant SpCas9–sgRNA complex (100 nM, molar ratio, 1:2), in 30 μL of Cas9 reaction buffer. Aliquots (10 μL) were taken at 5 and 30 min and mixed with 10 μL of quench buffer, then incubated at 60 °C for 5 min. The reaction products were analyzed using a MultiNA microchip electrophoresis system. In vitro cleavage assays were performed at least three times.
Plasmid preparation
The plasmid containing the selective marker HPT (hygromycin phosphotransferase) gene and CpYGFP (a yellow GFP-like protein derived from the marine copepod Chiridius poppei) controlled by the 35S promoter was purified by a QIAGEN plasmid Maxi Kit (QIAGEN Inc.). The OsPDS-targeted CRISPR/Cas9 vector for the sonication-assisted whisker method was constructed using pZH_gYSA_PubiMMCas9 and pU6_ccdB_gRNA34.
Cell culture
Calli were induced from mature rice seed (Oryza sativa L. ssp. Japonica cv. Nipponbare) and suspension-cultured as reported previously35. After suspension culture for 3 weeks, the cell clumps were crushed and passed through a one-mm stainless sieve immediately prior to use. The cell preparation is identical both in “with selection” experiment or “without selection” experiments.
Whisker preparation
Whiskers of potassium titanate fibers (Whisker LS20; TITAN KOGYO Inc.) with an average diameter of 0.5 nm and length ranging between 5 and 30 nm were used for transformation. Approximately 50 mg of dry fibers into tube were sterilized with 1 mL EtOH (100%) overnight and dried by leaving the tube with an opened cap on the clean bench. The sterilized whiskers were stored at 4 °C until use. The dry whiskers were handled in an exhaust fume hood to avoid inhalation. Sterile whiskers in liquid medium (1% w/v) containing 1/3MS basal salts and 30 g/L sucrose were mixed using a vortex mixer.
Transformation, selection, and regeneration
Approximately 250 µL in PCV of suspension-cultured rice calli after a 3-day subculture were dispensed into a 1.5-mL Eppendorf tube. The calli were dispersed in a fresh liquid medium containing 1/3 MS salts and 30 g/L sucrose. The 1% (w/v) whisker suspension (500 µL), vortexed immediately before use, was added to this tube and tapped for mixing. After centrifugation at 3000 rpm for 5 s, the supernatant was removed. The plasmid/polyornithine (POH) complex (70 µL) harboring antibiotics-resistant gene cassette was mixed with ice-cold 0–100 pmol RNPs in the tube (i.e., in case of 100 pmol RNP, 1 µM in final) and the tube was tapped strongly to mix except for the DNA-free experiments. For the DNA-free experiments, only the RNP with POH of 100 µL was mixed with the whisker. Immediately, the suspension was centrifuged for 5 min at 15,000 rpm at 4 °C and tapped. This process was repeated 3 times. After incubation on ice for 10 min, the tube containing the mixture of calli, whiskers, and the plasmid was subjected to supersonic treatment with a disrupter for 1 min (Bioruptor UCD-200, Sonicbio. Co. Ltd.) at room temperature. Next, the mixture was washed with a fresh liquid medium to remove the whiskers. The whisker-treated calli were then transferred into a 35 × 10 mm plastic Petri plate for subculture. Three ml of liquid R2 medium (1 × R2 basal salts, 2 mg/L 2, 4-D, and 30 g/L sucrose). The culture was grown on a rotary shaker (120 rpm) in the dark at 28 °C.
After 3–6 days, the whisker-treated calli were transferred onto the surface of the selection medium [N6 basal salts, 2 mg/L 2, 4-D, 30 g/L sucrose, 50 mg/L hygromycin B (Hyg), and 3 g/L Gelrite (Wako Chemicals Inc.)]. After 7 days of culture, the calli were manually split into pieces and subjected to DNA extraction (see below). After 30 days of culture, the growing calli were transferred into a regeneration medium (MS basal salts, 1 mg/L NAA, 2 mg/L BA, 20 g/L sucrose, 30 g/L sorbitol, 50 mg/L Hyg, and 4 g/L Gelrite) and cultured at 28 °C with 16 h of light per day.
PCR genotyping and Amplicon-seq analysis
DNA was extracted from the part of calli using the cetyltrimethylammonium bromide (CTAB) method. The target region was PCR-amplified using KOD One PCR master Mix or KOD FX Neo (TOYOBO Inc., Osaka, Japan) with the primer set of the first-round PCR (see Supplementary Table 1). PCR was carried out using the cycling conditions as follows: 98 °C for 2 min followed by 35 cycles of 98 °C for 10 s, 55 °C for 5 s, and 68 °C for 3 s. In the experiment of DNA-free genome editing, the restriction enzyme which recognizes the putative DSB site by CRISPR/Cas9 were utilized for first round PCR product. Since the DNA fragments with certain mutations at the target site should be resistant to the restriction enzyme, the genome-edited DNA fragments would be concentrated in the PCR product upon the treatment with the restriction enzyme. The PCR products were digested by PstI-HF (New England Biolab Inc., Massachusetts, USA) and gel-purified using FastGene Gel/PCR Extraction Kit (Nippon Genetics Inc., Tokyo, Japan) to reduce unedited OsPDS amplicons (See also Supplementary Fig. 6). In the second-round PCR, Illumina Combinatorial Dual Index sequences were attached as follows: 5′-AATGATACGGCGACCACCGAGATCTACACNNNNNNNNTCGTCGGCAGCGTC-3′ and 5′-CAAGCAGAAGACGGCATACGAGATNNNNNNNNGTCTCGTGGGCTCGG-3′. PCR was carried out using the cycling conditions as follows: 98 °C for 2 min followed by 30 cycles of 98 °C for 10 s, 55 °C for 5 s, and 68 °C for 3 s. The PCR products were purified using 1.2 times-volume of Sera-Mag Select beads (Cytiva Inc., Marlborough, MA, USA) with a supernatant substituted with 20% PEG-8000 and 2.5 M NaCl solution. The equal volume of purified DNA was pooled and its concentration was measured using a Qubit dsDNA HS Assay Kit and Qubit 3.0 Fluorometer (Thermo Fisher Scientific Inc., Waltham, MA, USA) and adjusted to 50 pM. The pooled library was used for 2 × 150-bp paired-end sequencing with iSeq100 (Illumina Inc., San Diego, CA, USA) after mixing with 0.25 volume of 50 pM PhiX control v3 (Illumina Inc.). The obtained fastq files were analyzed by CRISPResso236. Indels at the Cas9 cleavage site were considered as mutations induced by the introduced RNPs. The mutation quantification window was set as 10 bp.
Carotenoid identification and quantification
We performed carotenoid extraction from the calli (0.5 g) and shoots (0.25 g) with biological triplicates following a previously reported procedure23. The acetone supernatants of the extracts were dried and the samples were kept at − 20 °C. Right before analysis, the dried sample was resuspended in 1 mL acetone with 0.1% butylated hydroxytoluene (BHT). An aliquot (2.0–2.5 µL) of this sample was injected into a UPLC system (described below) and the carotenoids were identified by UV–Vis, MS, and MS/MS spectral data and by retention time in HPLC and compared to standard samples. Authentic β-carotene, lutein, and neoxanthin were purchased from Fujifilm Wako (#032-17991, #524-31401, and #523-31471, respectively). Standards for ε-carotene, δ-carotene, lycopene, and violaxanthin were prepared by extraction from recombinant E. coli producing each carotenoid.
The instrument used was a Waters ACQUITY UPLC H-Class PLUS System with Xevo TQ-S micro equipped with an electrospray ionization source operating in positive mode. Carotenoid separation was performed on a BEH UPLC C18 column (100 mm × 2.1 mm, 1.7 μm) (Waters Inc., Milford, CT) at 30 °C. The mobile phases consisted of solvents A (water:acetonitrile 10:90), B (100% acetonitrile), and C (acetonitrile:isopropanol 60:40) using the following gradient elution program at a flow rate of 0.2 mL/min: 0–2 min, 100% A; 2–4 min, gradient to 100% B; 4–6 min, gradient to 100% C; 6.01 min, back to 100% A, and hold for 3 min. The injection volume was 2–2.5 µL. The desolvation gas was set to 1000 L/h at 300 °C, the cone gas flow was at 50 L/h. The capillary voltage was set to 3.5 kV and the cone voltage at 30 V for all samples. The MS/MS experiments were performed using a collision energy of 20 V. The data were analyzed using the MassLynx 4.1 software equipped to the instrument. The carotenoid amounts were quantified as previously described37, 38 by calculating the UPLC peak area at 450 nm using the standard curve of authentic samples.
Data availability
Quantified carotenoid data were analyzed and visualized using R version 4.1.1 (R Core Team). The R script used for the data analyses and source data are available on Github (https://github.com/aist-pgrrg/whisker_RNP).
References
Knott, G. J. & Doudna, J. A. CRISPR-Cas guides the future of genetic engineering. Science 361, 866–869 (2018).
Metje-Sprink, J., Menz, J., Modrzejewski, D. & Sprink, T. DNA-free genome editing: Past, present and future. Front. Plant Sci. 9, 1957 (2018).
Ma, X., Zhang, X., Liu, H. & Li, Z. Highly efficient DNA-free plant genome editing using virally delivered CRISPR-Cas9. Nat. Plants 6, 773–779 (2020).
Ariga, H., Toki, S. & Ishibashi, K. Potato virus X vector-mediated DNA-free genome editing in plants. Plant Cell Physiol. 61, 1946–1953 (2020).
Camacho, A., Van Deynze, A., Chi-Ham, C. & Bennett, A. B. Genetically engineered crops that fly under the US regulatory radar. Nat. Biotechnol. 32, 1087–1091 (2014).
Zhang, Y., Iaffaldano, B. & Qi, Y. CRISPR ribonucleoprotein-mediated genetic engineering in plants. Plant Commun. 2, 100168 (2021).
Woo, J. W. et al. DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins. Nat. Biotechnol. 33, 1162–1164 (2015).
Toda, E. et al. An efficient DNA- and selectable-marker-free genome-editing system using zygotes in rice. Nat. Plants 5, 363–368 (2019).
Liang, Z. et al. Efficient DNA-free genome editing of bread wheat using CRISPR/Cas9 ribonucleoprotein complexes. Nat. Commun. 8, 14261 (2017).
Svitashev, S., Schwartz, C., Lenderts, B., Young, J. K. & Mark Cigan, A. Genome editing in maize directed by CRISPR-Cas9 ribonucleoprotein complexes. Nat. Commun. 7, 13274 (2016).
Kaeppler, H. F., Gu, W., Somers, D. A., Rines, H. W. & Cockburn, A. F. Silicon carbide fiber-mediated DNA delivery into plant cells. Plant Cell Rep. 9, 415–418 (1990).
Takahashi, W., Shimada, T., Matsushita, J. & Tanaka, O. Aluminium borate whisker-mediated DNA delivery into callus of rice and production of transgenic rice plant. Plant Prod. Sci. 3, 219–224 (2000).
Matsushita, J., Otani, M., Wakita, Y., Tanaka, O. & Shimada, T. Transgenic plant regeneration through silicon carbide whisker-mediated transformation of rice (Oryza sativa L.). Breed. Sci. 49, 21–26 (1999).
Frame, B. R. et al. Production of fertile transgenic maize plants by silicon carbide whisker-mediated transformation. Plant J. 6, 941–948 (1994).
Mizuno, K., Takahashi, W., Beppu, T., Shimada, T. & Tanaka, O. Aluminum borate whisker-mediated production of transgenic tobacco plants. Plant Cell, Tissue Organ Cult. 80, 163–169 (2005).
Asad, S. et al. Silicon carbide whisker-mediated embryogenic callus transformation of cotton (Gossypium hirsutum L.) and regeneration of salt tolerant plants. Mol. Biotechnol. 40, 161–169 (2008).
Terakawa, T., Hasegawa, H. & Yamaguchi, M. Efficient whisker-mediated gene transformation in a combination with supersonic treatment. Breed. Sci. 55, 465–468 (2005).
Khalafalla, M. M. et al. Efficient production of transgenic soybean (Glycine max L. Merrill) plants mediated via whisker-supersonic (WSS) method. Afr. J. Biotechnol. 5, 1 (2006).
Maruyama, N. et al. Stable accumulation of seed storage proteins containing vaccine peptides in transgenic soybean seeds. J. Biosci. Bioeng. 118, 441–447 (2014).
Taniguchi, R., Terakawa, T., Hasegawa, H., Kurita, M. & Kondo, T. Development of transgenetic Japanese cedar by whisker method. Jpn. For. Soc. Annu. Meet. Collect. 120, 645–645 (2009).
Shan, Q. et al. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat. Biotechnol. 31, 686–688 (2013).
Chin, D. P. et al. Generation of brilliant green fluorescent petunia plants by using a new and potent fluorescent protein transgene. Sci. Rep. 8, 16556 (2018).
Ishihara, A. et al. Biochemical and molecular characterization of orange- and tangerine-colored rice calli. Plant Biotechnol. 32, 193–203 (2015).
Fu, B. X. H., St Onge, R. P., Fire, A. Z. & Smith, J. D. Distinct patterns of Cas9 mismatch tolerance in vitro and in vivo. Nucleic Acids Res. 44, 5365–5377 (2016).
Bai, C. et al. A golden era—Pro-vitamin A enhancement in diverse crops. In Vitro Cell Dev. Biol. Plant 47, 205–221 (2011).
Wu, S. et al. Establishment of a PEG-mediated protoplast transformation system based on DNA and CRISPR/Cas9 ribonucleoprotein complexes for banana. BMC Plant Biol. 20, 425 (2020).
Liang, Z. et al. Genome editing of bread wheat using biolistic delivery of CRISPR/Cas9 in vitro transcripts or ribonucleoproteins. Nat. Protoc. 13, 413–430 (2018).
Lin, Y. et al. Non-viral delivery of the CRISPR/Cas system: DNA versus RNA versus RNP. Biomater. Sci. 10, 1166–1192 (2022).
Kaya, H., Ishibashi, K. & Toki, S. A split Staphylococcus aureus Cas9 as a compact genome-editing tool in plants. Plant Cell Physiol. 58, 643–649 (2017).
Brotosudarmo, T. H. P., Limantara, L., Chandra, R. D. & Heriyanto Chloroplast pigments: Structure, function, assembly and characterization. In Plant Growth and Regulation—Alterations to Sustain Unfavorable Conditions (eds Ratnadewi, D. & Hamim) (IntechOpen, 2018).
Kössler, S. et al. Lycopene β-cyclase expression influences plant physiology, development, and metabolism in tobacco plants. J. Exp. Bot. 72, 2544–2569 (2021).
Kusaba, M., Maoka, T., Morita, R. & Takaichi, S. A novel carotenoid derivative, lutein 3-acetate, accumulates in senescent leaves of rice. Plant Cell Physiol. 50, 1573–1577 (2009).
Gagnon, J. A. et al. Efficient mutagenesis by Cas9 protein-mediated oligonucleotide insertion and large-scale assessment of single-guide RNAs. PLoS ONE 9, e98186 (2014).
Mikami, M., Toki, S. & Endo, M. Comparison of CRISPR/Cas9 expression constructs for efficient targeted mutagenesis in rice. Plant Mol. Biol. 88, 561–572 (2015).
Urushibara, S., Tozawa, Y., Kawagishi-Kobayashi, M. & Wakasa, K. Efficient transformation of suspension-cultured rice cells mediated by Agrobacterium tumefaciens. Breed. Sci. 51, 33–38 (2001).
Clement, K. et al. CRISPResso2 provides accurate and rapid genome editing sequence analysis. Nat. Biotechnol. 37, 224–226 (2019).
Furubayashi, M., Maoka, T. & Mitani, Y. Promiscuous activity of β-carotene hydroxylase CrtZ on epoxycarotenoids leads to the formation of rare carotenoids with 6-hydroxy-3-keto-ε-ends. FEBS Lett. 596, 1921–1931 (2022).
Furubayashi, M. et al. Capsanthin production in Escherichia coli by overexpression of capsanthin/capsorubin synthase from Capsicum annuum. J. Agric. Food Chem. 69, 5076–5085 (2021).
Acknowledgements
The authors thank A. Kuwazawa, S. Tsujikawa, Y. Taguchi, and Y. Takiguchi for their technical assistance. They also thank A. Ohmiya, H.Yamamoto, and S. Sakamoto for the helpful discussions. They also thank the source image of RNP to TogoTV (DBCLS TogoTV, CC-BY-4.0).
Author information
Authors and Affiliations
Contributions
T.T., N.M., A.N. and S.S.S. conceived and designed the research. T.Y. performed most of the whisker experiments, plant regeneration, and extraction of carotenoids. A.N. performed gRNA design, SpCas9 purification, in vitro gRNA synthesis, and in vitro cleavage assay. S.S.S. and S.I. performed Amplicon-seq analyses. M.F. performed the identification and quantification of carotenoids. S.S.S., A.N., N.M., and T.T. wrote the manuscript with the input of all the authors.
Corresponding authors
Ethics declarations
Competing interests
T.Y and T.T. have financial interests in Inplanta Innovations Inc. Yokohama, Japan. S.I. has financial interests in TOPPAN INC. A patent, which was invented by A.N., S.S.S., N.M., T.Y., T.T., and S.I., has been filed on behalf of JP2021-189460 by AIST, Inplanta Innovations Inc., and TOPPAN INC. M.F. has no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nakamura, A., Yano, T., Mitsuda, N. et al. The sonication-assisted whisker method enables CRISPR-Cas9 ribonucleoprotein delivery to induce genome editing in rice. Sci Rep 13, 14205 (2023). https://doi.org/10.1038/s41598-023-40433-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-40433-w
This article is cited by
-
It takes two to tango with CRISPR: a history and overview of augmenting the technology for genetic engineering
Proceedings of the Indian National Science Academy (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.