Abstract
The liver is one of the most ordinary metastatic sites of gastroesophageal junction adenocarcinoma and significantly affects its prognosis. Therefore, this study tried to construct a nomogram that can be applied to predict the likelihood of liver metastases from gastroesophageal junction adenocarcinoma. 3001 eligible patients diagnosed with gastroesophageal junction adenocarcinoma between 2010 and 2015 in the Surveillance, Epidemiology, and End Results (SEER) database were involved in the analysis. Patients were randomly divided into a training cohort and an internal validation cohort using R software, with an allocation ratio of 7:3. According to the consequences of univariate and multivariate logistic regression, we constructed a nomogram for predicting the risk of liver metastases. The discrimination and calibration ability of the nomogram was appraised by the C-index, ROC curve, calibration plots, and decision curve analysis (DCA). We also used Kaplan–Meier survival curves to compare differences in overall survival in patients with gastroesophageal junction adenocarcinoma with and without liver metastases. Liver metastases developed in 281 of 3001 eligible patients. The overall survival of patients with gastroesophageal junction adenocarcinoma with liver metastases before and after propensity score matching (PSM) was obviously lower than that of patients without liver metastases. Six risk factors were finally recognized by multivariate logistic regression, and a nomogram was constructed. The C-index was 0.816 in the training cohort and 0.771 in the validation cohort, demonstrating the good predictive capacity of the nomogram. The ROC curve, calibration curve, and decision curve analysis further demonstrated the good performance of the predictive model. The nomogram can accurately predict the likelihood of liver metastases in gastroesophageal junction adenocarcinoma patients.
Similar content being viewed by others
Introduction
The incidence of gastroesophageal junction adenocarcinoma (GEJA) has increased markedly in Western countries over the past few decades1,2. Gastroesophageal junction cancer is a cancer in which the center of the tumor is situated in the gastroesophageal junction region. The widely used categorization of gastroesophageal junction cancer in Western countries is the Siewert classification3. The Siewert classification applies only to adenocarcinomas situated within 5 cm above or below the gastroesophageal junction. The Siewert classification divided GEJA into three types: type I is located about 1–5 cm above the esophagogastric junction; type II is located between 1 cm above and 2 cm below the esophagogastric junction; type III is located about 2–5 cm below the esophagogastric junction4,5. Cells at the gastroesophageal junction have histological features of both esophageal and gastric cells6. Therefore, its histological origin and appropriate treatment remain controversial7,8. In clinical practice, type I and type III GEJA are often treated and staged with reference to esophageal and gastric cancers9. Siewert type II GEJA is located along the borderline between the mediastinum and abdomen, they can metastasize to both thoracic and abdominal cavities. Therefore, the prognosis or metastasis of Siewert type II GEJA could be significantly different from other types of GEJA10,11,12. A previous study found that the poor prognosis of GEJA was largely attributable to early and frequent metastases13. Gastroesophageal junction carcinoma patients have a poor prognosis after metastases, with a 5-year survival rate of about 11%14. A population-based study showed that the liver is the most common site of metastasis for Siewert type II GEJA15. Consequently, it has important clinical value to construct a predictive model that can be applied to predict the risk of liver metastases from GEJA. This study tried to construct and validate a nomogram based on the SEER database for predicting the likelihood of liver metastases from Siewert type II GEJA.
Methods
Patients
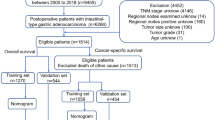
We screened 3001 GEJA patients newly diagnosed between 2010 and 2015 from the SEER database who met our inclusion criteria, of which 281 developed liver metastases. The exclusion process is shown in Fig. 1. Patients included in this study must meet the following criteria: (1) Tumor size does not exceed 100 mm (2) First malignant primary indicator (3) Age between 19 and 84 (4) The pathological type is adenocarcinoma. The exclusion criteria for GEJA patients were as follows: (1) Incomplete clinical and pathological features. (2) Patients who were identified by autopsy. We extracted race, gender, year of diagnosis, T stage, N stage, tumor size, age, and bone/brain/liver/lung metastases, as well as other follow-up data, from the SEER database. This study adopted the 7th edition of the American Joint Committee on Cancer (AJCC) TNM staging. All data used in this study were anonymized and de-identified from the SEER database. Therefore, approval by an institutional review board is not required, nor is informed consent of all subjects and/or their legal guardian(s). All methods of this study were performed in accordance with the relevant regulations and guidelines.
Statistical analysis and optimal cutoffs
We used x-tile v3.6.1 (Yale University) software to determine optimal cutoff values for tumor size and age16. By using X-tile, we transform all continuous variables into categorical variables. We used Fisher's exact test or chi-square to compare differences in categorical variables. To balance differences in other factors between GEJA patients with liver metastases and those without liver metastases, we performed a 1:3 PSM in R software v4.3.0. The Kaplan–Meier survival curves were applied to evaluate the difference in survival time between patients with liver metastases and those without liver metastases before and after PSM. We randomly divided the 3001 GEJA patients into a training group (n = 2101) and an internal validation group (n = 900) in a 7:3 ratio by R software. We performed univariate and multivariate logistic regression, from which we created a nomogram for predicting the possibility of liver metastases. We use ROC curve, C-index, calibration curve, and decision curve analysis to verify its validity. All statistical analyses were carried out using GraphPad Prism v8.0.2 (GraphPad Software, Inc.), SPSS v26.0 (SPSS Inc.), and R software v4.1.3 (https://www.r-project.org/). The difference is statistically significant at P < 0.05.
Results
Characteristics of GEJA patients
We included 3001 patients with GEJA diagnosed between 2010 and 2015 in this retrospective study, with 9.3% (n = 281) had liver metastases, 3.5% (n = 106) had lung metastases, 2.5% (n = 77) had bone metastases, 0.4% (n = 14) had brain metastases. Table 1 summarizes patient characteristics before and after PSM. As shown in Table 1, most of the variables have been balanced after PSM.
Survival analysis of liver metastases from gastroesophageal junction adenocarcinoma
Using R software v4.3.0, we performed a 1:3 propensity score matching of patients with gastroesophageal junction adenocarcinoma based on the presence or absence of liver metastases, and finally, 221 patients who had liver metastases were matched with 471 patients without liver metastases. The median follow-up for the pre-and post-PSM cohorts was 22 months (interquartile range: 10–44 months) and 11 months (interquartile range: 5–22 months), respectively. As shown in Table 1, other variables had been largely balanced. 1952 (65.0%) and 603 (87.1%) patients died during follow-up in the pre-and post-PSM cohorts, respectively. GEJA patients with liver metastases and those without liver metastases had median OS of 8.0 (95% CI 6.7–9.3) months and 30.0 (95% CI 27.7–32.3) months, respectively, in the pre-PSM cohort. (Fig. 2a) In the post-PSM cohort, they were 9.0 months (95% CI 6.5–9.5) and 14.0 months (95% CI 10.3–15.1), respectively. (Fig. 2b).
The diagnostic likelihood of liver metastases in GEJA patients
We randomly divided the patients into a training cohort and an internal validation cohort with an allocation of 7:3 ratio by R software v4.3.0. More information about the training and validation cohorts is shown in Table 2. As shown in Table 3, we performed univariate and multivariate logistic regression in the training cohort using SPSS v26.0 and finally identified age, T stage, bone metastasis, lung metastasis, and tumor size as risk factors for liver metastasis in GEJA patients.
Construction and validation of a predicted nomogram
Based on the risk factors for liver metastases identified by multivariate logistic regression, we created a nomogram to predict the risk of liver metastases in GEJA patients. (Fig. 3). The C-index in the training cohort is 0.816 and the C-index in the validation cohort is 0.771, which indicates that the prediction model has good discriminative ability as well as accuracy. As shown in Fig. 4, the AUC values of the nomogram in the training and validation cohorts are 0.816 and 0.771, respectively, which reflect the good predictive ability of our constructed prediction model. The calibration curves for both the training cohort (Fig. 5a) and the validation cohort (Fig. 5b) showed a good correlation between the predicted possibility of liver metastases and actually diagnosed liver metastases. A DCA was performed to determine the clinical utility of the nomogram. Figure 6 showed good positive net benefit in both the training cohort (Fig. 6a) and the internal validation cohort (Fig. 6b), indicating the good clinical applicability of the nomogram in predicting the presence of liver metastases in patients with adenocarcinoma of the gastroesophageal junction.
Discussion
The survival of patients with metastatic Siewert type II GEJA is influenced by multiple factors, such as pathological type, age, metastatic pattern, degree of differentiation, and treatment17,18. The liver is the most ordinary site of distant metastases in patients with Siewert type II GEJA, and it is also the most prognostic factor in all distant metastases19. The liver is also the most ordinary metastatic site of esophageal and gastric cancers20,21. Once metastasis occurs, the prognosis of GEJA patients will be poor. Surgery is the most common method of treatment for GEJA. However, once liver metastasis occurs, the comprehensive benefit of surgery for patients will be very low, and radiotherapy and chemotherapy will become the first treatment for patients. We found that 9.3% (n = 281) of GEJA patients included in this study developed liver metastases, and 18.3% (n = 551) of GEJA patients developed distant metastases. The survival rate of GEJA patients with liver metastases was obviously lower than those without liver metastases. For patients with liver metastases, early detection and early treatment can greatly improve their survival time and quality of life. Ultrasound, CT, and PET-CT are commonly used methods to detect distant metastases in GEJA, and PET-CT can accurately exclude distant metastases22,23. However, it has been shown that 15–20% of new esophageal cancers have distant metastases that are not identified by CT, the sensitivity and specificity of detection of distant metastasis by CT are 52% and 91%, respectively24,25. In addition, these imaging tests have radiation hazards and high prices, and not every patient can afford to undergo long-term or frequent imaging tests. Therefore, constructing a nomogram to predict the risk of liver metastases in GEJA patients can better guide clinical practice. Nomograms have long been widely used in oncology because they can provide visual predictions of patient outcomes based on relevant clinical variables26. The results obtained through data analysis can demonstrate the high predictive performance as well as clinical utility of nomograms, which can reduce examination costs on the one hand and avoid radiation hazards on the other. Besides, nomograms can be used as an early and low-cost screening tool for tumor metastasis, which can be a very meaningful guide for tumor diagnosis, treatment, and prognosis. Several nomograms have previously been used to predict prognosis in patients with gastroesophageal junction cancer. However, a nomogram to predict the likelihood of liver metastases from gastroesophageal junction adenocarcinoma has not been constructed6,27,28. Therefore, based on the SEER database, we constructed a nomogram that can be used to predict the likelihood of liver metastases in GEJA patients.
In this retrospective study, a nomogram that can predict the risk of liver metastases resulting from GEJA was constructed. And its accuracy was verified by the ROC curve, C index, and calibration curve. Through univariate and multivariate logistic regression, we finally identified age, tumor size, N stage, T stage, bone metastases, and lung metastases as factors affecting liver metastases in gastroesophageal junction adenocarcinoma.
We found that the likelihood of liver metastases in GEJA patients decreased with increasing age. A previous study found that colorectal cancer incidence increases with age, but metastatic spread decreases with age29,30. And they further analyzed the possible mechanism and believed that this conclusion is the result of the interaction between the tumor microenvironment, tumor biology, the immune system, and the genome30. This is consistent with our conclusion that age is an important factor affecting cancer spread, and the risk of cancer metastasis decreases with age.
We found that tumor size was also an important factor affecting the happening of liver metastases in GEJA patients, and the larger the tumor size, the higher the risk of liver metastases. It has been previously reported that the risk of lymph node metastasis increases with increasing tumor size in patients with Siewert type II T1-T3 GEJA31. It can be seen that tumor size is related to tumor invasion, which is further demonstrated by our findings.
As shown in Fig. 3, N staging is the least influential among the many factors that affect the occurrence of liver metastases in GEJA. Previous studies have found that in non-small cell lung cancer, the rate of multiorgan metastases increases with increasing N stage32. Therefore, for GEJA patients, a higher N stage is linked with a higher risk of liver metastases. The mechanism behind it remains to be revealed in future studies.
T staging of gastroesophageal junction malignancies is based on the degree of invasion. However, to our surprise, we found that the higher the T stage, the lower the risk of liver metastases in GEJA patients. We also found that the risk of liver metastases in gastric cancer decreased with increasing T stage. Nevertheless, more evidence is needed to confirm the relationship between the T stage and liver metastases in GEJA patients.
Malignant tumors can metastasize to other organs in the body through blood spread. Therefore, it is not surprising that there is a correlation between bone metastases and lung metastases, and liver metastases. However, we found no correlation between brain and liver metastases in our study (P = 0.108). We believe this is due to the low number of patients with brain metastases in the patients included in this study (n = 14, 0.4%).
Therefore, age, tumor size, T stage, N stage, lung metastasis, and brain metastasis all affect the occurrence of liver metastases in GEJA patients. The nomogram we constructed can accurately predict the likelihood of liver metastases in GEJA patients and better guide clinical practice.
Conclusion
The survival time of GEJA patients with liver metastases was obviously lower than that of GEJA patients without liver metastases. The nomogram model developed in our study can precisely predict the possibility of GEJA patients with liver metastases.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request. It can also be downloaded directly from the SEER database. (https://seer.cancer.gov/).
References
Keighley, M. R. Gastrointestinal cancers in Europe. Aliment. Pharmacol. Ther. 18(Suppl 3), 7–30 (2003).
Steevens, J., Botterweck, A. A., Dirx, M. J., van den Brandt, P. A. & Schouten, L. J. Trends in incidence of oesophageal and stomach cancer subtypes in Europe. Eur. J. Gastroenterol. Hepatol. 22(6), 669–678 (2010).
Manabe, N., Matsueda, K. & Haruma, K. Epidemiological review of gastroesophageal junction adenocarcinoma in Asian countries. Digestion 103(1), 29–36 (2022).
Siewert, J. R. & Stein, H. J. Classification of adenocarcinoma of the oesophagogastric junction. Br. J. Surg. 85(11), 1457–1459 (1998).
Roder, J.D., Stein, H.J. & Siewert, J.R. Carcinoma of the periampullary region: Who benefits from portal vein resection? Am. J. Surg. 171(1), 170–174 (1996); discussion 174–175.
Liu, X., Jiang, Q., Yue, C. & Wang, Q. Clinicopathological characteristics and survival predictions for adenocarcinoma of the esophagogastric junction: A SEER population-based retrospective study. Int. J. General Med. 14, 10303–10314 (2021).
Lagarde, S. M., ten Kate, F. J., Reitsma, J. B., Busch, O. R. & van Lanschot, J. J. Prognostic factors in adenocarcinoma of the esophagus or gastroesophageal junction. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 24(26), 4347–4355 (2006).
Anker, C. J. et al. Executive summary of the American radium society appropriate use criteria for operable esophageal and gastroesophageal junction adenocarcinoma: Systematic review and guidelines. Int. J. Radiat. Oncol. Biol. Phys. 109(1), 186–200 (2021).
Zhu, K. et al. Proximal gastrectomy versus total gastrectomy for Siewert type II adenocarcinoma of the esophagogastric junction: A comprehensive analysis of data from the SEER registry. Dis. Markers 2019, 9637972 (2019).
Hasegawa, S., Yoshikawa, T., Cho, H., Tsuburaya, A. & Kobayashi, O. Is adenocarcinoma of the esophagogastric junction different between Japan and western countries? The incidence and clinicopathological features at a Japanese high-volume cancer center. World J. Surg. 33(1), 95–103 (2009).
Curtis, N. J. et al. The relevance of the Siewert classification in the era of multimodal therapy for adenocarcinoma of the gastro-oesophageal junction. J. Surg. Oncol. 109(3), 202–207 (2014).
Cai, M. Z., Lv, C. B., Cai, L. S. & Chen, Q. X. Priority of lymph node dissection for advanced esophagogastric junction adenocarcinoma with the tumor center located below the esophagogastric junction. Medicine 98(51), e18451 (2019).
Mochizuki, K. et al. Esophagogastric junction carcinomas-discriminating histological types through immunohistochemistry. Anticancer Res. 37(12), 6855–6861 (2017).
Steup, W.H., De Leyn, P., Deneffe, G., Van Raemdonck, D., Coosemans, W. & Lerut, T. Tumors of the esophagogastric junction. Long-term survival in relation to the pattern of lymph node metastasis and a critical analysis of the accuracy or inaccuracy of pTNM classification. J. Thorac. Cardiovasc. Surg. 111(1), 85–94 (1996); discussion 94–85.
Chen, K. et al. Sites of distant metastases and the cancer-specific survival of metastatic Siewert type II esophagogastric junction adenocarcinoma: A population-based study. Expert Rev. Gastroenterol. Hepatol. 14(6), 491–497 (2020).
Camp, R. L., Dolled-Filhart, M. & Rimm, D. L. X-tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 10(21), 7252–7259 (2004).
Blank, S. et al. A reliable risk score for stage IV esophagogastric cancer. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 39(8), 823–830 (2013).
Badgwell, B. et al. Long-term survival in patients with metastatic gastric and gastroesophageal cancer treated with surgery. J. Surg. Oncol. 111(7), 875–881 (2015).
Chen, K. et al. Survival nomogram for patients with metastatic Siewert type II adenocarcinoma of the esophagogastric junction: A population-based study. Expert Rev. Gastroenterol. Hepatol. 14(8), 757–764 (2020).
Ai, D. et al. Patterns of distant organ metastases in esophageal cancer: A population-based study. J. Thorac. Dis. 9(9), 3023–3030 (2017).
Riihimäki, M., Hemminki, A., Sundquist, K., Sundquist, J. & Hemminki, K. Metastatic spread in patients with gastric cancer. Oncotarget 7(32), 52307–52316 (2016).
D’Journo, X. B. Clinical implication of the innovations of the 8(th) edition of the TNM classification for esophageal and esophago-gastric cancer. J. Thorac. Dis. 10(Suppl 22), S2671-s2681 (2018).
Miyazaki, T. et al. Effectiveness of FDG-PET in screening of synchronous cancer of other organs in patients with esophageal cancer. Anticancer Res. 34(1), 283–287 (2014).
Kato, H. et al. The incremental effect of positron emission tomography on diagnostic accuracy in the initial staging of esophageal carcinoma. Cancer 103(1), 148–156 (2005).
Flamen, P. et al. Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 18(18), 3202–3210 (2000).
Balachandran, V. P., Gonen, M., Smith, J. J. & DeMatteo, R. P. Nomograms in oncology: More than meets the eye. Lancet Oncol. 16(4), e173-180 (2015).
Guo, Z., Guo, H., Tian, Y., Zhang, Z. & Zhao, Q. Nomograms for predicting disease-free survival in patients with Siewert type II/III adenocarcinoma of the esophagogastric junction receiving neoadjuvant therapy and radical surgery. Front. Oncol. 12, 908229 (2022).
Chen, J. et al. Development and validation of a survival nomogram for patients with Siewert type II/III adenocarcinoma of the esophagogastric junction based on real-world data. BMC Cancer 21(1), 532 (2021).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics 2020. CA A Cancer J. Clin. 70(1), 7–30 (2020).
Pretzsch, E. et al. Age and metastasis—How age influences metastatic spread in cancer. Colorectal cancer as a model. Cancer Epidemiol. 77, 102112 (2022).
Feng, H. et al. The probability of Lymph node metastasis with a tumor size larger than and smaller than 4 cm is different in stages T1–T3 of Siewert type II adenocarcinoma of esophagogastric junction: A population-based study. J. Cancer 12(22), 6873–6882 (2021).
Yang, J. et al. The prognostic impact of lymph node metastasis in patients with non-small cell lung cancer and distant organ metastasis. Clin. Exp. Metas. 36(5), 457–466 (2019).
Acknowledgements
The authors thank the participants and their families in this study.
Funding
Supported by Natural Science Foundation of Gansu Province (21JR1RA118) and Gansu Provincial Youth Science and Technology Fund (21JR1RA107, 18JR3RA305).
Author information
Authors and Affiliations
Contributions
M.Z., W.Y., BH., C.C., D.Z., and Y.Y. made substantial contributions to conception and design; M.Z., W.Y., C.C., D.Z., and Y.Y. made substantial contributions to the acquisition, analysis, and interpretation of data. All authors made substantial contributions to the drafting of the manuscript and revising of the manuscript and the final approval of the manuscript to be published. All authors agree to be accountable for all aspects of the work and ensure its accuracy and its integrity.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, M., Yang, W., Yang, Y. et al. Nomogram for predicting the likelihood of liver metastases at initial diagnosis in patients with Siewert type II gastroesophageal junction adenocarcinoma. Sci Rep 13, 11032 (2023). https://doi.org/10.1038/s41598-023-37318-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-37318-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.