Abstract
Spontaneous testicular teratomas (STTs) are tumours comprising a diverse array of cell and tissue types, which are derived from pluripotent stem-like cells called embryonal carcinoma cells (ECCs). Although mouse ECCs originate from primordial germ cells (PGCs) in embryonic testes, the molecular basis underlying ECC development remains unclear. This study shows that the conditional deletion of mouse Dead end1 (Dnd1) from migrating PGCs leads to STT development. In Dnd1-conditional knockout (Dnd1-cKO) embryos, PGCs colonise the embryonic testes but fail to undergo sexual differentiation; subsequently, ECCs develop from a portion of the PGCs. Transcriptomic analyses reveal that PGCs not only fail to undergo sexual differentiation but are also prone to transformation into ECCs by upregulating the expression of marker genes for primed pluripotency in the testes of Dnd1-cKO embryos. Thus, our results clarify the role of Dnd1 in developing STTs and developmental process of ECC from PGC, providing novel insights into pathogenic mechanisms of STTs.
Similar content being viewed by others
Introduction
Testicular teratomas are tumours composed of a diverse array of cell and tissue types derived from all three germ layers, including erythrocytes, adipocytes, cartilage, muscle, hair, and glandular tissue, as well as a cluster of highly proliferative and pluripotent stem-like cells called embryonal carcinoma cells (ECCs). At the onset of spontaneous testicular teratomas (STTs), primordial germ cells (PGCs) transform into ECCs in embryonic testes and subsequently differentiate into various types of embryonic and adult cells constituting the tumours. In mice, the incidence rate of STTs varies according to the genetic background; the 129 family of inbred mouse strains causes STTs at a probability of 1–7% depending on the subline, whereas other strains, such as C57BL/6J, LTXBJ, and C3H/HeJ, rarely develop STTs1,2,3,4. Previous studies have identified several genes and genomic loci associated with high susceptibility to STTs in the genetic background of 129 strains5,6,7,8,9,10,11,12 and have attempted to analyse the function of these genetic factors in the development of STTs13,14. Consequently, these analyses recently suggested that ECCs developed from PGCs that failed to undergo sexual differentiation and retained early PGC characteristics even after colonising the embryonic testes15. However, the molecular mechanisms underlying the transformation of PGC to ECC remain unclear.
Dead end1 (Dnd1) encodes an RNA-binding protein specifically expressed in PGCs at embryonic stages. The expression of DND1 begins immediately after PGC development, continues during the migration and colonisation of the embryonic testes, and becomes restricted to spermatogonia in adult testes after birth, whereas its expression gradually decreases after colonisation of the embryonic ovary, as embryonic development progresses, and disappears during oogenesis16,17. In 2005, Dnd1 was identified as the gene responsible for a spontaneous mutation called Ter5, isolated in 1973 as a mutation that increased STT incidence to 17% and 94% in the heterozygotes and homozygotes, respectively, in the 129/Sv genetic background3. Ter was a single nucleotide polymorphism that generated a premature stop codon in the third exon of Dnd1, which is presumed to result in a null mutation of Dnd1 due to nonsense-mediated mRNA decay. Therefore, Dnd1 is a key to understanding the mechanisms underlying STT development. However, as STTs predominantly occur in the 129 strain genetic background, not only loss of Dnd1 but also unidentified genetic factors specific to the 129 strain family of mice are essential for STT development. In addition, the number of PGCs drastically decreases during migration and gonad colonisation owing to apoptotic cell death18 and some of the remaining PGCs transform to ECCs in the Dnd1Ter/Ter embryonic testes, suggesting that secondary effects of Dnd1-loss might cause ECC development in the 129 strain genetic background. Therefore, it is challenging to clarify the role of Dnd1 in the mechanisms of ECC development using Ter mutant.
We have previously shown that DND1 directly interacts with another RNA-binding protein, NANOS2, in PGCs colonising the embryonic testes and suppresses the expression of target genes by recruiting the CCR4-NOT deadenylase complex to their mRNAs through NANOS2 for degradation16. To elucidate the physiological significance of the DND1-NANOS2 complex, we generated a drug-inducible Dnd1-conditional knockout (Dnd1-cKO) mouse line and removed DND1 from PGCs in the embryonic testes after NANOS2 expression began. Consequently, PGCs failed to undergo sexual differentiation and were gradually lost by apoptotic cell death, even in the presence of NANOS2, resulting in small testes with no germ cells in the seminiferous tubules, as well as the testes of Nanos2 mutant mice. Therefore, we concluded that DND1 is an essential partner of NANOS2 to regulate the sexual differentiation of PGCs in embryonic testes. In addition, we conducted a functional analysis of DND1 in the spermatogonia of adult testes using Dnd1-cKO mice and showed that DND1 is required for the maintenance of undifferentiated and differentiating spermatogonia19. Thus, DND1 plays a critical role in various germline development steps. However, the physiological significance of DND1 expression in early PGCs remains unclear.
This study aimed to investigate the effect of the loss of Dnd1 in early PGCs and analyse the transcriptomic changes in PGCs to elucidate the mechanism underlying the transformation of PGC to ECC. Our results provide new insights on the developmental mechanisms of ECC from PGC.
Results
Dnd1 deletion in migrating PGCs induces spontaneous testicular teratomas
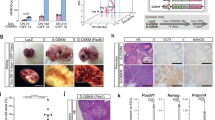
To examine whether DND1 plays a physiological role in the development of PGCs before sexual differentiation, we introduced the Oct4ΔPE-CreERT2 transgene into Dnd1flox/flox mice and administered tamoxifen to pregnant mothers at embryonic day (E) 9.5, to delete DND1 from PGCs at migrating stages (Fig. 1A). We then analysed the male offspring after birth. Notably, we detected larger testes in 4-week-old Dnd1-cKO mice than those in control mice (Fig. 1B). Section staining analysis revealed that the control testes showed normal spermatogenesis, whereas the Dnd1-cKO testes contained various tissues and cell types (Fig. 1C, D). We found tissue structures derived from all three germ layers, including hair follicles from the ectoderm, muscles from the mesoderm, and gut-like epithelium from the endoderm (Fig. 1E–G), indicating that teratomas developed in the testes.
Dnd1 deletion in migrating PGCs causes testicular teratomas. (A), Mating strategy for the generation of conditional knockout mice of Dnd1. (B), Comparison of testis size between 4-week-old littermates of control (Dnd1flox/flox) and Dnd1-cKO (Dnd1flox/flox; Oct4ΔPE-CreERT2) mice. (C–G), Testis sections from 4-week-old control (C) and Dnd1-cKO (D–G) mice were stained with haematoxylin and eosin. Hair follicles, muscles, and gut-like epithelium are observed in (E, F), and (G), respectively. (H, I), Testis from control (H) and Dnd1-cKO (I) embryos with Nanog-GFP transgene at E16.5, under a fluorescence microscope. The arrowheads in (I) indicate GFP-positive foci. (J–R), Testis sections from E16.5 Dnd1-cKO embryos with Nanog-GFP transgene were immunostained with antibodies against GFP (J, M, P), NANOG (K), POU5F1 (N), and OTX2 (Q). DNA was labelled with DAPI (blue) (L, O, R). Images correspond to one of n = 3 biological replicates. S, The incidence of male embryos with at least one ECC colony in the testes was analysed in control and Dnd1-cKO embryos with Nanog-GFP transgene after injection of tamoxifen at E10.5, E11.5, and E12.5. Scale bars: 5 mm in (B), 100 μm in (C) and (D), 100 μm in (E–G), 200 μm in (H) and (I), and 100 μm in (J–R).
To analyse the development of STTs at earlier stages, we visualised the onset of STTs by labelling tumour precursors known as embryonal carcinoma cells (ECCs). A reporter gene expressing GFP under the control of a pluripotent stem cell marker, Nanog, was introduced into Dnd1-cKO embryos, as NANOG was expressed not only in early PGCs, but also in ECCs20,21. This transgene showed distinct GFP-positive foci inside the Dnd1-cKO testes at E16.5 (Fig. 1H, I). Section immunostaining analysis revealed that these foci consisted of colony-forming cells expressing stem cell markers, such as NANOG and POU5F1, and a marker for ECCs, OTX213 (Fig. 1J–R). Meanwhile, the PGCs that were negative for these markers also faintly expressed GFP. These data indicate that the colony-forming cells strongly expressing GFP are putative ECCs that generate teratomas at later stages; therefore, the Nanog-GFP transgene allows us to promptly detect the initiation of teratoma development in the embryonic testes.
We then examined whether the timing of Dnd1 loss in PGCs contributed to the onset of teratoma because Dnd1-cKO mice did not develop STTs when tamoxifen was injected at E13.516. To this end, we injected tamoxifen to foster mothers at each stage from E10.5 to E12.5 and analysed the frequency of development of the GFP-positive foci in the embryonic testes using section-immunostaining or GFP fluorescence analysis. The GFP-positive foci were observed in the testes of Dnd1-cKO embryos at a frequency of 95.8% when tamoxifen was injected at E10.5; however, a gradual decrease to 46.2% and 0% was detected at E11.5 and E12.5, respectively (Fig. 1S).
Since NANOS2 expression begins at E12.522, these results indicate that DND1 suppresses the onset of STTs in PGCs that colonise the embryonic testes, independent of NANOS2.
Colony-forming cells are ECCs originating from PGCs in the testes of Dnd1-cKO embryos
ECCs originate from PGCs that colonise the embryonic testes at the onset of STTs23,24. To confirm that the Nanog-GFP-positive cell foci were indeed ECCs, we examined whether the cell foci were derived from PGCs in the Dnd1-cKO embryonic testes. We introduced the CAG-CAT-EGFP transgene into Dnd1flox/flox embryos with the Oct4ΔPE-CreERT2 transgene and traced the developmental fate of the PGCs in the absence of DND1 by injecting tamoxifen at E10.5 (Fig. 2A). As it has been reported that ECCs highly express NANOG and lose the expression of a germ cell marker deleted in azoospermia (DAZL)13, we conducted section-immunostaining analysis using antibodies against GFP, NANOG, and DAZL. GFP marked approximately 70% of DAZL-positive PGCs in the E17.5 control testes in this experimental system (Fig. 2B–E). In the testes of E17.5 Dnd1-cKO embryos, there were only a few DAZL-positive PGCs, and some NANOG-positive and DAZL-negative cell foci developed as expected (Fig. 2F–I). As the cells consisting of foci were marked by GFP, we concluded that these cells were ECCs derived from PGCs. In addition, these findings suggest that in the absence of DND1, only a fraction of PGCs transformed into ECCs, whereas most of the remaining PGCs disappeared by E17.5.
ECCs originated from PGCs in the Dnd1-cKO testes. (A), Strategy for lineage tracing of PGCs in control and Dnd1-cKO embryos. (B–I), Testis sections from E17.5 control (Dnd1+/flox; CAG-CAT-EGFP; Oct4ΔPE-CreERT2) and Dnd1-cKO (Dnd1flox/flox; CAG-CAT-EGFP; Oct4ΔPE-CreERT2) embryos were immunostained with antibodies against GFP (B, F), DAZL (C, G), and NANOG (D, H). The merged images are shown in (E) and (I). Enlarged views of the area enclosed by rectangles in (F, G, H), and (I) are shown in (F′, G′, H′), and (I′), respectively, to better visualise the expression of each protein. Images correspond to one of n = 4 biological replicates. Arrowheads in (F′, G′, H′), and (I′) indicate a ECC colony positive for GFP. Scale bars: 100 μm.
ECCs develop from a fraction of PGCs after E16.5 in the testes of Dnd1-cKO embryos
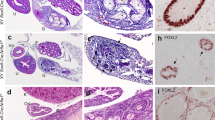
We next analysed when and how the ECCs developed from PGCs to further examine the onset of STTs. We used Rosa26-CreERT2, instead of Oct4ΔPE-CreERT2, to delete Dnd1 from PGCs more efficiently and conducted section-immunostaining analysis of the testes from the control and Dnd1-cKO embryos using the Nanog-GFP transgene at E14.5, E15.5, E16.5, and E17.5 with antibodies against DAZL, NANOG, and GFP. DAZL was continuously expressed in the PGCs of the control testes at these stages, whereas NANOG expression was detected in PGCs at E14.5 but gradually decreased and almost disappeared by E16.5 (Fig. 3A–L). GFP expression was, however, maintained in the PGCs until E17.5, possibly due to the higher stability of GFP than that of NANOG (Fig. 3M–P).
ECCs develop from a fraction of PGCs after E16.5 in Dnd1-cKO testes. (A–p), Testis sections from the control (Dnd1flox/flox; Nanog-GFP) (A–P) and Dnd1-cKO (Dnd1flox/flox; Nanog-GFP; Rosa26-CreERT2) (a–p) embryos at E14.5, E15.5, E16.5, and E17.5, were immunostained with antibodies against DAZL (A–D, a–d), NANOG (E–H, e–h), and GFP (M–P, m–p). Merged images of DAZL and NANOG are shown in (I–L) and (i–l), respectively. Enlarged views of the area enclosed by rectangles in (c, g, k), and (o) are shown in (c′, g′, k′), and (o′), respectively, to better visualise the expression of each protein. Images correspond to one of n > 3 biological replicates. Arrowheads in (c′, g′, k′), and (o′) indicate a ECC colony. Scale bars: 100 μm.
We found clear differences between the testes of Dnd1-cKO embryos and those of littermate controls. Although there were no apparent histological differences between the control and Dnd1-cKO testes at E14.5, the number of PGCs began to decrease at E15.5, and most of them disappeared by E17.5 in the Dnd1-cKO testes, as shown by DAZL staining (Fig. 3a–d, and Fig. S1A). In contrast, the expression of NANOG was maintained at a high level in some PGCs in Dnd1-cKO testes at E15.5 (Fig. 3e, f, and Fig. S1B ), whereas these cells co-expressed DAZL and did not form cell foci (Fig. 3i, j). However, some small cell foci began to develop at E16.5, where NANOG was expressed at a high level, while DAZL expression was not observed (Fig. 3g, k). The number of such cell increased at E17.5 (Fig. 3h, l, and Fig. S1C). Both PGCs and ECCs were marked with GFP during these stages (Fig. 3m–p).
Since ECCs are derived from PGCs, these results indicate that only a fraction of PGCs transform into colony-forming ECCs by E16.5, acquiring a high level of NANOG expression and a loss of DAZL expression during the initiation of STTs. In contrast, the other PGCs began to decrease in number after E15.5 and almost disappeared until E17.5 in the testes of Dnd1-cKO embryos.
Loss of DND1 causes accumulation of PGCs that failed to undergo sexual differentiation in embryonic testes
Next, we explored what happens to PGCs following Dnd1 loss during ECC development. Recently, ECCs are suggested to develop from PGCs that failed to undergo sexual differentiation in embryonic testes, in which case PGCs show retention of pluripotency gene expression, downregulation of male-type gene expression, failure of mitotic arrest, and upregulation of meiotic gene expression15,25. Therefore, we examined whether PGCs successfully underwent male-type differentiation in the testes of Dnd1-cKO embryos expressing the Nanog-GFP transgene. As the retention of pluripotency gene expression was already shown by NANOG expression (Fig. 3E–G, e–g, and S1B), the expression of NANOS2 was first analysed, because NANOS2 is a marker for male-type differentiation of PGCs; the loss of this protein causes failure of sexual differentiation, followed by apoptotic cell death25.
Immunostaining analysis revealed that NANOS2 was expressed in almost all PGCs in the control embryonic testes at E15.5, except for a few PGCs remaining in the mesonephros (Fig. 4A–C). In contrast, NANOS2 was rarely expressed in PGCs in the Dnd1-cKO embryonic testes, although a few PGCs appeared to normally express NANOS2 (Fig. 4D–F).
Accumulation of PGCs that failed sexual differentiation in Dnd1-cKO testes. A–L and N–e, Testis sections from E15.5 control (Dnd1flox/flox; Nanog-GFP) (A–C, G–I, N–P, T–V, and Z–b) and Dnd1-cKO (Dnd1flox/flox; Nanog-GFP; Rosa26-CreERT2) (D–F, J–L, Q–S, W–Y, and c–e) embryos were immunostained with antibodies against GFP (A, D, G, J, N, Q, T, W, Z, and c), NANOS2 (B, E), KI67 (H, K), SYCP3 (O, R), LAMININ (U, X), and cleaved CASPASE3 (a, d). DNA was labelled with DAPI (C, F, I, L, P, S, V, Y, b, and e). Enlarged views in (G–L) and (Q–S) are shown to better visualise KI67-positive and SYCP3-positive PGCs, respectively. Note that PGCs exhibit some aggregates of SYCP3 in the nucleus in (R) as previously reported25,47. Images correspond to one of n > 3 biological replicates. Arrowheads in Y indicate PGCs outside seminiferous tubules. Scale bars: 100 μm. (M, f), Ratios of (M) KI67- and (f) cleaved CASPASE3-positive PGCs per GFP-positive PGC in the E15.5 control and Dnd1-cKO testes. Three embryos were analysed for each genotype. Black bars represent the mean ± SD. **p < 0.01, ***p < 0.001 (Student’s t-test).
To examine the reason for the expression of NANOS2 in a few PGCs of the Dnd1-cKO embryonic testes, we analysed the expression of DND1. We detected its expression in some NANOS2-positive PGCs, indicating that Dnd1 was retained in these cells because of the inefficiency of Cre recombinase (Supplementary Fig. S2A–H). However, we also found that some PGCs expressed considerable amounts of NANOS2 despite the lack of DND1, whereas most PGCs expressed NANOS2 at a very low level (Supplementary Fig. S2I–L). These data indicate that NANOS2 begins to be expressed even in the absence of DND1 but requires DND1 for its full expression in most PGCs. However, even if NANOS2 is expressed, PGCs are presumed to fail to undergo male-type differentiation in Dnd1-cKO testes because NANOS2 is not fully functional without DND116. Consistent with this notion, we found several phenotypes corresponding to the failure of sexual differentiation in the PGCs of E15.5 Dnd1-cKO testes. The percentage of KI67-positive PGCs was increased from 24.0% in the control testes to 71.6% in the Dnd1-cKO testes (Fig. 4G–M), indicating that mitotic arrest was disrupted. A meiotic protein, SYCP3, was highly expressed and exhibited several nuclear aggregates (Fig. 4N–S), which may be located in the nucleoli, as accumulation of SYCP3 in the nucleoli can be a marker of meiotic progression, as previously reported26,27. A few PGCs were found outside the testis cords (Fig. 4T–Y). In addition, the percentage of activated CASPASE3-positive PGCs was drastically increased from 2.97% in the control testes to 15.7% in the Dnd1-cKO testes (Fig. 4Z, and a–f), accounting for the reduction in the PGC number in the Dnd1-cKO testes after E15.5. All these phenotypes were also observed in Nanos2-KO embryonic testes28, indicating that loss of DND1 in migrating PGCs causes accumulation of PGCs that are unable to undergo sexual differentiation in the embryonic testes.
Increase in embryonic germ cell (EGC) derivation from Dnd1-cKO PGCs
PGCs can be reprogrammed into pluripotent stem cells, also known as EGCs, when cultured with specific cytokines, such as leukaemia inhibitory factor (LIF) and basic fibroblast growth factor (bFGF) on feeder cells expressing membrane-bound steel factor29,30. The efficiency of EGC derivation is the highest immediately after PGC development and gradually decreases with increasing embryonic age; eventually, PGCs lose the capacity to generate EGCs after E15.531,32. It was reported that in Dazl-null mice, PGCs failed to undergo sexual differentiation in the embryonic testes and retained a high capacity to generate EGC compared to wild-type PGCs, even at E15.58, suggesting that pluripotency is less restrictive in the PGCs failing sexual differentiation in embryonic testes. Therefore, we speculated that the PGCs also maintain a relatively high capacity to generate EGC in the testes of Dnd1-cKO embryos, since they fail to undergo sexual differentiation as well as the PGCs in the testes of Dazl-null embryos.
To test this hypothesis, we attempted to derive EGCs from PGCs in the control and Dnd1-cKO embryos using the Nanog-GFP transgene. PGCs were isolated using fluorescence-activated cell sorting (FACS) (Supplementary Fig. S3A–N), plated with Sl/Sl4-m220 feeder cells expressing membrane-bound steel factor, and cultured with the medium containing LIF and bFGF as shown in Fig. 5A. The PGCs isolated from E13.5 testes of the control embryos gave rise to EGC colonies, with a mean derivation efficiency of 7.99 ± 1.37% per EGFP-positive PGCs plated; however, the efficiency drastically decreased to 0.44 ± 0.13% at E14.5, reaching 0.13 ± 0.02% at E15.5 (Fig. 5B–D, and H, control). These observations were roughly consistent with previous reports, although the efficiency was relatively high at each stage31,32. In contrast, PGCs from E14.5 to E15.5 testes of the Dnd1-cKO embryos retained a significantly higher capacity to generate EGC colonies than those of control embryos (1.62 ± 0.41% at E14.5 and 0.50 ± 0.06% at E15.5), while PGCs from E13.5 testes of the Dnd1-cKO embryos gave rise to EGCs with comparable derivation efficiency to those of control PGCs (7.90 ± 1.11%) (Fig. 5E–G, and H, Dnd1-cKO). There was no apparent difference in EGC derivation efficiency from PGCs between Dnd1-cKO and control female embryos during these stages (Supplementary Fig. S4A–G).
Increase in EGC formation in Dnd1-cKO PGCs. (A), Schematic representation of the culture conditions for deriving EGCs from PGCs. (B–G), Representative images of EGC colonies derived from EGFP-positive PGCs in the testes of control (Dnd1flox/flox; Nanog-GFP) (B–D) and Dnd1-cKO (Dnd1flox/flox; Nanog-GFP; Rosa26-CreERT2) (E–G) embryos at E13.5 (B, E), E14.5 (C, F), and E15.5 (D, G). EGC colonies were stained by alkaline phosphatase (AP) activity. Scale bars: 10 mm. (H), Rate of EGC derivation from EGFP-positive PGCs in the testes of control (Dnd1flox/flox; Nanog-GFP) and Dnd1-cKO (Dnd1flox/flox; Nanog-GFP; Rosa26-CreERT2) embryos. Black bars represent the mean ± SD. **p < 0.01, ***p < 0.001 (Student’s t-test).
These results indicate that DND1 restricts the pluripotency of PGCs in embryonic testes after E14.5, which subsequently makes it easier for PGCs to become pluripotent in the absence of DND1.
Transcriptomic analysis of Dnd1-cKO PGCs in the embryonic testes
We investigated the transcriptomic changes in Dnd1-cKO PGCs relative to control PGCs to determine the cause of the transformation from PGC to ECC. Dnd1flox/flox; Nanog-GFP females were crossed with Dnd1flox/flox; Rosa26-CreERT2 males and injected with tamoxifen at E10.5. PGCs were then isolated from the testes of five independent Dnd1-cKO and control embryos by FACS at E11.5 and subjected to RNA-seq analysis, as previously described33. The numbers of Dnd1 transcripts were drastically decreased in Dnd1-cKO PGCs compared with those in control PGCs (Fig. 6A), and 180 upregulated and 140 downregulated differentially expressed genes (DEGs) were identified in the Dnd1-cKO PGCs (Fig. 6B and Supplementary Table S1). However, Gene Ontology (GO) analysis revealed that GO terms were not significantly enriched in the DEGs, and principal component analysis (PCA) showed that the Dnd1-cKO PGC clustered closely with the control PGC (Fig. 6C), suggesting that these PGCs have relatively similar transcriptomes.
Transcriptomic analysis of PGCs in testes from the control and Dnd1-cKO embryos. (A), Expression levels of Dnd1 transcript in each PGC from five independent control and Dnd1-cKO embryos are indicated by read counts in the sequence analyses. (B), Volcano plot of RNA-seq data of PGCs from the control and Dnd1-cKO embryos at E11.5. Red: p < 0.05, fold change > 1.5; blue: p < 0.05, fold change < 1.5. (C) PCA of the transcriptomes of PGCs from control and Dnd1-cKO mice at E11.5. Each dot represents a single transcriptome. Transcriptomes from five biological replicates are plotted. (D), PCA of the transcriptomes of PGCs in testes from control (Dnd1flox/flox; Nanog-GFP) and Dnd1-cKO (Dnd1flox/flox; Nanog-GFP; Rosa26-CreERT2) embryos at E12.5 and E13.5. Each dot represents a single transcriptome. Transcriptomes from three replicates were plotted. (E, F), Heatmap representation of the expression and GO enrichment of genes upregulated (E) and downregulated (F) in PGCs from Dnd1-cKO embryos compared to those from control embryos at E12.5 and E13.5. The heatmap was generated using the ‘heatmap.2’ function in the gplot package (ver.3.1.3, https://rdocumentation.org/packages/gplots/versions/3.1.3) after performing gene clustering using ‘dist’, ‘hclust’, and ‘cutree’ functions in the stats package (ver.3.6.2, https://rdocumentation.org/packages/stats/versions/3.6.2).
Therefore, we further analysed the transcriptomic changes by comprehensive microarray analyses using PGCs isolated from three independent male Dnd1-cKO and control embryos at E12.5 and E13.5. PCA analysis showed that Dnd1-cKO PGCs were separated from those of control at E12.5 and then progressed in opposite directions from control PGCs as the embryonic stage progress to E13.5, suggesting that the transcriptional profile in these mutant PGCs continue to diverge from the normal trajectory (Fig. 6D). To further investigate these differences, we extracted significant DEGs in Dnd1-cKO PGCs by using a threshold of 1.5 fold change as the cut-off value and subsequently clustered them (Fig. 6E, F, and Supplementary Table S2). Clusters 1 and 2 contained upregulated DEGs, in which the expression of the DEGs in cluster 1 was downregulated from E12.5 to E13.5, whereas the DEGs in cluster 2 were upregulated at E13.5, compared to E12.5 (Fig. 6E). GO analysis of the DEGs in cluster 1 revealed an enrichment of genes mainly involved in chemotaxis, suggesting that the migratory character of early PGCs is highly retained in Dnd1-cKO PGCs at E12.5 and down-regulated slowly to E13.5, compared with control PGCs. In contrast, the DEGs in cluster 2 were enriched for GO terms associated with multicellular organism development and cell differentiation, which included genes such as Otx2, Sox3, Lefty1, Fgf5 and Fgf15. These genes are also known as markers for the primed state of pluripotency34,35,36, suggesting that PGCs may be inclined to become pluripotent ECCs in the absence of DND1.
On the contrary, down-regulated DEGs were also divided into two clusters (Fig. 6F). GO analysis revealed that DEGs in cluster 3 were enriched for GO terms associated with the meiotic cell cycle, piRNA metabolic processes, and spermatogenesis. In contrast, GO terms were not significantly enriched in cluster 4. In the normal development of PGCs, the expression of meiotic genes such as Sycp3, Syce2, Spo11, Meiob, and Meikin gradually increases in both male and female PGCs after colonising embryonic gonads and is highly upregulated only in female PGCs after E14.5 (Supplementary Fig. S5A). However, male PGCs also show increased expression of these genes at relatively lower levels after E14.5 (Supplementary Fig. S5B), and subsequently begin to express genes involved in piRNA metabolic processes and spermatogenesis such as Piwil1 and Nanos237. Therefore, these data indicated that PGCs failed to enter the normal developmental pathway in male PGCs.
Collectively, these analyses support our hypothesis that in the absence of Dnd1, PGCs fail to undergo sexual differentiation, retain early PGC characteristics, and begin to enter the transformation pathway to ECC.
Comparison of transcriptomic changes between Dnd1-cKO and Ter mutant PGCs
Lastly, to further investigate the cause for transformation of ECC from PGC, we attempted to reveal common transcriptomic changes in Dnd1-cKO and Ter mutant PGCs. To this end, we extracted DEGs in the Dnd1Ter/Ter PGCs at E12.5 and E13.5 from the RNA-seq results reported by Ruthig et al.14 using a threshold of 1.5 fold change as the cut-off value, and compared them with the DEGs in the Dnd1-cKO PGCs. These analyses identified 384 and 228 genes commonly up and downregulated in both Dnd1Ter/Ter and Dnd1-cKO PGCs, respectively (Fig. 7A, B, and Supplementary Table S2). GO analysis revealed that the commonly downregulated genes were enriched for GO terms associated with the meiotic cell cycle, spermatogenesis, and piRNA metabolic process (Fig. 7C), which were consistent with the GO terms of downregulated DEGs in Dnd1-cKO PGCs, indicating that failure of sexual differentiation is a common feature of PGCs before the transformation to ECCs. On the other hand, the commonly upregulated genes were enriched for GO terms mainly associated with apoptosis, reflecting intensive apoptotic cell death in both mutant PGCs. In addition, we found a GO term associated with SMAD protein signal transduction (Fig. 7D), indicating that TGF-β superfamily signaling is active in both Dnd1Ter/Ter and Dnd1-cKO PGCs immediately before transformation to ECCs. As a previous study reported that TGF-β superfamily signaling was also active in ECCs13, these results, therefore, raise a possibility that this signaling promotes the transformation of PGCs to ECCs.
Comparison of transcriptomic changes between Dnd1-cKO and Dnd1Ter/Ter PGCs. (A, B), The DEGs upregulated (A) and downregulated (B) in Dnd1-cKO PGCs at E12.5 and E13.5 are compared with those in Dnd1Ter/Ter PGCs reported by Ruthig et al., respectively. (C, D), GO analysis of commonly upregulated (C) and downregulated (D) DEGs in both Dnd1-cKO and Dnd1Ter/Ter PGCs.
Discussion
In this study, we showed that deletion of Dnd1 from migrating PGCs led to the accumulation of PGCs that failed to undergo sexual differentiation in the embryonic testes and found that ECCs were visibly developed from a portion of them after E16.5. Our transcriptomic analyses confirmed the failure of sexual differentiation and additionally revealed the upregulation of marker genes for the primed state of pluripotency, suggesting that PGCs are prone to becoming pluripotent ECCs in the testes of Dnd1-cKO embryos. In addition, we compared the DEGs in Dnd1-cKO PGCs with those in Dnd1Ter/Ter PGCs and found enrichment of genes associated with a GO term of SMAD protein signal transduction in the commonly upregulated DEGs, suggesting the involvement of TGF-β signaling pathway in the ECC development. However, it remains unclear how only a portion of these PGCs is selected as ECCs. One possibility is that the interaction with specific tissue structures, such as basement membranes and mesonephros, and the paracrine factors from them would provide niches for PGCs to transform into ECCs. In this model, only PGCs in the niche can be transformed into ECCs. Alternatively, considering that ECC development is similar to that of induced pluripotent stem cells (iPSCs) in the context of dedifferentiation into a pluripotent state, there might be a subgroup of PGCs transcriptomically close to ECCs, from which ECCs might develop stochastically as well as iPSCs38. In either case, comparative transcriptomic analyses of PGCs transforming into ECCs and PGCs that do not transform into ECCs through the timing of ECC development would be an effective method for analysing the mechanism of ECC development. In this context, our Dnd1-cKO mouse line can be a useful tool for performing single-cell RNA sequence analyses of PGCs transformed into ECCs in the future.
We have previously shown that when Dnd1 was deleted by tamoxifen injection at E13.5, PGCs failed to undergo male-type differentiation and were subsequently removed by apoptotic cell death, despite a comparable expression of NANOS2 with that of control PGCs16. This was probably due to NANOS2 dysfunction because DND1 is an essential partner for NANOS2 to regulate the male-type differentiation of PGCs in embryonic testes. Therefore, we presumed that even when Dnd1 was removed from PGCs at migrating stages, small testes without germ cells could be observed due to NANOS2 dysfunction, as in the deletion of Dnd1 at E13.5. However, we were surprised to find that PGCs transformed into ECCs and then generated teratomas in adult testes, which was never observed with the loss of Dnd1 at E13.5. These data indicate that the deletion of Dnd1 at the migrating stages makes PGCs more prone to transformation into ECCs than the deletion of Dnd1 at E13.5. Given that ECCs are derived from PGCs that fail to undergo sexual differentiation, retaining the characteristics of migrating PGCs, the timing of Dnd1 loss at E13.5 may be too late for transformation into ECCs since PGCs begin sexual differentiation by upregulating NANOS2 expression. In fact, it was recently reported that ECCs are derived from PGCs that do not express NANOS2 during embryonic testes15. Alternatively, the functions of DND1 in migrating PGCs may be different from those in PGCs that colonise the embryonic testes. Since only NANOS3, but not NANOS2, is expressed in migrating PGCs and DND1 is directly associated with NANOS3 in vitro16, NANOS3 may be a partner for DND1 in migrating PGCs. Therefore, the difference in target RNAs between the NANOS3-DND1 complex and the NANOS2-DND1 complex might generate the stage-dependent functional difference in DND1, resulting in susceptibility to STTs in the deletion of Dnd1 at migrating stages. However, as DND1 is also implicated in mRNA protection from miRNA-mediated RNA degradation in other contexts, NANOS-independent DND1 function might play an important role in the suppression of STTs in migrating PGCs. Identification of RNAs associated with DND1 and DND1-partner protein in migrating PGCs is required to understand the mechanisms of transformation from PGC to ECC.
Mouse STTs occur predominantly on a genetic background of the 129 family of inbred mouse strains; although several kinds of gene mutation have been reported to increase the frequency of STT development in mice, STTs occurred only after introducing these mutations into a 129 strain genetic background by crossbreeding with 129 strain mice5,6,7,8,9. Therefore, genetic factors specific to the 129 strains, in addition to these gene mutations, is critical for the occurrence of STTs. However, the genetic factors specific to the 129 strains remains unidentified, making it difficult to analyse the mechanisms underlying the development of STTs on the 129 strain genetic background. In this study, we found that the conditional deletion of Dnd1 in migrating PGCs caused STTs at a frequency of almost 100%, which was independent of the genetic factor of the 129 strains because the Dnd1-cKO mice were generated without 129 strain mice9,16. This result may indicate that STTs are solely caused by the loss of Dnd1 in migrating PGCs. However, it is possible that the genetic background of the Dnd1-cKO mouse line itself has some factors that stimulate the generation of STTs. In fact, the ratio of the number of PGC in the cell cycle phase other than G0 (Fig. 4M, 24.01% in control) and the efficiency of EGC formation derived from PGC (Fig. 5H, 7.99% in control at E13.5) were both considerably higher than those previously described31,39. These data suggest that PGC development is delayed in this mouse line, leading to STT development at a high frequency13; however, we cannot exclude the possibility that the floxed alleles of Dnd1 have some effect on the expression of DND1, thereby resulting in these phenomena. More critically, conventional deletion of Dnd1 in the same genetic background of the Dnd1-cKO mouse line caused STTs, with a high probability of approximately 60% (Supplementary Fig. S6). This data indicates that some genetic factor stimulating STT development are present in the Dnd1-cKO mouse line, because C57BL/6J and MCH/ICR mice did not develop STTs or developed STTs at a very low frequency, even after the conventional deletion of Dnd19. Therefore, unidentified genetic factors specific to the Dnd1-cKO mouse line might contribute to STT development, along with the deletion of Dnd1 in migrating PGCs. To elucidate the effect of Dnd1 loss on STT development in more detail, assays for STT development in the Dnd1-cKO mice should be performed on various genetic backgrounds. Alternatively, if other genetic factors are involved in the onset of STT in the Dnd1-cKO mouse line, identification of these factors using forward genetics should be considered to elucidate the mechanisms of STT development.
Methods
Animal experiments
All mouse experiments were conducted according to the protocol approved by the President of Yokohama National University after review by the Institutional Animal Care and Use Committee (Approval No. 2022-04). All methods were carried out in accordance with relevant guidelines and regulations. All methods are reported in accordance with ARRIVE guidelines.
Mice
Dnd1-flox, Oct4ΔPE-CreERT2, and Rosa26-CreERT2 mice have been previously described9,16. In brief, the Dnd1_flox mouse line was established by using TT2 ES cells and maintained via intercrosses generating Dnd1flox/flox mice, while the Oct4ΔPE-CreERT2 transgenic mouse line was generated by injecting the transgene into fertilised eggs of B6C3F1 mice and maintained by crossing with MCH/ICR mice. The Rosa26-CreERT2 mouse line was maintained on a genetic background of MCH/ICR strain.
The transgenic mouse line CAG-CAT-EGFP40 was provided by Dr. Junichi Miyazaki and maintained on a MCH/ICR genetic background, while Nanog-GFP transgenic mouse line21 was provided by RIKEN BRC through the National Bio-Resource Project of MEXT, Japan, on a mixed background of C57BL/6J and DBA, and subsequently crossed with the Dnd1flox/flox mice. The PCR primer pair used for genotyping these lines was as follows: EGFP-Fw (5′-CTCGTGACCACCCTGACCTA-3′) and EGFP-Rv (5′-GTCCATGCCGAGAGTGATCC-3′).
Tamoxifen treatment
Tamoxifen (T006000, Toronto Research Chemicals Inc., Canada) was dissolved in sesame oil (S3547, Sigma Aldrich, CA) at 20 mg/ml in a light-blocking tube at 55 °C. After filtration, the solution was administered to the pregnant mice in a single oral dose of 3 mg per mouse at E9.5 and E10.5, or 5 mg per mouse at E11.5 and E12.5.
Haematoxylin and eosin staining
Testes were fixed with Bouin’s solution overnight, embedded in paraffin, and sectioned at 6 μm. After deparaffinisation, the sections were stained with haematoxylin and eosin according to standard procedures. Observations were performed under a microscope (AxioImager.M2; Carl Zeiss, Germany) equipped with a camera (Eos Kiss X2; Canon, Japan).
Immunostaining
Embryonic testes were fixed with 4% paraformaldehyde overnight, embedded in paraffin, and sectioned at 4 µm. After deparaffinisation, the sections were autoclaved for 15 min at 105 °C with Antigen Unmasking Solution (H-3300, Vector Laboratories, CA) followed by blocking with 5% skim milk for 30 min, and then incubated overnight at 4 °C with primary antibodies against DAZL (1:2,000) produced by a guinea pig16, DND1 (1: 2,000) produced by a guinea pig16, NANOS2 (1:200) produced by a rabbit22, GFP (1: 1000; NB100-1770, Novus Biological, CO), NANOG (1:1000; IHC-00205, BETHYL Laboratories, MA), OCT4/POU5F1 (1:2000; sc-5279, Santa Cruz, TX), OTX2 (1:2,400; EPR20375, Abcam, UK), KI67 (1:2,000; RM-9106-S0, Thermo Fisher Scientific, MA), LAMININ (1:1,000; LAM-1, ICN Biomedicals, PA), cleaved CASPASE3 (1: 900; Asp175, Cell Signaling Technology, MA), or SYCP3 (1: 400; a gift from Dr. Chuma)41.
After washing with primary antibodies, the sections were incubated with donkey anti-guinea pig, anti-goat, anti-mouse, or anti-rat IgG conjugated with Aquamarine, Alexa488, or Alexa594 (1:500) (Jackson ImmunoResearch Laboratories Inc., PA, USA). Each section was counterstained with 0.5 μg/ml 4′,6-diamidino-2-phenylindole (DAPI) for 7 min, enclosed in Fluoromount™ (Diagnostic Biosystems Inc., CA), and then observed using fluorescence microscopy (AxioImager.M2; Carl Zeiss) and a CCD camera (AxioCam; Carl Zeiss). All antibodies were diluted with Can Get Signal Immunoreaction Enhancer Solution (Toyobo, Japan).
Surveys for ECC colonies
Testes were excised from control and Dnd1-cKO embryos at E16.5, E17.5, and E18.5, and surveyed for ECC development by GFP fluorescence under a stereomicroscope (SZX16; Olympus, Japan) equipped with a CCD camera (AxioCam; Carl Zeiss). The incidence of ECC development was calculated as the percentage of male embryos in at least one ECC colony. Section immunostaining analysis using antibodies against GFP and NANOG was used to confirm ECC colonies that were ambiguous during the observation of whole testes.
FACS analysis
Single-cell suspensions were prepared from the testes of embryos by incubation with Accutase (SF006, Merck Millipore, MA) at 37 °C for 40 min. After incubation, 10% newborn calf serum (S0750; Biowest, France) was added. After centrifugation at 1,800 rpm for 5 min, cells were re-suspended in sorting medium containing 2% newborn calf serum, 1% Glutamax (35,050,061, Thermo Fisher Scientific), 1% non-essential amino acids (11,140,050, Thermo Fisher Scientific), and DNA was labelled with 7-AAD (Beckman Coulter, CA, USA). FACS was performed using a Cell Sorter (MoFlo Astrios, Beckman Coulter, CA, USA).
EGC culture
EGC was established as described previously42. Briefly, sorted PGCs were cultured on Mitomycin C (Kyowa Kirin, Japan)-inactivated Sl/Sl4-m220 feeder cells43 in DMEM (D6429, Sigma-Aldrich) supplemented with 15% KSR (10828010, Thermo Fisher Scientific), 1% Glutamax (35050061, Thermo Fisher Scientific), 1% non-essential amino acids (11140050, Thermo Fisher Scientific), 0.1 mM β-mercaptoethanol (198-15781, FUJIFILM Wako, Japan), 1000 unit/mL mLIF (ESG1107, Merck Millipore), and 12 ng/mL bFGF (450-33, PeproTech, NJ) until day 3. On day 3, the media was replaced with fresh media containing 1 μM PD325901 (04-0006, ReproCELL), 1 μM CHIR99021 (04-0004, ReproCELL, Japan) and 250 nM A83-01(039-24111, FUJIFILM Wako, Japan).
Cells were cultured under 20% O2 and 5% CO2 at 37 °C and the media were refreshed after 1, 3, and 5 days of culture. The efficiency of colony formation was determined after 7 days in culture as the number of colonies per seeded cell in a culture well.
RNA-seq analysis
RNA-seq analyses were performed according to a method called Quartz-seq as previously described33. In brief, 100 PGCs from the testes of control and Dnd1-cKO embryos at E11.5 were directly sorted in a lysis buffer, and then reverse transcription (RT) was performed using Super Script III (12574018, Thermo Fisher Scientific) and an RT primer containing oligo-dT, T7 promoter, and PCR target region sequences. After removal and digestion of the RT primers using AMPure XP (A63880, Beckman Coulter) and exonuclease (2650, Takara Bio, Japan), a poly-A tail was added to the 3′ ends of the first-strand cDNA using terminal transferase (03289869103, Roche, Switzerland). Second-strand DNA was then synthesised using MightyAmp DNA polymerase (R076, Takara Bio) and a tagging primer. PCR amplification was performed using suppression primers. The amplified double-stranded cDNA was purified using a MinElute PCR Purification Kit (28004; Qiagen, Netherlands). Library preparation for RNA-seq was performed by ligation-based Illumina multiplex library preparation (LIMprep), as previously described33. Next-generation sequencing of cDNA libraries was performed using an Illumina HiSeq1500 (Illumina, CA).
Read files were mapped to the mouse reference genome (GRCm38 p6) using STAR aligner44 and only uniquely mapped reads were used for expression level estimation. Further analyses were conducted using R software (ver. 4.1.1). Gene counts were obtained using the ‘featureCounts’ function in the Rsubread package with ENSEMBL gene annotation (release98) and the genes showing log2(RPM + 1) values > 4 [greater than = 10–20 copies per cell]45 in at least one sample were used. The principal component analysis (PCA) was performed using the ‘prcomp’ function in the stats package (ver.3.6.2, https://rdocumentation.org/packages/stats/versions/3.6.2) with scaling. Gene ontology analysis was performed using DAVID as previously described46.
Microarray analysis
Total RNA was extracted from sorted 120,000 PGCs in the testes of control and Dnd1-cKO embryos at E12.5 and E13.5, using the RNeasy Plus Micro kit (74034, Qiagen) according to the manufacturer’s instructions. The quality of the purified total RNA was verified using an Agilent 2100 Bioanalyzer (Agilent Technologies, CA). Conversion of isolated total RNA into cDNA and labelling of cDNA by cyanine-3 were performed using a Low Input Quick Amp Labelling Kit (One-Colour) (5190-2305, Agilent Technologies, CA). Labelled cDNA was fragmented, hybridised to SurePrint G3 Mouse GE 8 × 60 K v2 Microarray (Agilent Technologies) for 17 h at 65 °C and washed by using Gene Expression Hybridisation Kit and Gene Expression Wash Pack (Agilent Technologies). The microarray was scanned using Agilent SureScan G2600D, and the scan image data were analysed using Feature Extraction (11.5.1.1) software.
The raw gene expression data were processed using the limma package (ver. 3.28.14, https://www.rdocumentation.org/packages/limma/versions/3.28.14) for R (ver. 4.1.1). Briefly, raw data were first read using the ‘read.maimages’ function; then, background correction of microarray expression intensities and log2 transformation were performed using ‘backgroundCorrect’ and ‘normalizeBetweenArrays’ functions. After normalisation, control probes, probes with no gene symbols, and probes that were above the background on less than three arrays were filtered out. To perform gene-level interpretation, whole probes were collapsed into gene symbols by taking the maximum intensity among probe sets targeting the same gene. Differential gene expression analysis was performed using ‘lmFit’ and’ eBayes’ functions. Gene expression changes of > 1.5-fold and p value < 0.05 were considered significant. The principal component analysis (PCA) was performed using ‘prcomp’ function. GO analysis was conducted using DAVID, as previously described46 and GO terms with p values less than 1.0E-4 were considered significantly enriched. The heatmap was generated using the ‘heatmap.2’ function in the gplot package (ver.3.1.3, https://rdocumentation.org/packages/gplots/versions/3.1.3) after performing gene clustering using ‘dist’, ‘hclust’, and ‘cutree’ functions in the stats package. A Venn diagram were generated using the ‘venn.diagram’ function in the VennDiagram package (ver.1.7.3, https://www.rdocumentation.org/packages/VennDiagram/versions/1.7.3).
Statistical analysis
The statistical significance of the differences in the ratios of cleaved CASPASE3-positive germ cells and KI67-positive germ cells and the efficiency of EGC formation was assessed using two-tailed t-tests. Statistical significance was set at p < 0.05. Statistical analyses were performed using Microsoft Excel.
Data availability
The datasets analysed during the current study are available in the GEO repository; https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE218770 for RNA-seq analysis, and https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE218744 for microarray analysis.
References
Stevens, L. C. & Little, C. C. Spontaneous testicular teratomas in an inbred strain of mice. Proc. Natl. Acad. Sci. USA 40, 1080–1087 (1954).
Stevens, L. C. & Hummel, K. P. A description of spontaneous congenital testicular teratomas in strain 129 mice. J. Natl. Cancer Inst. 18, 719–747 (1957).
Stevens, L. C. A new inbred subline of mice (129-terSv) with a high incidence of spontaneous congenital testicular teratomas. J. Natl. Cancer Inst. 50, 235–242 (1973).
Noguchi, M. et al. The ter mutation responsible for germ cell deficiency but not testicular nor ovarian teratocarcinogenesis in ter/ter congenic mice. Dev. Growth Differ. 38, 59–69 (1996).
Youngren, K. K. et al. The ter mutation in the dead end gene causes germ cell loss and testicular germ cell tumours. Nature 435, 360–364 (2005).
Krentz, A. D. et al. Interaction between DMRT1 function and genetic background modulates signaling and pluripotency to control tumor susceptibility in the fetal germ line. Dev. Biol. 377, 67–78 (2013).
Schemmer, J. et al. Transcription factor TFAP2C regulates major programs required for murine fetal germ cell maintenance and haploinsufficiency predisposes to teratomas in male mice. PLoS ONE 8, e71113 (2013).
Nicholls, P. K. et al. Mammalian germ cells are determined after PGC colonization of the nascent gonad. Proc. Natl. Acad. Sci. USA 116, 25677–25687 (2019).
Imai, A. et al. Mouse Dead end1 acts with Nanos2 and Nanos3 to regulate testicular teratoma incidence. PLoS ONE 15, e0232047 (2020).
Matin, A., Collin, G. B., Asada, Y., Varnum, D. & Nadeau, J. H. Susceptibility to testicular germ-cell tumours in a 129.MOLF-Chr 19 chromosome substitution strain. Nat. Genet. 23, 237–240 (1999).
Miyazaki, T. et al. Identification of genomic locus responsible for experimentally induced testicular teratoma 1 (ett1) on mouse Chr 18. Mamm. Genome 25, 317–326 (2014).
Miyazaki, T. et al. Identification of two additional genomic loci responsible for experimentally induced testicular teratoma 2 and 3 (ett2 and ett3). Zool. Sci. 35, 172–178 (2018).
Dawson, E. P. et al. Delayed male germ cell sex-specification permits transition into embryonal carcinoma cells with features of primed pluripotency. Development 145, dev156612 (2018).
Ruthig, V. A. et al. The RNA-binding protein DND1 acts sequentially as a negative regulator of pluripotency and a positive regulator of epigenetic modifiers required for germ cell reprogramming. Development 146, dev175950 (2019).
Webster, N. J. et al. Testicular germ cell tumors arise in the absence of sex-specific differentiation. Development 148, dev197111 (2021).
Suzuki, A. et al. Dead end1 is an essential partner of NANOS2 for selective binding of target RNAs in male germ cell development. EMBO Rep. 17, 37–46 (2016).
Ruthig, V. A. et al. A transgenic DND1GFP fusion allele reports in vivo expression and RNA-binding targets in undifferentiated mouse germ cellsdagger. Biol. Reprod. 104, 861–874 (2021).
Cook, M. S., Coveney, D., Batchvarov, I., Nadeau, J. H. & Capel, B. BAX-mediated cell death affects early germ cell loss and incidence of testicular teratomas in Dnd1(Ter/Ter) mice. Dev. Biol. 328, 377–383 (2009).
Niimi, Y. et al. Essential role of mouse Dead end1 in the maintenance of spermatogonia. Dev. Biol. 445, 103–112 (2019).
Chen, Y., Du, Z. & Yao, Z. Roles of the Nanog protein in murine F9 embryonal carcinoma cells and their endoderm-differentiated counterparts. Cell Res. 16, 641–650 (2006).
Okita, K., Ichisaka, T. & Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 448, 313–317 (2007).
Suzuki, A., Tsuda, M. & Saga, Y. Functional redundancy among Nanos proteins and a distinct role of Nanos2 during male germ cell development. Development 134, 77–83 (2007).
Stevens, L. C. Origin of testicular teratomas from primordial germ cells in mice. J. Natl. Cancer Inst. 38, 549–552 (1967).
Bustamante-Marin, X., Garness, J. A. & Capel, B. Testicular teratomas: An intersection of pluripotency, differentiation and cancer biology. Int. J. Dev. Biol. 57, 201–210 (2013).
Suzuki, A. & Saga, Y. Nanos2 suppresses meiosis and promotes male germ cell differentiation. Genes Dev. 22, 430–435 (2008).
Ortega, S. et al. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat. Genet. 35, 25–31 (2003).
Gonzalez-Sanz, S., Barrenada, O., Rial, E., Brieno-Enriquez, M. A. & Del Mazo, J. The antiandrogenic vinclozolin induces differentiation delay of germ cells and changes in energy metabolism in 3D cultures of fetal ovaries. Sci. Rep. 10, 18036 (2020).
Suzuki, H., Tsuda, M., Kiso, M. & Saga, Y. Nanos3 maintains the germ cell lineage in the mouse by suppressing both Bax-dependent and -independent apoptotic pathways. Dev. Biol. 318, 133–142 (2008).
Matsui, Y., Zsebo, K. & Hogan, B. L. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell 70, 841–847 (1992).
Resnick, J. L., Bixler, L. S., Cheng, L. & Donovan, P. J. Long-term proliferation of mouse primordial germ cells in culture. Nature 359, 550–551 (1992).
Matsui, Y. & Tokitake, Y. Primordial germ cells contain subpopulations that have greater ability to develop into pluripotential stem cells. Dev. Growth Differ. 51, 657–667 (2009).
Matsui, Y. et al. The majority of early primordial germ cells acquire pluripotency by AKT activation. Development 141, 4457–4467 (2014).
Sasagawa, Y. et al. Quartz-Seq: A highly reproducible and sensitive single-cell RNA sequencing method, reveals non-genetic gene-expression heterogeneity. Genome Biol. 14, R31 (2013).
Kurek, D. et al. Endogenous WNT signals mediate BMP-induced and spontaneous differentiation of epiblast stem cells and human embryonic stem cells. Stem Cell Rep. 4, 114–128 (2015).
Hackett, J. A. & Surani, M. A. Regulatory principles of pluripotency: From the ground state up. Cell Stem Cell 15, 416–430 (2014).
Boroviak, T. et al. Lineage-specific profiling delineates the emergence and progression of naive pluripotency in mammalian embryogenesis. Dev. Cell 35, 366–382 (2015).
Saga, Y. How germ cells determine their own sexual fate in mice. Sex Dev., 1–13 (2022).
Hochedlinger, K. & Jaenisch, R. Induced pluripotency and epigenetic reprogramming. Cold Spring Harb. Perspect. Biol. 7 (2015).
Shimada, R., Koike, H., Hirano, T., Kato, Y. & Saga, Y. NANOS2 suppresses the cell cycle by repressing mTORC1 activators in embryonic male germ cells. Science 24, 102890 (2021).
Kawamoto, S. et al. A novel reporter mouse strain that expresses enhanced green fluorescent protein upon cre-mediated recombination. FEBS Lett. 470, 263–268 (2000).
Chuma, S. & Nakatsuji, N. Autonomous transition into meiosis of mouse fetal germ cells in vitro and its inhibition by gp130-mediated signaling. Dev. Biol. 229, 468–479 (2001).
Nagamatsu, G. & Suda, T. Conversion of primordial germ cells to pluripotent stem cells: Methods for cell tracking and culture conditions. Methods Mol. Biol. 1052, 49–56 (2013).
Matsui, Y. et al. Effect of Steel factor and leukaemia inhibitory factor on murine primordial germ cells in culture. Nature 353, 750–752 (1991).
Dobin, A. et al. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Nakamura, T. et al. SC3-seq: A method for highly parallel and quantitative measurement of single-cell gene expression. Nucleic Acids Res. 43, e60 (2015).
da Huang, W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Heaney, J. D. et al. Germ cell pluripotency, premature differentiation and susceptibility to testicular teratomas in mice. Development 139, 1577–1586 (2012).
Acknowledgements
We thank S. Chuma and J. Miyazaki for providing antibodies against SYCP3 and a CAG-CAT-EGFP transgenic mouse line, respectively. We also thank M. Torimoto, K. Suzuki, A. Sakai, and L. Xu for their technical assistance with EGC culture, and S. Shigenobu, K. Yamaguchi, and A. Akita for their technical assistance with RNA-seq analysis. This work was supported by the Naito Foundation, NIBB Collaborative Research Program (16-414), and Japan Society for the Promotion of Science (KAKENHI grant numbers 17H05046 and 22K06040) to A. Suzuki.
Author information
Authors and Affiliations
Contributions
A.S. developed the concept of the study. A.I., K.M., and Y.N. performed the histological analyses. A.I. and Y.N. conducted the EGC culture. A.I., K.M., and Y.N. carried out RNA-seq and microarray analyses, respectively. A.S. wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Imai, A., Matsuda, K., Niimi, Y. et al. Loss of Dead end1 induces testicular teratomas from primordial germ cells that failed to undergo sexual differentiation in embryonic testes. Sci Rep 13, 6398 (2023). https://doi.org/10.1038/s41598-023-33706-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-33706-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.