Abstract
Wind energy has significant growth potential and applicability on a global scale, but approximately 2.4% of wind turbine blades must be decommissioned annually. The majority of blade components can be recycled; however, wind blades are rarely recycled. In the present study, an alternative method was presented involving a small molecule-assisted technique based on a dynamic reaction that dissolves waste composite materials containing ester groups to recycle end-of-life wind turbine blades. This effective process requires temperatures below 200 °C, and the major component, i.e., resin, can be easily dissolved. This method can be applied to recycle composite materials, such as wind turbine blades and carbon fibre composites comprising fibres and resins. Depending on the waste, up to 100% of the resin degradation yield can be achieved. The solution used for the recycling process may be reused multiple times and can be reused to obtain resin-based components and create a closed loop for this type of material.
Similar content being viewed by others
Introduction
Wind is a fully renewable energy source with infinite resources and efficient technology for its utilization. Europe, China, and offshore wind turbines set new records in 2020, installing over 93 GW for a total of 742.7 GW1. The EU projects that new builds will raise wind energy capacity to 323 GW by 2030 from 205 GW2. Wind energy supplies 15% of EU electricity, and by 2030, it will supply 30%. Between 2020 and 2030, many 2000-s-era wind turbines will be directed to undergo decommissioning and dismantling3,4. Germany, Spain, and Denmark had 41–57% of Europe's installed wind turbines with exploitation time reaching over 15 years old in 20205,6. In 2021, total power of 4 GW wind turbines (6000 turbines) may be decommissioned due to the 20-year support expiration7. Annually, 2.4% of all wind turbine blades in Europe are replaced8. Large composite materials such as wind blades are rarely recycled9,10,11,12,13, and many dismantles and landfilled blades strain the environment, resulting in a loss of chemical energy and material potential for recycling.
Wind turbine blades possess a complex composition, containing thermoplastic coatings, thermoset/glass and carbon fibre composites14, carbon fibre, balsa wood, and adhesives15. This composition makes the materials separation and further reuse of the separated fractions very difficult16,17,18,19. An additional 1 kW of installed wind power requires 12–15 kg of composites, including blade materials20. The crosslinked thermoset polymers of the outer layer composites cannot be melted or remoulded, making even the early stages of recycling problematic21,22,23,24,25,26. Mechanical27,28,29,30, thermal31, and chemical32,33,34,35,36 thermoset composite recycling methods have been developed by researchers. Pyrolysis and gasification thermal recycling techniques have TRL ratings of 9 and 5/6, respectively37,38. Unfortunately, pyrolysis conditions with temperatures exceeding 500 °C can damage fibres by retaining oxidation residues, char, or chemical structure. It is also not always economical, and its suitability is dependent on the technology used. If the thermal conversion process is to be performed as an auto-thermal process, some or all of the volatiles emitted by the process must be used. As a result, some, if not the majority, of the organic compounds recoverable from such a stream are lost. Furthermore, thermal conversion processes produce complex mixtures that necessitate additional high-temperature processes like distillation, hydrodeoxygenation, or hydrocracking before use. Solvolysis, used in this work, recovers clean, intact fibres and reuses resin, and this could close the fibre-reinforced resin composites loop39. Due to the high temperature (yet lower than pyrolysis or gasification) and high-pressure conditions, which allow significant volumes of solvents to be collected and reintroduced, this technique is inefficient and energy-intensive. This method offers the best cost-to-value ratio of the items despite a TRL of 5/626,40.
According to a design of experiments (DOE) plan, the WTBW, GFC and CFC were solvolyses at 100–190 °C for 1–3 h under 0–60 bar of inert N2 in a Parr 4650 batch reactor with a 500 mL capacity chemically recycled glass, carbon fibre, and epoxy resin oligomers. All tests were carried out by the experimental design matrix of a central composite design (CCD), and the data were analysed via analysis of variance (ANOVA) to determine which factors have a statistically significant effect on the resin removal efficiency. As a catalyst, strongly basic bicyclic guanidine (TBD) was added to the process. Various amounts of TBD (0.015 or 0.025 mol ratio to fixed 1:1 mol ratio of EG: NMP) were tested to analyse their catalytic effect on the recycling process.
The main novelty presented in this paper is the identification of variables statistically significantly affecting the resin removal process from the matrix of both glass and carbon fibre-reinforced composites. Furthermore, using a design of experiments (DOE) approach, the process conditions were determined and optimised. The presented method has the significant advantage of being able to reuse the reaction mixture multiple times. Some composite waste components, particularly those derived from wind turbine waste, contain polystyrene, which can be separated by sedimentation from the reaction mixture containing ethylene glycol and n-methyl pyrrolidone. Furthermore, preliminary tests have shown that condensation in the reaction mixture can be used to separate epoxy resin precursors. In comparison to previous reports41,42,43, the presented method allows carbon and glass fibres to be recovered with greater efficiency and without additional degradation. Furthermore, monomers and dimers, which are components of the composite waste being processed, can be separated.
This is the first report to dissolve industrial epoxy elements of wind turbine blades and commercial composite debris at low temperatures and pressures. This method greatly reduces the amount of composite waste, and thermoset polymer breakdown is also revealed.
Materials and methods
Materials
Commercial carbon fibre composite (Fig. 1d, e) and wind turbine blades (supplied as sheets with dimensions of approximately 25 × 30 cm; Fig. 1a) were used as waste samples in the solvolysis process. The samples were trimmed into thin strips (~ 0.5 cm) to capture the profile of the blade (Fig. 1b). Smaller samples (0.5 × 1 cm) were cut from the strips for solvolysis (Fig. 1c). The GFC and CFC were solvolysis as received.
The following chemicals were used in the solvolysis process and for resin synthesis from the solvolysis product stream: ethylene glycol (EG; molecular weight [MW] = 62.07 g/mol), 1-methyl-2-pyrrolidinone (NMP; MW = 99.13 g/mol), TBD (MW = 139.20 g/mol), epichlorohydrin (EPI; MW = 92.52 g/mol), isopropanol (i-Pr), sodium hydroxide (NaOH), acetic acid, propylene carbonate (PC; MW = 102.09 g/mol), and glycerol triacetate (TAG; MW = 218.21 g/mol). All reagents were purchased from Sigma‒Aldrich (St. Louis, MO, USA) and used as received.
Analytical methods
Test samples were analysed using the Fourier transform infrared spectroscopy (FTIR) by Thermo Nicolet, model IS50. Sample spectra were recorded with the step size was 0.05/cm, and the range was 400–4000/cm. All measurements were carried out at room temperature, and a total of 64 scans were acquired for each sample under consideration. The spectral processing was carried out with the help of OMNIC version 9.
In addition, to better understand the composition of the resin matrix, samples were subjected to analytical pyrolysis by Pyrolyzer EGA/PY-3030D Multi-Shot Pyrolyzer for Py-GC/MS analysis (Frontier Laboratories Ltd, Fukushima Japan). The pyrolysis temperature was set at 500 °C, while the GC oven temperature was gradually raised from 45 to 275 °C at a rate of 5 degrees Celsius per minute. A portion of the sample vapours generated in the furnace was divided (at a ratio of 1/50), and a portion was sent to the column at a flow rate of 1.91 mL/min and a pressure of 27.3 kPa, while the remainder was vented. The vapours were separated using a Shimadzu QP-2010 Ultra Plus (Japan) gas chromatogram with a temperature-programmed capillary column and analyzed at 70 eV using a Shimadzu MS-QP2010SE mass spectrometer. The Zebron ZB-5 capillary column from Phenomenex was used (with a 5 percent diphenyl and a 95% dimethylpolysiloxane stationary phase, a column length of 30 m, a column ID of 0.32 mm, and a thickness of 0.10 m). The mass spectrometer should be configured to the following settings: ion source heater 250 °C, interface temperature 300 °C, vacuum 10–5 Pa, m/z range 45–300, and scan speed 1428. Shimadzu (NIST17.0) post-run software was utilized to further examine each experiment's chromatograms and spectra.
Furthermore studied samples were subjected to NMR analyses: soluble fractions, separated during extraction with chloroform in a Soxhlet apparatus; the products of the solvolysis process and the condensation product of one of the latter with epichlorohydrin. 1H NMR and 13C NMR spectra were recorded at 25 °C with the aid of a 600 MHz Varian spectrometer in CDCl3 as a solvent. Tetramethylsilane (TMS) was used as an internal reference.
Recycling of glass, carbon fibre, and epoxy resin oligomers
Chemical recycling was performed at 100–190 °C for 60–180 min under 30–60 bar of an inert N2 atmosphere in a 500 mL Parr 4650 batch reactor with the transesterification catalyst, TBD, ethylene glycol and 1-methyl-2-pyrrolidinone in a 1:1 mol ratio. Under each set of solvolysis conditions, 1 g of sample and 20 mL of solution were used. To avoid overheating, the Parr 4650 batch reactor was calibrated before each test. After each experiment, the sample was filtered to extract fibre residues. After filtration, the liquid sample was analysed by nuclear magnetic resonance spectroscopy (NMR), and the solid residue on the filter was washed, dried, and weighed. After weighing the fibre sample content, it was burned to analyse the contents of the ash. The solvolysis process resin degradation yield (RDY) was determined using Eq. (1),
where w1 is the sample weight before solvolysis, w2 is the sample weight after solvolysis, and w0 is the epoxy resin weight before solvolysis42.
After process condition optimization, the solvolysis was scaled up to a 2 L unpressurized batch reactor with a reflux condenser and a nitrogen supply. The liquid sample after solvolysis was subjected to an addition reaction to investigate the potential for resin recovery from the composite materials. Specifically, 50 mL of liquid solvolysis products, 20.0 mL of EPI, and 10.0 mL of i-Pr were added to a 100 mL triple-neck flask equipped with a reflux condenser, thermometer, and dropper for the addition reaction. To ensure complete mixing, the reaction mixture was heated at 50 °C for 5 min. The addition reaction was allowed to proceed for 15 min at 67–70 °C by adding 1.5 mL of NaOH (0.15 mol). After cooling to 55 °C, 15.5 mL of NaOH was added to the reaction mixture, and condensation occurred for 90 min. After complete condensation, water was added to the mixture, which was then heated to 80 °C for 10 min to dissolve the generated sodium chloride. After 30 min, the reaction mixture was added to a separatory funnel to separate the aqueous phase from the organic phase.
Recycling mechanism
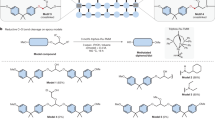
The degradation of the resin-composite network is a crucial step in the recycling of waste composite materials. Figure 2 shows the mechanism of the small molecule-assisted dissolution approach. The recycling solvent comprises EG and organic solvent (i.e., NMP) in a molar ratio of 1:1, along with various amounts of the transesterification catalyst (i.e., TBD) ranging from 0.015 to 0.025 mol. The transesterification-type bond exchange process occurs between hydroxy groups in the solvent and ester groups in the resin-composite material. EG cleaves ester bonds in the polymer network, causing the thermoset substrate to dissolve into oligomers and monomers. A surface layer model with three layers (solid swelling layer, gel layer, and pure polymer layer) was used to characterize the thermosetting polymer dissolution kinetics (Fig. 2)44. The diffusion of the organic solvent (i.e., NMP) accelerates the swelling of the thermosetting resin, which allows significant amounts of EG and TBD to enter the polymer network. A thicker gel layer forms at the surface layer, where the bond exchange reaction occurs, and the resin surface gradually erodes.
Recyclability of resin-based waste composite
The small molecule-assisted waste composite recycling method includes two basic steps: (1) the recycling solution dissolves the resin of the waste composite, and (2) the carbon or glass fibre-based waste composite is removed from the products. In the first step, the solution changes from transparent to yellow and dark orange following the reaction between the transesterification catalyst, resin, and other chemicals present in the samples as present in Fig. 3.
A total of 29 solvolysis experiments were carried out under various processing conditions, according to the experimental design, i.e., using a temperature range of 100–190 °C, a pressure range of 0–60 bar, a duration range of 1–3 h, and a TBD catalyst concentration range of 0.015–0.025 mol. Owing to the deep penetration of small molecules, the thermosetting polymer substrate eventually deteriorates. At high temperatures, polymers with low melting points were also dissolved. After the dissolution process, the sample was filtered to separate the liquid from the glass fibres, which were then washed with isopropanol, dried at 105 °C, and weighed.
Based on the results from these 29 experiments under various conditions, the optimal conditions in terms of maximizing the resin removal from the WTB composite samples are as follows: processing temperature = 190 °C, duration = 2.5–3 h, pressure = 60 bar, and TBD content = 0.025 mol (Fig. 4). The conducted analysis showed small, statistically significant effects of the solvolysis duration and catalyst concentration (alpha confidence interval = 0.05) on the standard deviation of the RDY value; this relationship is related to the variable composition of the tested WTB samples. Specifically, this variability is due to the inconsistent amount of glass fibre used in the composite construction.
The experimental plan and ANOVA method revealed the statistically significant variables that affect the solvolysis process in this study. We also evaluated the interactions between the selected variables and determined whether they had statistically significant effects on the degree of resin removal from WTB samples. The optimized processing conditions led to resin removal yields ranging from 4.36 to 34.43%. The factors with the most statistically significant effect on the resin removal yield were temperature, pressure, and time. ANOVA confirmed that there was a negative correlation between time and pressure and a positive correlation between the reaction time and the amount of catalyst. The negative impact of the interaction between processing time and increasing pressure on the RDY could be avoided by conducting the process at atmospheric pressure. Under these conditions, there is a local maximum for the RDY value, and the interaction between the processing time and increasing pressure does not negatively impact the processing efficiency.
After the optimization of solvolysis for WTBW the cascade approach was applied. In the WTBW case, the cascade process more than doubled the resin removal rate from the material. Additionally, after the second step, the overall amount of RDY resin that was removed reached 80% (Fig. 5b). Comparable results were obtained for the CFC using waste from WTBW that had been subjected to the described process only once (32.5%; Fig. 5a).
After that, the solvolysis of commercial carbon fibre composites was then scaled up to 2 L and run out of an unpressurized batch reactor. At this time, the solvolysis process was carried out in the same optimal conditions and at twice the time—up to 6 h. After that, very good results, close to 100% resin degradation yield, were obtained. These results are presented in Fig. 5c and d. After washing, very clear fibres were obtained.
It could be seen that after washing, very clear fibres were obtained, which was also confirmed by SEM–EDS analysis (Fig. 6). SEM–EDS clearly show the smooth surface of larger-scale fibres.
Based on Py-GC–MS as well as NMR analysis, it was found that in both cases the composite matrices contained a crosslinked and uncrosslinked fraction of epoxy resin, 1,2-benzene dicarboxylic acid (phthalic acid) and phthalic acid derivatives, and in the case of waste from wind turbine blade also styrene and polystyrene. The main compounds identified in the pyrogram from wind turbine blades were: styrene (3.05 min), 1,2-benzene dicarboxylic acid (12.79 min), cyclopropyl phenylmethane (21.97 min), 3-(2-Phenylethyl)benzonitrile (35.07 min) 1-docosene (46.45 min) and heptacosane (49.44 min). The results of the analytical pyrolysis of studied composites correspond to epoxy resin and unsaturated polyester resin45,46,47. The ATR-FTIR spectra indicate that at high wavenumbers, the FTIR spectrum contains an absorption band at 3400 cm-1 attributable to the O–H stretching mode of hydroxyl groups, showing the existence of dimers or high molecular weight species. Bands at 3060/cm correspond to the C–H stretching vibration mode of the epoxide group, while those at 2960 and 2872/cm relate to the –CH2 and –CH3 stretching vibration modes of aromatic and aliphatic chains, respectively. Bands at 1722/cm are assigned to the C=O stretching mode. Some weak intensity bands at 1600, 1493, 1452, and 1374/cm indicate the presence of N–H functional groups of amines, as well as C–N bonds of amine and imide groups presumably belonging to the catalyst and modifier added to the modified epoxy resin48. Furthermore, the presence of bands at 1232, 1153, and 1063/cm confirms the existence of C–O–C and C–O stretching vibrations due to ether linkage. The epoxide groups in the resin are identified by the characteristic absorption bands cantered at 984 and 908/cm, which are connected with the glycidyl ether functionality and the C–O stretching vibration mode of the oxirane ring, respectively. Finally, bands at 742/cm were assigned to C–H out-of-plane absorptions of substituted aromatic rings.
After liquefaction, the liquid products were analysed via nuclear magnetic resonance to ascertain their viability for further application. The NMR analyses were performed on the following samples: soluble fractions of WTB, which were separated during extraction with chloroform in a Soxhlet apparatus; products of WTB and condensation products of WTB solvolysis with EPI. 1H- and 13C-NMR spectra at 25 °C were recorded on a 600 MHz Varian spectrometer using CDCl3 as the solvent and tetramethyl silane (TMS) as an internal reference. 1H-NMR examinations of the soluble portion of wind turbine blade samples revealed the presence of styrene, 1,2-benzenedicarboxylic acid (phthalic acid), and phthalic acid derivatives (Supplementary materials S 1a). Additionally, the uncross-linked fraction of epoxy resin was detected. In the liquid samples obtained after the recycling process (Supplementary materials Sb, c), monomers and dimers were detected after the transesterification process. Bisphenol A, which is a substrate used in the production of epoxy resins, is one of the most important compounds identified in the analyzed samples.
It is important to note that the manufacturing technology for wind turbine blades has undergone changes over time, resulting in alterations to the composition, quantity, and variety of epoxy resins and additives utilised. But, the epoxy resins as a group have remained unaltered, as can be asserted.
Based on the chemical analyses, it was calculated that during the liquefaction process under optimised conditions 80.04% of the polymer matrix, i.e., 8.58 g, was removed from the 30.06 g composite waste sample containing 64.35% of the fibres (19.34 g). On the fibre surface, 2.14 g of undissolved polymer matrix remained. The NMR analysis allowed determine that the post-liquefaction sample contained an unsaturated polyester resin comprising bis(2-ethylhexyl) phthalate and maleic anhydride (on the basis of Py-GC–MS and NMR analysis), which, when converted into an oligomer, was cured with styrene in an amount of 30–40 wt.%. This amount was confirmed by Py-GC–MS analysis. This analysis yielded 39.46% styrene in the pyrolysis products of the composite material. Due to the fact that the samples tested were real waste and no exact information was available on the raw materials used for the production of the wind turbine blades, it was assumed in the results presented below that 30 wt.% styrene could have been used to cure the unsaturated polyester resin, and not nearly 40 wt.% as shown by the Py-GC–MS analysis. This is because, during pyrolysis, some of the styrene may additionally come from phthalate decomposition. In addition, NMR analysis showed that the molar ratio of the phthalate-containing oligomer to NMP is 0.0054. Assuming an average oligomer mass of 1107 g/mol, it was calculated that there is almost 6 g of oligomer (5.978 g) in the liquid product, which makes it possible to close the mass balance presented in Fig. 7 quite satisfactorily. It can therefore be concluded that the recovery efficiency for the oligomers (phthalate oligomer, styrene, and polystyrene) from the polymer matrix in the case studied was approximately 80%.
The method described above allows the isolation of 19.34 g of glass fibre with a yield of 90%, while the residual 2.14 g, equivalent to 10%, represents the resin that is incapable of dissolving in the composite matrix under the experimental conditions employed. Moreover, the technique allowed the isolation of 8.58 g of oligomers, corresponding to an 80% weight yield of the polymer matrix in the experimental residue (excluding glass fibre). The oligomer composition comprises of styrene/polystyrene and phthalate oligomers, constituting approximately 30% and 70% of the total mixture, respectively.
Synthesis of resin precursors from liquid solvolysis products via condensation
The presence of bisphenol An in the liquid solvolysis products made it possible to test the hypothesis that a resin could be obtained by condensing bisphenol A with EPI without first purifying the reaction mixture. Obtaining a resin from such an intermediate would demonstrate that resins used in industry can be recovered and reused. The condensation of bisphenol A from the solid products after solvolysis in the presence of EPI indicated that polycondensation could produce an epoxy resin transition product known as bisphenol A propoxate. The NMR spectrum of the solid condensation products contains signals attributed to polystyrene. Polystyrene was most likely produced through the polymerization of styrene (which was present in the WTB samples) under the applied solvolysis conditions.
Comparison with other methods
There are ways in the literature that belong to chemical recycling procedures that use different low-molecular-weight compounds as potential solvents than the one used in this case. Acetone, low-molecular-weight alcohols such as ethyl alcohol or 2-propanol, acetic acid, ethylene glycol (EG), propylene glycol (PG), n-methyl pyrrolidone (NMP), propylene carbonate (PC) and glycerol triacetate (TAG) are examples of these molecules. To suggest a non-pressurised technique that could be employed more broadly and securely on a bigger scale than subcritical or supercritical systems, low-boiling solvents were omitted from the comparisons, and only high-boiling solvents were evaluated. The comparison also included propylene carbonate, a relatively safe and frequently used solvent in the polymer industry. For comparison, a 1 g sample of wind turbine blade waste was employed, and solvolysis operations were carried out under reproducible process conditions: 190 °C temperature, 3 h process time, with and without the presence of a catalyst. The results of the comparative analyses are shown in Table 1.
When utilising an identical molar ratio of ethyl glycol and propylene carbonate, it was discovered that the resulting mixture, even when heated, is rather dense and severely impedes proper catalyst dissolving (TBD). Forced stirring (EG/PC*) of the entire mixture was required in this case to achieve an acceptable result. It has been observed that in the case of wind turbine blade waste, the cross-linked resins utilised appear to be too stable to be degraded using merely low-molecular-weight compounds, as can be shown with some of the solvents successfully used by another researcher41,42,43. The process times of up to 16 h stated in the literature may be a severe impediment to the feasibility of the recycling process. Furthermore, the literature42 does not indicate under what pressure the processes were carried out in some cases, as the study used both ethylene glycol and propylene glycol, which have boiling points of 197 °C and 188.2 °C, respectively, so chemical recycling using them for 16 h at 270 °C had to be carried out under pressure in a suitably adapted reactor.
Conclusions
This study proposes a flexible reaction approach for recycling waste wind turbine blades that is effective and environmentally friendly. At temperatures below 200 °C, a transesterification reaction efficiently dissolved thermoset ester-containing resins in waste wind turbine blades and carbon fibre composites. The glass and carbon fibre materials that were obtained were easily separated from the product solution. Experiment results showed that this method could recycle a wide range of genuine carbon and glass fibre resin composites with high efficiency (almost 100%), including wind turbine blades made from epoxy-anhydride and polyester resin substrates. Because the concept described herein can be applied to other types of ester-containing substrate polymers, incorporating ester groups into the substrate polymer network could be a promising strategy for environmentally friendly recycling. Importantly, after processing, the liquid fraction can be used to separate monomers from epoxy resins, such as styrene and epoxy resin precursors.
To the best of our knowledge, this is the first time this approach has been used to process a complex real-world matrix of this type with a complex DOE approach. Once fully optimized, this technology could be used to recover the glass fibres that comprise the composite or to produce epoxy resin precursors for use elsewhere. Our study showed that in the case of WTB composite which was cross-linked by styrene, without stabilization styrene self-polymerises in the post-processing mixture, which facilitates its removal. The post-processing mixture can be recycled until it is saturated with the leached oligomer and then regenerated by recovering the oligomers.
Based on the findings presented here, future research should concentrate on further optimizing the solvolysis process, such as by using a specific solvent mixture based on the composition of the composite, using other transesterification catalysts, or changing the technical sequence. The developed approach is also compared to previously reported strategies for chemically recycling waste-stained glass turbine blades in this paper. In terms of reducing the amount of waste produced by wind turbine blades and carbon fibre composites, the strategy used in this study was the most successful. Scientists will continue to look for other possible catalysts that will speed up the breakdown of the epoxy resin-composite matrix as research on this topic progresses. Furthermore, further research will focus on determining the commercial feasibility of the presented solution depending on the type of waste materials.
Data availability
The datasets generated and analysed during the current study are available in the Mendeley Data repository49, “Chemical recycling of End-of-Life wind turbine blades”, V1, https://doi.org/10.17632/7hjmb2bxdh.1, https://data.mendeley.com/datasets/7hjmb2bxdh/1.
Abbreviations
- WTBW:
-
Wind turbine blade waste
- GFC:
-
Glass fibre composites
- CFC:
-
Carbon fibre composites
- EG:
-
Ethylene glycol
- NMP:
-
1-Methyl-2-pyrrolidinone
- TBD:
-
1,5,7-Triazabicyclo[4.4.0]dec-5-ene
- EPI:
-
Epichlorohydrin
- i-Pr:
-
Isopropanol
- NaOH:
-
Sodium hydroxide
- NMR:
-
Nuclear magnetic resonance
References
Wind, G. & Council, E. GWEC|GLOBAL WIND REPORT 2021. https://gwec.net/wp-content/uploads/2021/03/GWEC-Global-Wind-Report-2021.pdf (2021).
Wind energy in Europe: Outlook to 2023. https://www.anev.org/wp-content/uploads/2019/10/Market-outlook-2019.pdf (2019).
WindEurope. Repowering and Lifetime Extension: Making the Most of Europe’s Wind Resource. windeurope.org. https://windeurope.org/wp-content/uploads/files/policy/position-papers/WindEurope-Repowering-and-Lifetime-Extension.pdf (2017).
Jacoby, M. Recycling wind turbine blades. Chem. Eng. News 100, 26–30 (2022).
Ziegler, L., Gonzalez, E., Rubert, T., Smolka, U. & Melero, J. J. Lifetime extension of onshore wind turbines: A review covering Germany, Spain, Denmark, and the UK. Renew. Sustain. Energy Rev. 82, 1261–1271 (2018).
Volk, R., Stallkamp, C., Herbst, M. & Schultmann, F. Regional rotor blade waste quantification in Germany until 2040. Resour. Conserv. Recycl. 172, 105667 (2021).
Knight, S. What to do with turbines after they leave support system. Wind Power Monthly https://www.windpowermonthly.com/article/1671616/turbines-leave-support-system (2020).
Andersen, N., Eriksson, O., Hillman, K. & Wallhagen, M. Wind turbines’ end-of-life: Quantification and characterisation of future waste materials on a national level. Energies 9, 999 (2016).
Mishnaevsky, L. Sustainable end-of-life management of wind turbine blades: Overview of current and coming solutions. Materials 14, 1124 (2021).
Chien, C. F., Aviso, K., Tseng, M. L., Fujii, M. & Lim, M. K. Solid waste management in emerging economies: Opportunities and challenges for reuse and recycling. Resour. Conserv. Recycl. 188, 106635 (2023).
Singh, A., Charak, A., Biligiri, K. P. & Pandurangan, V. Glass and carbon fiber reinforced polymer composite wastes in pervious concrete: Material characterization and lifecycle assessment. Resour. Conserv. Recycl. 182, 106304 (2022).
Chen, Y. et al. Modeling waste generation and end-of-life management of wind power development in Guangdong, China until 2050. Resour. Conserv. Recycl. 169, 105533 (2021).
Chen, J. et al. Green development strategy of offshore wind farm in China guided by life cycle assessment. Resour. Conserv. Recycl. 188, 106652 (2023).
Upadhyayula, V. K. K. et al. Wind turbine blades using recycled carbon fibers: An environmental assessment. Environ. Sci. Technol. 56, 1267–1277 (2022).
Mishnaevsky, L. et al. Materials for wind turbine blades: An overview. Materials 10, 1–24. https://doi.org/10.3390/ma10111285 (2017).
Ding, A., Li, S., Wang, J. & Ni, A. A new analytical solution for spring-in of curved composite parts. Compos. Sci. Technol. 142, 30–40 (2017).
Ding, A., Wang, J., Ni, A. & Li, S. A new analytical solution for cure-induced spring-in of L-shaped composite parts. Compos. Sci. Technol. 171, 1–12 (2019).
Jubinville, D., Esmizadeh, E., Saikrishnan, S., Tzoganakis, C. & Mekonnen, T. A comprehensive review of global production and recycling methods of polyolefin (PO) based products and their post-recycling applications. Sustain. Mater. Technol. 25, e00188 (2020).
Khalid, M. Y., Arif, Z. U., Ahmed, W. & Arshad, H. Recent trends in recycling and reusing techniques of different plastic polymers and their composite materials. Sustain. Mater. Technol. 31, e00382 (2022).
WindEurope. Accelerating wind turbine blade circularity. Them. Rep. 2020, 11–13 (2020).
Bomgardner, M. & Scott, A. Recycling renewables. Chem. Eng. News 96, 34–41 (2018).
Delaney, E. L. et al. An integrated geospatial approach for repurposing wind turbine blades. Resour. Conserv. Recycl. 170, 105601 (2021).
Joustra, J., Flipsen, B. & Balkenende, R. Structural reuse of high end composite products: A design case study on wind turbine blades. Resour. Conserv. Recycl. 167, 105393 (2021).
Cooperman, A., Eberle, A. & Lantz, E. Wind turbine blade material in the United States: Quantities, costs, and end-of-life options. Resour. Conserv. Recycl. 168, 105439 (2021).
Baturkin, D., Hisseine, O. A., Masmoudi, R., Tagnit-Hamou, A. & Massicotte, L. Valorization of recycled FRP materials from wind turbine blades in concrete. Resour. Conserv. Recycl. 174, 105807 (2021).
Liu, P., Meng, F. & Barlow, C. Y. Wind turbine blade end-of-life options: An economic comparison. Resour. Conserv. Recycl. 180, 106202 (2022).
Beauson, J., Lilholt, H. & Brøndsted, P. Recycling solid residues recovered from glass fibre-reinforced composites—a review applied to wind turbine blade materials. J. Reinforced Plast. Compos. 33, 1542–1556. https://doi.org/10.1177/0731684414537131 (2014).
Guo, J., Cao, B., Guo, J. & Xu, Z. A plate produced by nonmetallic materials of pulverized waste printed circuit boards. Environ. Sci. Technol. 42, 5267–5271 (2008).
Guo, J., Li, J., Rao, Q. & Xu, Z. Phenolic molding compound filled with nonmetals of waste PCBs. Environ. Sci. Technol. 42, 624–628 (2008).
Nakagawa, T. et al. Effects of aging on the mechanical and fracture properties of chopped fiber composites made from repurposed aerospace prepreg scrap and waste. Sustain. Mater. Technol. 33, e00470 (2022).
Yang, Y. et al. Recycling of composite materials. Chem. Eng. Process. 51, 53–68 (2012).
Pimenta, S. & Pinho, S. T. Recycling carbon fibre reinforced polymers for structural applications: Technology review and market outlook. Waste Manage. 31, 378–392 (2011).
Ma, X. et al. Closed-loop recycling of both resin and fiber from high-performance thermoset epoxy/carbon fiber composites. ACS Macro Lett. 10, 1113–1118 (2021).
Worch, J. C. & Dove, A. P. 100th Anniversary of macromolecular science viewpoint: Toward catalytic chemical recycling of waste (and future) plastics. ACS Macro Lett. 2020, 1494–1506. https://doi.org/10.1021/ACSMACROLETT.0C00582/ASSET/IMAGES/LARGE/MZ0C00582_0005.JPEG (2020).
Lo, J. N., Nutt, S. R. & Williams, T. J. Recycling benzoxazine-epoxy composites via catalytic oxidation. ACS Sustain. Chem. Eng. 6, 7227–7231 (2018).
Rani, M., Choudhary, P., Krishnan, V. & Zafar, S. Development of sustainable microwave-based approach to recover glass fibers for wind turbine blades composite waste. Resour. Conserv. Recycl. 179, 106107 (2022).
Devic, A.-C., Ierides, M., Fernandez, V., Verbenkov, M. & Bax, L. Polymer composites circularity. Suschem 21, 256 (2018).
Zhang, J., Chevali, V. S., Wang, H. & Wang, C. H. Current status of carbon fibre and carbon fibre composites recycling. Compos. B Eng. 193, 108053 (2020).
Hong, H., Part, F. & Nowack, B. Prospective dynamic and probabilistic material flow analysis of graphene-based materials in Europe from 2004 to 2030. Environ. Sci. Technol. 2022, 13798–13809 (2022).
Vollmer, I. et al. Beyond mechanical recycling: Giving new life to plastic waste. Angew. Chem. Int. Ed. 59, 15402–15423 (2020).
Wu, M., Jin, B. C., Li, X. & Nutt, S. Advanced manufacturing: Polymer & composites science a recyclable epoxy for composite wind turbine blades. Adv. Manufact. Polymer Compos. Sci. 5, 114–127 (2019).
Mattsson, C., André, A., Juntikka, M., Trnkle, T. & Sott, R. Chemical recycling of End-of-Life wind turbine blades by solvolysis/HTL. IOP Conf. Ser. Mater. Sci. Eng. 942, 012013 (2020).
Oliveux, G., Dandy, L. O. & Leeke, G. A. Degradation of a model epoxy resin by solvolysis routes. Polymer Degrad. Stability https://doi.org/10.1016/j.polymdegradstab.2015.04.016 (2015).
Kuang, X. et al. Dissolution of epoxy thermosets via mild alcoholysis: The mechanism and kinetics study. RSC Adv. 8, 1493–1502 (2018).
Milczarek, J. M., Dziadosz, M. & Ziêba-Palus, J. Way to distinguish car paint traces based on epoxy primer layers analysis by pyrolysis-gas chromatography-mass spectrometry. Chem. Anal. (Warsaw) 54, 173 (2009).
Shih, Y. F., Jeng, R. J. & Wei, K. M. Carbon black containing interpenetrating polymer networks based on unsaturated polyester/epoxy III: Thermal and pyrolysis analysis. J. Anal. Appl. Pyrolysis 70, 129–141 (2003).
Evans, S. J., Haines, P. J. & Skinner, G. A. Pyrolysis-gas-chromatographic study of a series of polyester thermosets. J. Anal. Appl. Pyrolysis 55, 13–28 (2000).
Musto, P., Martuscelli, E., Ragosta, G., Russo, P. & Scarinzi, G. An interpenetrated system based on a tetrafunctional epoxy resin and a thermosetting bismaleimide: Structure-properties correlation. J. Appl. Polym. Sci. 69, 1029 (1998).
Sajdak, M. Chemical recycling of end-of-life wind turbine blades—mendeley data. https://doi.org/10.17632/7hjmb2bxdh.1. https://data.mendeley.com/datasets/7hjmb2bxdh (2022).
Acknowledgements
Publication supported by the Excellence Initiative - Research University program carried out, at the Silesian University of Technology, 2021 “Disposal and recovery of raw materials from end-of-life wind turbine blades by chemical processes” 08/020/SDU/10-21-01. This work was also supported by the Faculty of Energy and Environmental Engineering at the Silesian University of Technology (statutory research). Publication supported by the Rector’s pro-quality grant. The Silesian University of Technology, grant number 08/020/RGJ23/0033.
Author information
Authors and Affiliations
Contributions
The manuscript was written through the contributions of all authors. All authors have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Muzyka, R., Sobek, S., Korytkowska-Wałach, A. et al. Recycling of both resin and fibre from wind turbine blade waste via small molecule-assisted dissolution. Sci Rep 13, 9270 (2023). https://doi.org/10.1038/s41598-023-36183-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-36183-4
This article is cited by
-
Advances in green chemistry and engineering
Scientific Reports (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.