Abstract
Indirect somatic embryogenesis (ISE) is a morphogenetic pathway in which somatic cells form callus and, later, somatic embryos (SE). 2,4-dichlorophenoxyacetic acid (2,4-D) is a synthetic auxin that promotes the proliferation and dedifferentiation of somatic cells, inducing the ISE. However, 2,4-D can cause genetic, epigenetic, physiological and morphological disorders, preventing the regeneration and/or resulting abnormal somatic embryos (ASE). We aimed to evaluate the toxic 2,4-D effect during the Coffea arabica and C. canephora ISE, assessing the SE morphology, global 5-methylcytosine levels (5-mC%) and DNA damage. Leaf explants were inoculated in media with different 2,4-D concentrations. After 90 days, the friable calli were transferred to the regeneration medium, and the number of normal and abnormal SE was monthly counted. The increase of the 2,4-D concentration increased the number of responsive explants in both Coffea. At 9.06, 18.08 and 36.24 μM 2,4-D, C. arabica presented the highest values of responsive explants, differing from C. canephora. Normal and abnormal SE regeneration increased in relation to the time and 2,4-D concentration. Global 5-mC% varied at different stages of the ISE in both Coffea. Furthermore, the 2,4-D concentration positively correlated with global 5-mC%, and with the mean number of ASE. All ASE of C. arabica and C. canephora exhibited DNA damage and showed higher global 5-mC%. The allotetraploid C. arabica exhibited greater tolerance to the toxic effect of 2,4-D than the diploid C. canephora. We conclude that synthetic 2,4-D auxin promotes genotoxic and phytotoxic disorders and promotes epigenetic changes during Coffea ISE.
Similar content being viewed by others
Introduction
Indirect somatic embryogenesis (ISE) is a morphogenetic pathway that involves the callus formation and, later, somatic embryos (SE) regeneration. ISE is established in a sterile and appropriate in vitro condition, from the inoculation of tissue from explant donors1. The first in vitro approach involving Coffea was carried out by Staritsky2, which established somatic embryogenesis. The ability of a given tissue to generate SE is a characteristic restricted to a limited fraction of the cells3. For differentiation, cells need to acquire competence and follow a new morphogenetic pathway1. However, a new hypothesis has been proposed by Campos et al.4, in which meristematic cells are able to differentiate into SE without cell dedifferentiation. This debate is based on in vitro approaches that have elucidated the biological issues involved in the dedifferentiation, proliferation, and regeneration of callus, SE and plantlets. These issues provide the detailed analysis of the “omics” at different levels, as well as their interaction5,6,7,8,9,10,11.
2,4-dichlorophenoxyacetic (2,4-D) growth regulator is widely used in direct embryogenesis and in ISE, playing a crucial role in the cell dedifferentiation and SE recovering12. This growth regulator is related to the cell division at the S input and the G2-M transition13, and also to the cell expansion processes14. So, 2,4-D is the most used in Coffea ISE, which requires the initial presence of this auxin in the pre-incubation period or induction stages8,9,10,15,16. However, the high explant exposure to 2,4-D results in the formation of abnormal somatic embryos (ASE), due to the interruption of the genetic and physiological processes of the cells. This disruption causes a rapid efflux of protons, enzymatic activation, protein transcription and translation, and polysaccharide synthesis, resulting in loss of cell wall stability, depletion of reserve substances and inactivation of cell repair mechanisms12,17.
2,4-D can also cause genetic and epigenetic changes17. This is undesirable in Coffea propagation due to loss of genetic fidelity and ASE formation, compromising superior genotypes18,19,20. Phenotypic variants of C. arabica trees, which were regenerated in vitro, were induced by aneuploidy19. C. arabica plants regenerated in vitro showed genetic differences in relation to plant explant donors, since the somaclonal variation was revealed by differential fragments amplified by sequence-related amplified polymorphism (SRAP) primers20. Landey et al.18 reported that is low the frequency of genetic and epigenetic changes during somatic embryogenesis in C. arabica. According to these authors, the aneuploidy is main genome modification in most phenotypic variants, indicating that missegregation during the anaphase plays a major role in somaclonal variation in C. arabica.
Cell reprogramming is complex and often concomitant with significant changes in chromatin status. Chromatin change is characterized by DNA methylation and histone chemical modifications (mainly methylation or acetylation)21. In Coffea, epigenetic changes occur during somatic embryogenesis characterized by cytosine methylation6,8,9,10. These epigenetic changes occur due to the in vitro environment, promoting an important role to regulate and control genes involved with the morphogenetic pathway6. Epigenetic variations can be transient and altered through DNA methylation, as well as acetylation, phosphorylation, methylation and ubiquitination of the histones1. However, cell proliferation and SE and plantlets recovering are influenced by coordination of genetic and epigenetic factors22. When these factors are not coordinated, the main consequence is non-establishment of the ISE or the large-scale production of ASE17. The main abnormalities exhibited in SE are fusion of two or more embryos, absence of apical and root meristems, translucent embryos, multiple cotyledons and loss of bipolarity10,17. Generally, embryonic anomalies are associated with point mutations19,20, aneuploidy18 and/or epigenetic changes10. 2,4-D is one of the precursors for the emergence of these abnormalities because this herbicide can be toxic even in minimal concentrations17. Therefore, it is essential to quantify global methylation levels (5-mC%) during ISE and to evaluate the toxic effect of 2,4-D on SE regeneration in Coffea.
Comet Assay evidences DNA single-strand and/or double-strand breaks in individual cells23. For this reason, Comet Assay has been applied to assess the genotoxic effect of chemical, physical and biological agents. For example, Özkul et al.24 verified the 2,4-D genotoxic effect on Allium cepa L. roots from seeds germinated in semisolid medium supplement with ~ 18.08 μM 2,4-D for 48 h. However, the Comet Assay has not applied to assess DNA integrity in SE regenerated via ISE, in which 2,4-D is used to induce friable callus proliferation. From C. arabica and C. canephora, we aimed to: (a) induce ISE from different 2,4-D concentrations, (b) quantify the 5-mC% during ISE, (c) verify the effect of the 2,4-D and 5-mC% during ISE, and (d) assess the genotoxicity and phytotoxicity of the remaining 2,4-D on regenerated SE.
Results
Callogenesis
C. arabica and C. canephora exhibited a distinct mean number of responsive explants (leaf fragment with callus) influenced by 2.4-D (Fig. 1, SI Fig. 1, SI Table 1). The first responsive explants of C. arabica and C. canephora were observed at 30 days for all media. C. arabica exhibited the higher mean number of responsive explants than C. canephora in 9.06 – 36.24 μM 2,4-D at all times. In 54.36 μM 2,4-D, C. arabica and C. canephora showed the same mean number of responsive explants (Fig. 1a).
Friable callus induction (mean values and standard deviation) from C. arabica and C. canephora leaf explants in different 2,4-D concentrations over 90 days. C. arabica and C. canephora exhibited distinct values of responsive explants in 9.06–54.36 μM 2,4-D over 90 days (a). 2,4-D concentrations influenced the callus inductions over time in C. arabica (b) and in C. canephora (c). *Mean values followed by the same letter do not present a significant difference.
Considering the 2,4-D concentration, 18.08 µM after 30 days provided the highest mean number of C. arabica responsive explants than 9.06 and 54.36 μM. The concentration of 36.24 μM 2,4-D resulted the same mean number of responsive explants than the other concentrations. After 30 days, the mean number of C. arabica responsive explants was not influenced by 2,4-D (Fig. 1b). For C. canephora, 54.36 μM 2,4-D had the highest mean number of responsive explants at all times. 18.08 and 36.24 μM 2,4-D were statistically equal at 30 days, as well as these concentrations were superior to the 9.06 μM 2,4-D. After 30 days, 36.24 μM 2,4-D exhibited more responsive explants than 9.06 μM 2,4-D. In the same period, 36.24 μM 2,4-D was equal to 18.08 μM 2,4-D, which showed the same mean number than 9.06 μM (Fig. 1c).
The mean number of responsive explants of C. arabica increased over time in 9.06, 36.24 and 54.36 μM 2,4-D (SI Fig. 1a). In C. canephora, the mean number of responsive explants increased over time for all 2,4-D concentrations (SI Fig. 1b). All callus showed a pale yellow and friable appearance (SI Fig. 1). Briefly, induction media with different 2,4-D concentrations in C. arabica influenced the callus induction only up to 30 days. In C. canephora, the best medium to induce responsive explants was with 54.36 μM 2,4-D.
Somatic embryo regeneration
C. arabica and C. canephora exhibited different mean numbers of normal mature cotyledon somatic embryo (MCSE). The origin of the friable callus (9.06, 18.08, 36.24 or 54.36 μM 2,4-D) and time (30, 60, 90, 120, 150, 180, 210 and 240 days) influenced the MCSE regeneration (SI Table 2). The first MCSE were observed at 90 days for C. arabica friable callus induced in all 2,4-D concentrations, at 90 days for C. canephora friable callus in 54.36 μM 2,4-D, and at 150 days for 9.06, 18.08 and 36.24 μM 2,4-D. C. arabica friable callus originated in 9.06 and 54.36 μM 2,4-D regenerated more MCSE than C. canephora friable callus at all evaluated times. C. arabica friable callus originated in 18.08 and 36.24 μM 2,4-D regenerated more MCSE than C. canephora at 150 days, and less after 180 days (Fig. 2).
The origin of the friable callus (9.06–54.36 μM 2,4-D) influenced the MCSE regeneration in C. arabica and C. canephora over 240 days. C. arabica and C. canephora exhibited distinct MCSE values (mean values and standard deviation), considering 2,4-D concentrations and time. *Mean values followed by the same letter do not present a significant difference.
C. arabica friable callus origin influenced the MCSE regeneration, since after 90 days the friable callus from 54.36 μM 2,4-D resulted in the higher mean MCSE number, followed by 9.06 μM 2,4-D and, later, 18.08 and 36.24 μM 2,4-D (Fig. 3a). In C. canephora, the origin of friable callus at different concentrations of 2,4-D influenced the regeneration of MCSE after 90 days. The greatest regeneration of MCSE was observed in friable callus induced in 54.36 μM 2,4-D. Differently, the MCSE regeneration was identical at 120 and 150 days for friable callus originating at 9.06, 18.08 and 36.24 μM 2,4-D. After 150 days, the greatest MCSE regeneration was observed in friable callus from 18.08 μM, followed by 36.24 μM and finally 9.06 μM 2,4-D (Fig. 3b). Therefore, MCSE regeneration increased over time, and the best friable calli were induced in 54.36 μM 2,4-D for both species (SI Fig. 2).
MCSE regeneration from C. arabica and C. canephora friable callus (mean values and standard deviation). The 2,4-D concentrations, which gave rise to friable calli, influenced MCSE regeneration during the ISE in C. arabica (a) and in C. canephora (b). *Mean values followed by the same letter do not present a significant difference.
C. arabica and C. canephora exhibited different mean numbers of ASE. The origin of the friable callus (9.06, 18.08, 36.24 or 54.36 μM 2,4-D) and time (30, 60, 90, 120, 150, 180, 210 and 240 days) influenced the ASE regeneration (SI Table 3). The first ASE were observed at 90 days for C. arabica friable callus induced in all 2,4-D concentrations and for friable callus of C. canephora at 54.36 μM 2,4-D, and at 180 days for 9.06, 18.08 and 36.24 μM 2,4-D. C. arabica friable callus from 9.06 to 54.36 μM 2,4-D regenerated more ASE than C. canephora friable callus at all times evaluated. Friable callus of C. arabica from 18.08 μM 2,4-D regenerated more ASE than of C. canephora during 180 days. After 180 days, C. canephora friable callus provided more ASE. The C. arabica friable callus from 36.24 μM 2,4-D regenerated more ASE than C. canephora friable callus during 180 days. At 210 days, ASE regeneration was equal and, at 240 days, ASE regeneration was higher in the friable callus of C. canephora (Fig. 4).
The origin of the friable callus (9.06–54.36 μM 2,4-D) influenced the ASE regeneration of C. arabica and C. canephora over 240 days. C. arabica and C. canephora exhibited distinct ASE values (mean values and standard deviation), considering 2,4-D concentrations and time. *Mean values followed by the same letter do not present a significant difference.
In C. arabica, the friable callus of 54.36 μM 2,4-D regenerated more ASE at all times evaluated. At 90 and 120 days, the friable callus of 18.08 μM 2,4-D regenerated more ASE than the 9.06 and 36.24 μM 2,4-D. ASE regeneration increased in the friable callus of 9.06 μM 2,4-D, surpassing the friable callus of 36.24 μM 2,4-D at 120 days. At 150 days, ASE regeneration increased in friable callus of 9.06 μM 2,4-D, equaling with friable callus of 18.08 μM 2,4-D. After 150 days, ASE regeneration continued to increase in friable callus of 9.06 μM 2,4-D, overcoming the friable callus of 18.08 μM 2,4-D. Also, the friable callus of 18.08 μM 2,4-D resulted more ASE than friable callus of 36.24 μM 2,4-D in all time evaluated (Fig. 5a). In C. canephora, ASE regeneration was equal at 90 days for all friable callus originated in the induction medium with 9.06, 18.08, 36.24 and 54.36 μM 2,4-D. After 90 days, ASE regeneration increased in friable callus of 54.36 μM 2,4-D, overcoming the others and differing over time. ASE regeneration in friable callus of 9.06, 18.08 and 36.24 μM 2,4-D was equal to 120 and 150 days. After 150 days, the friable callus of 36.24 μM 2,4-D regenerated more ASE than the friable callus of 18.08 μM 2,4-D. In the same time, the friable callus of 18.08 μM 2,4-D regenerated more ASE than friable callus of 9.06 µM 2,4-D (Fig. 5b). As well as observed for MCSE, the ASE regeneration increases over time, and more ASE was observed in friable callus from 54.36 μM 2,4-D for both species (SI Fig. 3).
ASE regeneration from C. arabica and C. canephora friable callus (mean values and standard deviation). The 2,4-D concentrations that gave rise to friable calli influenced ASE regeneration during ISE in C. arabica (a) and in C. canephora (b). *Mean values followed by the same letter do not present a significant difference.
Global 5-mC%
Although statistically equal between the Coffea explant donors, the 5-mC% varied between C. arabica and C. canephora during the ISE (friable callus, embryogenic callus, ASE and MCSE) (SI Table 4). C. canephora and C. arabica explant donors (control) exhibited mean values of 20.60 and 16.37 5-mC% and did not differ statistically (Fig. 6a). Friable callus of C. arabica and C. canephora induced at different concentrations of 2,4-D exhibited distinct 5-mC%. In the induction media with 9.06 and 36.24 μM 2,4-D, friable callus of C. arabica and C. canephora did not differ statistically in relation to 5-mC%. However, in the induction media with 18.08 and 54.36 μM 2,4-D, the friable callus of C. arabica and C. canephora exhibited distinct values of 5-mC% (Fig. 6b). Friable callus of C. arabica showed a mean value of 21.76 5-mC% in induction medium with 18.08 μM 2,4-D and differed from friable callus of C. canephora, which showed a mean value of 12.84 5-mC%. In the induction medium with 54.36 μM 2,4-D, the opposite was observed. The friable callus of C. canephora exhibited a mean value of 24.20 5-mC%, differing from the friable callus of C. arabica, which showed a mean value of 11.50 5-mC%. 2,4-D concentrations influenced the 5-mC% in the friable calli of each species. In C. arabica, friable callus induced in culture medium with 18.08 μM 2,4-D exhibited a mean value of 21.76 5-mC% and differed from friable callus induced with 54.36 μM 2,4-D, which exhibited a mean value of 11.48 5-mC%. In C. canephora, the friable callus induced in a culture medium with 54.36 μM 2,4-D showed the highest 5-mC%, with a mean of 24.20, differing from the friable callus induced with 18.08 and 36.24 μM 2.4-D, with a mean of 12.80 and 14.60, respectively (Fig. 6c).
5-mC% during C. arabica and C. canephora ISE (mean values and standard deviation). Global 5-mC% in C. arabica and C. canephora leaf explant (control) donors (a). Comparison of the 5-mC% between the C. arabica and C. canephora friable callus originated in the induction medium with 9.06–54.36 μM 2,4-D (b). Comparison of the 5-mC% of the friable callus originated from 9.06 to 54.36 μM 2,4-D for C. arabica and C. canephora (c). 5-mC% in embryogenic callus of C. arabica and C. canephora (d). 5-mC% in MCSE (control) and ASE of C. arabica regenerated from friable callus originated in 9.06–54.36 μM of 2,4-D (e). *Mean values followed by the same letter do not present a significant difference.
Global 5-mC% of C. arabica and C. canephora embryogenic calli were not influenced by 2,4-D concentrations. Furthermore, embryogenic callus of C. canephora had a mean value of 43.97 5-mC%, differing from C. arabica that exhibited a mean value of 26.78% (Fig. 6d). Regarding the 5-mC% of MCSE and ASE in C. arabica, the origin of friable callus influenced the response. The 5-mC% increases in the ASE according to the concentrations of 2,4-D that gave rise to friable callus. MCSE and ASE that were regenerated from friable callus of 9.06 μM 2,4-D exhibited a lower 5-mC%, with a mean of 8.91 and 11.33, and differed from ASE that were regenerated from friable callus of 36.24 and 54.36 μM 2,4-D, with a mean of 18.44 and 24.45 5-mC%, respectively. In addition, ASE regenerated from friable callus of 18.08 μM 2,4-D exhibited a mean value of 15.70 5-mC%, differing from ASE that were regenerated from friable callus of 54.36 μM 2,4-D (Fig. 6e). It was not possible to determine the level of 5-mC% in ASE of C. canephora that were regenerated from friable callus of 9.06, 18.08 and 36.24 μM 2,4-D, since the biological material was not sufficient (data are not shown).
During ISE in C. arabica and C. canephora, in general, the level of 5-mC% varied in the different stages analyzed (friable callus, embryogenic callus, ASE and MCSE). In C. arabica, the explant donor (control) presented a mean value of 20.60 5-mC%. During the callus induction and proliferation step, it was observed that the 5-mC% was statistically equal, since friable callus exhibited a mean value of 16.64. In the SE induction step, a 5-mC% increase was observed as the embryogenic callus exhibited a mean value of 26.77. The regenerated ASE and MCSE (control in relation to ASE) also exhibited distinct values of 5-mC%. ASE showed a higher 5-mC%, with a mean of 17.36. Furthermore, ASE were identified due to their apical-basal pattern abnormalities, such as deformation and/or absence of the root apical meristem, hypocotyl, shoot apical meristem and/or cotyledons (Fig. 7a). In C. canephora, the explant donor (control) exhibited a mean value of 16.40 5-mC%. Global 5-mC% remained constant in the callus induction and proliferation stage, since the friable calluses had a mean value of 18.10. During the SE induction step there was an increase in the 5-mC%, since the embryogenic callus exhibited a mean value of 44.00. ASE exhibited higher 5-mC%, with a mean of 22.00, differing from MCSE (control in relation to ASE), which exhibited a mean value of 11.00. C. canephora ASE were also identified due to their apical-basal pattern abnormalities (Fig. 7b).
Comparison of the 5-mC% (mean values and standard deviation) in C. arabica (a) and in C. canephora (b) during ISE: explant donor (control), friable callus, embryogenic callus, ASE and MCSE (control in relation to the ASE). *Mean values followed by the same letter do not present a significant difference.
Comet assay
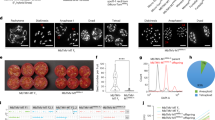
2,4-D had a genotoxic effect on ASE in C. arabica and C. canephora, due to the higher occurrence of DNA damage compared to MCSE (control) (Fig. 8, SI Table 5). C. canephora ASE showed more DNA damage than C. arabica ASE at all 2,4-D concentrations from which friable calluses originated. C. canephora was more sensitive to the 2,4-D than C. arabica, since C. arabica ASE regenerated from friable callus induced at 9.06 µM had less DNA damage (Fig. 8a). Furthermore, regenerated ASE of C. canephora showed the same mean DNA damage rate for friable callus induced in all 2,4-D concentrations (9.06 µM–54.36 µM). In C. arabica, ASE from the 9.06 μM 2,4-D exhibited less DNA damage compared to 18.08, 36.24 and 54.36 μM (Fig. 8b).
Percentage of DNA damage (mean values and standard deviation) in MCSE (control) and ASE of C. arabica and C. canephora, which are regenerated from friable callus originated from 9.06 to 54.36 μM 2,4-D. Comparison of the percentage of DNA damage between MCSE and ASE of C. arabica and C. canephora considering each 2,4-D concentration (a). Comparison of percentage of DNA damage in MCSE and ASE recovered from each 2,4-D concentration for C. arabica and C. canephora (b). Without DNA damage (c). With DNA damage (d,e). *Mean values followed by the same letter do not present a significant difference. Bar = 5 μm.
Discussion
C. arabica and C. canephora ISE was established based on a tissue culture procedure proposed by Sanglard et al.16, involving the stages of callus induction and proliferation (callogenesis), followed by SE regeneration (embryogenesis). Basically, we modified the 2,4-D concentration, adding 18.08, 36.24 and 54.36 μM in the callus induction and proliferation medium. We verified the immediate 2,4-D effect in the induction and proliferation of the C. arabica and C. canephora friable callus, as well as its remaining genotoxic effect during the SE regeneration stage in the embryogenic callus from the mean number of MCSE and ASE. In addition, we measured, compared, and associated global 5-mC% with 2,4-D concentrations during the in vitro response. Our results showed that 2,4-D has a genotoxic and phytotoxic effects on SE regeneration in both species, however C. canephora is more sensitive to the action of 2,4-D, since ASE showed more DNA damage. Despite this, C. canephora requires relatively higher 2,4-D concentration than C. arabica to ISE establishment.
2,4-D is a synthetic auxin widely used in plant tissue culture to induce ISE14,16. This compound triggers the cellular transition processes from the differentiated cell to embryogenic cell. This process is complex and includes dedifferentiation and cell division, which are modulates to metabolic and developmental reprogramming, conferring competence to the cells of the explants1. The 2,4-D concentration and time influenced the rate and cell proliferation of friable callus of the two Coffea, as well as the global 5-mC%. 2,4-D induces ISE in C. arabica and C. canephora and genetic, epigenetic factors and endogenous auxin levels in the cell can influence establishment in vitro. According to Vondráková et al.12, endogenous auxin level in the cell and its homeostasis affect the exogenous auxin effectiveness in ISE. Homeostasis is controlled by several mechanisms, such as auxin biosynthesis, as well as its degradation, transport and conjugation25.
For C. canephora, exogenous auxin increases both free indole-3-acetic acid (IAA) and IAA amide conjugates during the callus induction, due to novel synthesis26. IAA is synthesized by the enzymes tryptophan aminotransferase of Arabidopsis (TAA) and YUCCA (YUC) from one of the tryptophan-dependent pathways. Uc-Chuc et al.27 identified 10 members of the CcYUC gene family in C. canephora. Quintana-Escobar et al.28 showed differences in the concentration of auxin homeostasis proteins in C. arabica, with a more significant accumulation of ABCs, GH3.17, UGT75C1 and IBR1 proteins. Karyotypic/genetic differences influence several traits of an individual, including the biosynthesis of auxins. C. arabica is an allotetraploid with 2C = 2.62 pg and 2n = 4x = 44 chromosomes. C. canephora is diploid with 2C = 1.41 pg and 2n = 2x = 22 chromosomes. We expected that C. arabica has more family members or copies of genes that encode proteins involved in auxin metabolism and, as a result, require fewer exogenous auxins. However, further investigations should be conducted to understand auxin biosynthesis in polyploids, as well as its spatial and temporal pattern of distribution during ISE.
The use of synthetic auxins, mainly 2,4-D, is necessary for the ISE induction and SE multiplication in Coffea, consequently potentiating the regeneration of plantlets in vitro9,10,16. 2,4-D can cause epigenetic and genetic changes in cells during ISE, even at very low concentrations1,29,30. In the present study, 2,4-D and, consequently, the global variations of 5-mC% influenced the in vitro response (acquisition of competence) in C. arabica and C. canephora. In addition, ASE regeneration was observed for both species. Corroborating with Oliveira et al.9,10, variations in 5-mC% in ISE in Coffea were associated with species and in vitro environment. The formation of ASE was related to the prolonged effect of 2,4-D, which influences the expression, development and maturation of the SE and its subsequent conversion into a plant. The long exposure or accumulation of 2,4-D in tissue interferes in establishing internal auxin gradients and inhibits cell polarization31. As a consequence, 2,4-D disrupts normal endogenous auxin balance and polar auxin transport, inducing embryonic abnormalities32,33.
In addition to 2,4-D, embryonic abnormalities may be associated with other chemical compounds required in plant tissue culture. These compounds may also cause mutations or epigenetic changes (somaclonal variation) and, consequently, influence embryonic development, SE morphology and, consequently, prevent plant recovering17. The formation of ASE has already been reported in Coffea10,34 and in different species such as Medicago truncatula35, Theobroma cacao36 and Melia azedarach37, being recurrent and common in plant tissue culture. For example, the ASE of the autoallohexaploid “Híbrido de Timor” (Coffea) exhibited the same ploidy level and chromosome number as the explant donor plants, and the high concentrations of activated charcoal in the regeneration medium has been appointed as one of the causes of these abnormalities due to phytotoxic effect10. The pattern of development of a normal SE in eudicotiledonea is characterized by the differentiation of a bipolar structure consisting of stem and root apex, passing through the stages of pre-embryonic and embryonic development, globular, cordiform, torpedo and cotyledonary38. However, ASE from C. arabica and C. canephora passed through these developmental stages, showing higher global 5-mC% and DNA damage due to the genotoxic action of 2,4-D.
DNA damage can be induced by exogenous chemical and physical factors by the action of genotoxic agents. Maintenance of the DNA integrity is necessary for the proper development of the organism and for the faithful transmission of the genetic information from one generation to the next39. Plants have specific mechanisms that repair DNA damage of the nuclear and organellar genomes. Our data show that C. canephora is more sensitive to 2,4-D, as ASE exhibited more DNA damage and higher values of 5-mC%. C. arabica is more tolerant to 2,4-D, probably because it is a polyploid. In addition, C. canephora ASE, which are regenerated from friable calli induced in all 2,4-D concentrations, showed the same mean DNA damage rate. Therefore, Coffea species react differently to the in vitro environment, especially to 2,4-D. As reviewed by Schifino-Wittmann40 and Sattler et al.41, polyploids have a greater buffering effect in relation to adaptability, as they have more genomic copies than diploids, and can accumulate more hidden variability. Based on this and our results, we hypothesized that the euploidy and its outcomes, as well as the evolution of the species, influence the in vitro response. However, this hypothesis should be verified in further studies. We show, for the first time, that 2,4-D promotes DNA damage during ISE and its short-term consequences in vitro, such as the formation of ASE that present morphological and/or physiological disturbances, preventing plantlet regeneration.
The addition of 2,4-D to the culture medium stimulates the induction and proliferation of friable callus in C. arabica and C. canephora and consequently increases the regeneration of SE. However, 2,4-D promotes morphological and epigenetic changes and still causes DNA damage, compromising the development of SE. Therefore, the establishment of somatic embryogenesis requires complex cellular, biochemical and molecular processes. Epigenetics plays a key role in somatic embryogenesis, as it is a genetic regulatory mechanism that influences morphogenetic processes in vitro. Maintaining the integrity of the genome of plantlets regenerated in vitro is desirable, as DNA damage can lead to loss of genetic fidelity, alter methylation patterns and cause oxidative stress, functional disturbances and cell death.
Material and methods
Coffea plant materials were collected from in vitro plantlets in accordance to relevant guidelines and legislation. We declare that all methods were performed in accordance with the relevant guidelines.
Biological material
Three plantlets of C. arabica and of C. canephora kept in vitro (Universidade Federal do Espírito Santo, Espírito Santo, Brazil) were used as explant donors. These plantlets have been cultivated in 4.3 g L−1 basal Murashige and Skoog (MS) salts, 10 mL L−1 B5 vitamins, 30 g L−1 sucrose, 2.8 g L−1 Phytagel and pH = 5.6, at 25 ± 2 °C under a 16/8 h light/dark regime with 36 μmol m−2 s−1 light radiation provided by two fluorescent lamps (20 W, Osram)16. These plantlets were used as control for global 5-mC%. Leaves were excised to establish the ISE and quantify 5-mC%.
Callogenesis
Five leaf fragments (2 cm2) from C. arabica and from C. canephora explant donor plantlets were randomly inoculated in Petri dishes (60 × 15 mm, Olen) containing 15 mL friable callus induction medium constituted with 2.15 g L−1 1/2 MS basal, 10 mL L−1 B5 vitamins, 30 g L−1 sucrose, 0.08 g L−1 L-cysteine, 0.4 g L−1 malt extract, 0.1 g L−1 hydrolyzed casein, 4.44 μM 6-benzylaminopurine (BAP), 2.8 g L−1 Phytagel, pH = 5.6, and supplemented with 9.06, 18.08, 36.24 or 54.36 μM 2,4-D. The 2,4-D concentrations were defined according to the studies about Coffea ISE2,4,5,6,8,9,10,15,16,18,19. Ten Petri dishes for each 2,4-D concentration were prepared for C. arabica and for C. canephora. The culture media were sterilized at 121 °C and 1.5 atm for 20 min. Petri dishes were maintained in the dark at 25 ± 2 °C for 90 days. Friable callus formation was evaluated biweekly, and after 90 days some samples of friable calli were collected to extract genomic DNA and determine the 5-mC%.
Indirect somatic embryogenesis
Friable callus of C. arabica and C. canephora were individually inoculated in Petri dishes (60 × 15 mm, Olen) dishes containing 15 mL of SE regeneration medium identical to Sanglard et al.16, differing with 4 g L−1 activated charcoal, totaling 60 repetitions for C. arabica and 44 for C. canephora. Petri dishes were kept in the dark at 25 ± 2 °C for 240 days. The regeneration of MCSE and ASE was monthly evaluated. Samples of embryogenic callus, MCSE and ASE were collected to extract genomic DNA and determine the 5-mC%. In addition, the MCSE and ASE samples were used for the comet assay.
Global 5-mC%
The collected samples (leaf from the explant donors, friable callus, embryogenic callus, MCSE and ASE) were macerated separately in the MagNALyser (Roche, Germany) for 60 s at 7000 rpm. Genomic DNA was extracted according to Doyle and Doyle42, with modifications adding 7.5 M ammonium acetate and excluding night precipitation. 30 μg of genomic DNA was diluted in 100 μL of dH2O and then acid hydrolysis was performed9,10. The pH of the hydrolysates was adjusted between 3 and 5 with KOH 1.0 mol L−1, and centrifuged at 10,000 rpm for 5 min. The supernatant was collected and transferred to a new microtube, placed in SpeedVac (Eppendorf) and subsequently stored in a refrigerator. To determine the 5-mC%, the lyophilized samples were suspended in 100 μL dH2O and analyzed in high performance liquid chromatography (HPLC Shimadzu, model LC-20AT) equipped with a photodiode matrix detector (SPD – M20A) using a column of reverse phase C18 based on silica (4.6 × 250 mm, 5 μm)9,10.
From 9.06, 18.08, 36.24 and 54.36 μM 2,4-D, the 5-mC% was measured for: (a) 20 samples of friable callus of C. arabica and C. canephora, totaling 5 repetitions for each treatment; (b) 12 samples of embryogenic callus of C. arabica and C. canephora, totaling 3 replicates for each treatment; (c) 12 ASE samples from C. arabica, totaling 3 replicates for each treatment; (d) 3 samples of C. canephora ASE from 54.36 μM 2,4-D because in other concentrations there was not enough biological material; (e) 3 MCSE samples from C. arabica and C. canephora; and (f) 3 samples of explant donors from C. arabica and C. canephora.
Comet assay
Slides were immersed in normal 1% agarose at 50 °C, dried and stored in 4 °C for 24 h. The MCSE (control) and the ASE of C. arabica and C. canephora were individually collected and chopped with a razor blade in 300 μL of cell suspension solution (400 mM Tris–HCl pH 7.5, 20% polyethylene glycol). The resulting suspension was filtered through a 50, 30 and 20 µm nylon membrane. 40 µL of this cell suspension was soaked in 60 µL of low melting point agarose at a concentration of 1% at 37 °C and placed on a microscope slide pre-coated with agarose. Then, the slides were covered with a microscope coverslip (24 × 40 mm) previously cleaned and placed in a refrigeration system at 4 °C for 20 min. The coverslips were removed and incubated in a lysis solution (2.5 M NaCl; 100 mM EDTA; 10 mM Trizma base, pH = 10; 1% Triton X-100 and 10% dimethyl sulfoxide) for 1 h at 4 °C. The slides were transferred to a horizontal electrophoresis chamber containing alkaline buffer (250 mM Trizma base, 10 mM NaOH and 1 mM EDTA, pH > 13) at 4 °C, where they were incubated for 20 min for DNA unfolding and then subjected to electrophoresis at 18 V for 20 min. After electrophoresis, the slides were incubated in neutralization buffer (400 mM Tris–HCl, pH 7.5) for 15 min and then stained with 100 μL of 50 μM acridine orange for 15 min, and immersed three times in dH2O at 4 °C. The slides were analyzed under a 20 × objective accoupled in a fluorescence microscope Olympus BX-60 (Olympus, Japan) and nuclei images were captured with a Photometrics CoolSNAP Pro cf (Roper Scientific, Tucson, AZ). 300 nuclei per slide were evaluated, being four slides for the MCSE of C. arabica and C. canephora and 4 repetitions for the ASE of C. arabica and C. canephora regenerated from friable callus in 9.06, 18.08, 36.24 and 54.36 μM 2,4-D. C. arabica and C. canephora MCSE exhibited nuclei with DNA damage, but these observed damages, below 10%, are within the acceptable range for processing the core isolation.
Statistical analysis
The ISE responses of C. arabica and C. canephora were compared during the callus formation and SE regeneration stages. The number of responsive explants was evaluated every 2 weeks for 90 days. The number of MCSE and ASE were evaluated monthly for 240 days. For both SE, 9.06, 36.24, 54.36 and 54.36 μM 2,4-D were considered. The data followed a normal pattern and were subjected to analysis of variance (ANOVA). The means were compared using the Tukey’s test (P < 0.05). A quadratic regression analysis was applied. To assess the 5-mC% and the genotoxic effect of 2,4-D during ISE, the normality of the data was verified. Then, an ANOVA was performed, taking into account the 9.06, 18.08, 36.24 and 54.36 μM 2,4-D. Mean values were compared by Tukey’s test (P < 0.05). For the 5-mC% and DNA damage statistical analyses, our controls were the C. canephora and C. arabica explant donors (5-mC%), and MCSE (5-mC% and DNA damage).
Abbreviations
- ISE:
-
Indirect somatic embryogenesis
- SE:
-
Somatic embryos
- 2,4-D:
-
2,4-Dichlorophenoxyacetic
- ASE:
-
Abnormal somatic embryos
- 5-mC%:
-
Global 5-methylcytosine levels
- SRAP:
-
Sequence-related amplified polymorphism
- MCSE:
-
Mature cotyledon somatic embryo
- MS:
-
Murashige and Skoog
- BAP:
-
6-Benzylaminopurine
References
Fehér, A. Somatic embryogenesis stress-induced remodeling of plant cell fate. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 1849(4), 385–402. https://doi.org/10.1016/j.bbagrm.2014.07.005 (2015).
Staritsky, G. Embryoid formation in callus tissues of coffee. Plant Biol. 19, 509–514. https://doi.org/10.1111/j.1438-8677.1970.tb00677.x (1970).
Queiroz-Figueroa, F. R., Rojas-Herrera, R., Galaz-Avalos, R. M. & Loyola-Vargas, V. M. Embryo production through somatic embryogenesis can be used to study cell differentiation in plants. Plant Cell Tissue Organ Cult. 86(3), 285–301. https://doi.org/10.1007/s11240-006-9139-6 (2006).
Campos, N. A., Panis, B. & Carpentier, S. C. Somatic embryogenesis in coffee: The evolution of biotechnology and the integration of omics technologies offer great opportunities. Front. Plant Sci. 8, 1460. https://doi.org/10.3389/fpls.2017.01460 (2017).
Arroyo-Herrera, A. et al. Expression of WUSCHEL in Coffea canephora causes ectopic morphogenesis and increases somatic embryogenesis. Plant Cell Tissue Organ Cult. 94, 171–180. https://doi.org/10.1007/s11240-008-9401-1 (2008).
Nic-Can, G. I. et al. New insights into somatic embryogenesis: Leafy cotyledon1, baby boom1 and wuschel-related homeobox4 are epigenetically regulated in Coffea canephora. Plos One 8, e72160. https://doi.org/10.1371/journal.pone.0072160 (2013).
Torres, L. F., Diniz, L. E. C., Do Livramento, K. G., Freire, L. L. & Paiva, L. V. Gene expression and morphological characterization of cell suspensions of Coffea arabica L. cv. Catiguá MG2 in different cultivation stages. Acta Physiol. Plant. 37(9), 1–7. https://doi.org/10.1007/s11738-015-1924-6 (2015).
Amaral-Silva, P. M. et al. Global 5-methylcytosine and physiological changes are triggers of indirect somatic embryogenesis in Coffea canephora. Protoplasma https://doi.org/10.1007/s00709-020-01551-8 (2020).
Oliveira, J. P. M., Sanglard, N. A., Ferreira, A. & Clarindo, W. R. Genomic methylated cytosine level during the dedifferentiation and cellular competence in Coffea arabica lines: Insights about the different in vitro responses. Forests 12(11), 1536. https://doi.org/10.3390/f12111536 (2021).
Oliveira, J. P. M., Sanglard, N. A., Ferreira, A. & Clarindo, W. R. Ploidy level, epigenetic and in vitro environment influence the indirect somatic embryogenesis of the new synthetic autoallohexaploid Coffea. Plant Cell Tissue Organ Cult. 146, 553–563. https://doi.org/10.1007/s11240-021-02093-4 (2021).
Zanin, F. C. et al. The SAUR gene family in coffee: Genome-wide identification and gene expression analysis during somatic embryogenesis. Mol. Biol. Rep. 49, 1973–1984. https://doi.org/10.1007/s11033-021-07011-7 (2022).
Vondráková, Z., Eliášová, K., Fischerová, L. & Vágner, M. The role of auxins in somatic embryogenesis of Abies alba. Open Life Sci. 6, 587–596. https://doi.org/10.2478/s11535-011-0035-7 (2011).
Teale, W. D., Paponov, I. A. & Palme, K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 7, 847–859. https://doi.org/10.1038/nrm2020 (2006).
Perrot-Rechenmann, C. Cellular responses to auxin: Division versus expansion. Cold Spring Harb. Perspect. Biol. https://doi.org/10.1101/cshperspect.a001446 (2010).
van Boxtel, J. & Berthouly, M. High frequency somatic embryogenesis from coffee leaves. Plant Cell Tissue Organ Cult. 44, 7–17. https://doi.org/10.1007/BF00045907 (1996).
Sanglard, N. A. et al. Indirect somatic embryogenesis in Coffea with different ploidy levels: A revisiting and updating study. Plant Cell Tissue Organ Cult. 136, 255–267. https://doi.org/10.1007/s11240-018-1511-9 (2019).
Garcia, C. et al. Abnormalities in somatic embryogenesis caused by 2,4-D: An overview. Plant Cell Tissue Organ Cult. 137, 193–212. https://doi.org/10.1007/s11240-019-01569-8 (2019).
Landey, R. B., Cenci, A. & Guyot, R. Assessment of genetic and epigenetic changes during cell culture ageing and relations with somaclonal variation in Coffea arabica. Plant Cell Tissure Organ Cult. 122, 517–531. https://doi.org/10.1007/s11240-015-0772-9 (2015).
Etienne, H. et al. Are genetic and epigenetic instabilities of plant embryogenic cells a fatality? The experience of coffee somatic embryogenesis. Hum. Genet. Embryol. https://doi.org/10.4172/2161-0436.1000136 (2016).
Bychappa, M., Mishra, M. K., Jingade, P. & Huded, A. K. C. Genomic alterations in coding region of tissue culture plants of Coffea arabica obtained through somatic embryogenesis revealed by molecular markers. Plant Cell Tissue Organ Cult. 139, 91–103. https://doi.org/10.1007/s11240-019-01666-8 (2019).
Salaün, C., Lepiniec, L. & Dubreucq, B. Genetic and molecular control of somatic embryogenesis. Plants 10(7), 1467. https://doi.org/10.3390/plants10071467 (2021).
Miguel, C. & Marum, L. An epigenetic view of plant cells cultured in vitro: Somaclonal variation and beyond. J. Exp. Bot. 62(11), 3713–3725. https://doi.org/10.1093/jxb/err155 (2011).
Tice, R. R. et al. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 35(3), 206–221 (2000).
Özkul, M., Özel, Ç. A., Yüzbaşıoğlu, D. & Ünal, F. Does 2,4-dichlorophenoxyacetic acid (2,4-D) induce genotoxic effects in tissue cultured Allium roots?. Cytotechnology 68(6), 2395–2405. https://doi.org/10.1007/s10616-016-9956-3 (2016).
Ludwig-Müller, J. et al. Two auxinic herbicides affect Brassica napus plant hormone levels and induce molecular changes in transcription. Biomolecules 11(8), 1153. https://doi.org/10.3390/biom11081153 (2021).
Ayil-Gutiérrez, B., Galaz-Ávalos, R. M., Peña-Cabrera, E. & Loyola-Vargas, V. M. Dynamics of the concentration of IAA and some of its conjugates during the induction of somatic embryogenesis in Coffea canephora. Plant Signal. Behav. 8(11), e26998. https://doi.org/10.4161/psb.26998 (2013).
Uc-Chuc, M. A., Kú-González, Á. F., Jiménez-Ramírez, I. A. & Loyola-Vargas, V. M. Identification, analysis, and modeling of the YUCCA protein family genome-wide in Coffea canephora. Proteins Struct. Funct. Bioinform. 90(4), 1005–1024. https://doi.org/10.1002/prot.26293 (2022).
Quintana-Escobar, A. O. et al. Differences in the abundance of auxin homeostasis proteins suggest their central roles for in vitro tissue differentiation in Coffea arabica. Plants 10(12), 2607. https://doi.org/10.3390/plants10122607 (2021).
LoSchiavo, F. et al. DNA methylation of embryogenic carrot cell cultures and its variations as caused by mutation, differentiation, hormones and hypomethylating drugs. Theor. Appl. Genet. 77, 325–331. https://doi.org/10.1007/BF00305823 (1989).
Fraga, H. P. F. et al. 5-Azacytidine combined with 2,4-D improves somatic embryogenesis of Acca sellowiana (O. Berg) Burret by means of changes in global DNA methylation levels. Plant Cell Rep. 31, 2165–2176. https://doi.org/10.1007/s00299-012-1327-8 (2012).
Nissen, P. & Minocha, S. C. Inhibition by 2,4-D of somatic embryogenesis in carrot as explored by its reversal by difluoromethylornithine. Physiol. Plant. 89(4), 673–680. https://doi.org/10.1111/j.1399-3054.1993.tb05272.x (1993).
Venkatesh, K., Rani, R. A., Baburao, N. & Padmaja, G. Effect of auxins and auxin polar transport inhibitor (TIBA) on somatic embryogenesis in groundnut (Arachis hypogaea L.). Afr. J. Plant Sci. 3, 277–282 (2009).
Verma, S. K., Das, A. K., Gantait, S., Gurel, S. & Gurel, E. Influence of auxin and its polar transport inhibitor on the development of somatic embryos in Digitalis trojana. 3 Biotech 8, 1–8. https://doi.org/10.1007/s13205-018-1119-0 (2018).
Sattler, M. C., Carvalho, C. R. & Clarindo, W. R. Regeneration of allotriploid Coffea plants from tissue culture: Resolving the propagation problems promoted by irregular meiosis. Cytologia 81, 125–132. https://doi.org/10.1508/cytologia.81.125 (2016).
Tvorogova, V. E., Lebedeva, M. A. & Lutova, L. A. Expression of WOX and PIN genes during somatic and zygotic embryogenesis in Medicago truncatula. Russ. J. Genet. 51, 1189–1198. https://doi.org/10.1134/S1022795415120121 (2015).
Chanatásig, CI. Inducción de la embriogénesis somática en clones superiores de cacao (Theobroma cacao L.), con resistencia a enfermedades fungosas. CATIE 1–86. http://orton.catie.ac.cr/repdoc/A0275e/A0275e.pdf (2004).
Vila, S., Gonzalez, A., Rey, H. & Mroginski, L. Effect of morphological heterogeneity of somatic embryos of Melia azedarach on conversion into plants. Biocell 34, 7–13 (2010).
Guerra, M. P., Torres, A. C. & Teixeira, J. B. Embriogênese somática e sementes sintéticas. Culturas de tecidos e transformação genética em plantas 2, 533–568 (1999).
Nisa, M. U., Huang, Y., Benhamed, M. & Raynaud, C. The plant DNA damage response: Signaling pathways leading to growth inhibition and putative role in response to stress conditions. Front. Plant Sci. 10, 653. https://doi.org/10.3389/fpls.2019.00653 (2019).
Schifino-Wittmann, M. T. Poliploidia e seu impacto na origem e evolução das plantas silvestres e cultivadas. Curr. Agric. Sci. Technol. https://doi.org/10.18539/cast.v10i2.904 (2004).
Sattler, M. C., Carvalho, C. R. & Clarindo, W. R. The polyploidy and its key role in plant breeding. Planta 243, 281–296. https://doi.org/10.1007/s00425-015-2450-x (2016).
Doyle, J. J. & Doyle, J. L. Isolation of plant DNA from fresh tissue. Focus 12, 13–15 (1990).
Acknowledgements
We would like to thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brasília – DF, Brazil, grant: 443801/2014-2), Fundação de Amparo à Pesquisa do Espírito Santo (FAPES, Vitória – ES, Brazil, grants: 65942604/2014 and 82/2017), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brasília – DF, Brazil, grants: 001) for financial support.
Funding
The funding was provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, 001, Conselho Nacional de Desenvolvimento Científico e Tecnológico, 316474/2021-5, Fundação de Amparo à Pesquisa e Inovação do Espírito Santo, 82/2017.
Author information
Authors and Affiliations
Contributions
The authors J.P.M.O. and W.R.C. designed this work, conducted all the experiments and wrote the manuscript. A.J.S. assisted in Assay Comet analysis of this work. M.N.C. assisted in the 5-mC% analysis. J.P.M.O. did the statistical analysis. All authors contributed equally to the editing and review of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Morais Oliveira, J.P., Silva, A.J.d., Catrinck, M.N. et al. Embryonic abnormalities and genotoxicity induced by 2,4-dichlorophenoxyacetic acid during indirect somatic embryogenesis in Coffea. Sci Rep 13, 9689 (2023). https://doi.org/10.1038/s41598-023-36879-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-36879-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.