Abstract
Tinnitus is the perception of a ringing, buzzing or hissing sound “in the ear” without external stimulation. Previous research has demonstrated changes in resting-state functional connectivity in tinnitus, but findings do not overlap and are even contradictory. Furthermore, how altered functional connectivity in tinnitus is related to cognitive abilities is currently unknown. Here we investigated resting-state functional connectivity differences between 20 patients with chronic tinnitus and 20 control participants matched in age, sex and hearing loss. All participants underwent functional magnetic resonance imaging, audiometric and cognitive assessments, and filled in questionnaires targeting anxiety and depression. Significant differences in functional connectivity between tinnitus patients and control participants were not obtained. However, we did find significant associations between cognitive scores and functional coupling of the default mode network and the precuneus with the superior parietal lobule, supramarginal gyrus, and orbitofrontal cortex. Further, tinnitus distress correlated with connectivity between the precuneus and the lateral occipital complex. This is the first study providing evidence for disruptions of default mode network and precuneus coupling that are related to cognitive dysfunctions in tinnitus. The constant attempt to decrease the tinnitus sensation might occupy certain brain resources otherwise available for concurrent cognitive operations.
Similar content being viewed by others
Introduction
Tinnitus is mostly perceived as ringing, buzzing or hissing “in the ear” without external stimulation and affects 10 to 25% of the adult population and as many as 45% of the elderly population1,2,3,4. Chronic tinnitus significantly impacts quality of life and mental health as it comes with increased distress, fatigue, depression and anxiety, and may also result in problems with sleeping and concentration5,6.
Previous studies have applied resting-state functional magnetic resonance imaging [fMRI] to explore functional abnormalities in tinnitus patients. During a resting-state measurement, participants do not perform a specific task, but their brains are scanned while they are in a so-called ‘resting-state’. Thereby spontaneous low frequency fluctuations (< 0.1 Hz) in the BOLD signal are assessed to identify resting-state networks7. This enables the analysis of functional connectivity—the temporal correlation of the BOLD response time courses of two brain regions that may be anatomically separate but are functionally related. Resting-state functional connectivity is particularly interesting within the framework of tinnitus because the perception of tinnitus can potentially be better determined without a distracting task. Up to 85% of chronic tinnitus patients perceive the tinnitus sensation permanently [though possibly at varying strength] and, therefore, resting-state fMRI measurements seem suitable to capture this perception8.
Although there are some studies on changes in resting-state functional connectivity in tinnitus patients, findings are scarce and vary in their outcomes. Briefly, these studies show that tinnitus is related to an atypical functional network comprising auditory and non-auditory brain regions9. Further, tinnitus is associated with decreases as well as increases in functional connectivity in frontal, parietal, cerebellar, auditory and limbic regions9,10,11,12,13,14. Similarly, typical resting-state networks, like default mode and dorsal attention networks, as well as their connections with the precuneus, are affected by tinnitus15,16,17,18. Additionally, tinnitus distress correlated with decreased functional connectivity between middle temporal gyrus and middle frontal gyrus19 and with increased functional connectivity between middle frontal gyrus and the precuneus20. A recent meta-analysis identified consistently increased resting-state functional connectivity in the insula, middle temporal gyrus, inferior and superior frontal gyri, parahippocampal gyrus and cerebellum, as well as decreased resting-state connectivity in the cuneus und thalamus21. Although presenting a relatively heterogeneous picture, these studies suggest an interaction of multiple brain areas and networks that are involved in tinnitus perception, and relate differently to tinnitus distress such as anxiety or depression.
Along alterations in resting-state functional connectivity, previous research provided evidence for cognitive deficits, such as reduced control of attention22,23,24, altered inhibitory control24,25,26, and a decline in general cognitive abilities like short-term memory, concentration and orientation27. Hence, it seems that tinnitus affects specifically those cognitive abilities that require executive control of attention and inhibition28,29. A failure in top-down cognitive control may result in a diminished ability to switch attention away from the tinnitus signal, and therefore the awareness of the tinnitus signal is maintained or even increased24. However, how cognitive abilities are associated with changes in resting-state functional connectivity in tinnitus has not been investigated. A complicating factor in previous research is that many studies did not control for age and hearing loss and thus it is not clear which underlying functional connectivity changes relate to increasing age, hearing impairment or solely to development of chronic tinnitus. Hence, the question of which pathophysiological mechanisms in the human brain are involved in tinnitus, still needs to be answered. Completing the clinical profile of tinnitus patients by investigating cognitive abilities and their relationship to functional brain alterations may play a crucial role in evaluating and advancing tinnitus interventions and treatment options.
The aim of the current study was to investigate resting-state functional connectivity changes associated with the tinnitus signal but also in relation to tinnitus distress and general cognitive abilities. Based on the above results, we hypothesized that tinnitus would disrupt functional resting-state connectivity in auditory cortex, thalamic, limbic, and prefrontal brain regions9,10,11,12,13 as well as in typical resting-state networks, like default-mode and dorsal attention networks, as well as their connections with the precuneus15,16,17,18. Further, we expected that tinnitus patients exhibit deficits in working memory and may also present with general cognitive decline22,23,24,25,27,29,30. These decreased cognitive abilities may be reflected in decreased resting-state connectivity between the default mode and dorsal attention networks16,24. Moreover, we hypothesized that tinnitus distress is related to decreased functional resting-state connectivity between right middle temporal gyrus and middle frontal gyrus19 and with increased functional connectivity between middle frontal gyrus and the precuneus20.
Results
Behavioral assessments
Mean values for the MoCA were 27.1 (± 2.14) for the tinnitus patients and 26.9 (± 2.37) for the control participants. The mean performance in the two-back task was 89.7 (± 7.6) % in the tinnitus group and 86.6 (± 7.1) % in the control participants. No significant differences between the groups were obtained for any of the cognitive tasks or in anxiety or depression scores (p > 0.1).
The mean values obtained for the tinnitus assessment questionnaires were 20 (± 11) for the THI and 123 (± 74.7) for the TFI. The mean perceived pitch frequency of the tinnitus was 8 kHz (range 3–12,5 kHz, n = 8 perceived the pitch at 8 kHz and n = 7 at 10 kHz) and the mean perceived tinnitus intensity was 5 dB SL. There was no significant correlation in our sample between tinnitus assessment questionnaires and depression or anxiety scores. Similarly, the values in perceived tinnitus pitch and intensity were not related to any of the depression and anxiety scores (p > 0.1) in our sample. Tinnitus duration ranged from half a year to 50 years (mean duration was 15 years) and did not significantly correlate with any of the behavioral scores. Three patients reported a pulsatile tinnitus, the others reported no pulsation. Four patients indicated their tinnitus sounded like wide-band, high-frequency noise; the others indicated they perceived it as a tone. Four tinnitus patients reported unilateral tinnitus, all others reported a bilateral tinnitus sensation.
Resting-state functional connectivity
For the resting-state functional connectivity analysis, two different analyses were conducted. First, a between-group comparison was computed to determine differences between tinnitus patients and control participants. Second, a linear regression analysis across tinnitus participants investigating the relation between resting-state functional connectivity and cognitive abilities (MoCA score, two-back task performance) as well as tinnitus distress (THI and TFI scores) was conducted.
In the between-group comparison, we did not obtain a significant difference in resting-state functional connectivity between tinnitus patients and control participants.
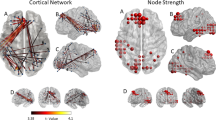
The subsequent multiple regression analysis in the tinnitus group showed several positive associations between resting-state functional connectivity and MoCA scores as well as TFI scores. An overview including peak coordinates is given in Table 1. First, we obtained a significant positive association between MoCA scores and resting-state functional connectivity of the default mode network and (1) left superior parietal lobule (r = 0.809; p < 0.001) and (2) orbitofrontal cortex (r = 0.825; p < 0.001) in tinnitus patients (Fig. 1). Second, a positive correlation between MoCA scores and resting-state functional connectivity of the precuneus and (1) left (r = 0.754; p < 0.001) and (2) right superior parietal lobule (r = 0.755; p < 0.001), (3) orbitofrontal cortex (r = 0.847; p < 0.001), and (4) supramarginal gyrus (r = 0.749; p < 0.001) was found in tinnitus patients (Fig. 2). Lastly, a significant positive relation between TFI scores and resting-state functional connectivity of the precuneus and the right lateral occipital cortex was apparent in the tinnitus group (r = 0.69; p < 0.001; Fig. 3).
Significant association between MoCA scores and resting-state functional connectivity of the default mode network with the left superior parietal lobule (SPL; top row) and the right orbitofrontal cortex (OFC; bottom row) in tinnitus patients [p < 0.05; FWE cluster-corrected threshold]. Significant brain areas were displayed on an inflated brain by CONN version 20b31.
Significant association between MoCA scores and resting-state functional connectivity of the precuneus with the left and right superior parietal lobules (SPL; top row), the right orbitofrontal cortex (OFC; middle row), and the left supramarginal gyrus (SMG; bottom row) in tinnitus patients [p < 0.05; FWE cluster-corrected threshold]. Significant brain areas were displayed on an inflated brain by CONN version 20b31.
Significant association between TFI scores and resting-state functional connectivity of the precuneus with the right lateral occipital cortex (LOC) in tinnitus patients [p < 0.05; cluster-corrected threshold]. Significant brain areas were displayed on an inflated brain by CONN version 20b31.
When correcting for multiple comparisons (Bonferroni correction), only three correlations survived: The correlation between the MoCA and resting-state functional connectivity of (1) the default mode network with the superior parietal lobule and of the precuneus with (2) the orbitofrontal cortex, and (3) with the right superior parietal lobule (this is also indicated in Table 1).
We also computed correlations between cognitive scores and resting-state functional connectivity for the control group. However, no significant correlations were obtained. In order to assess any significant differences between the correlation coefficients of the tinnitus patients and the control participants, we conducted Fisher r-to-z transformations. This analysis demonstrated that correlation coefficients for control participants and tinnitus patients were significantly different from each other for associations of MoCa scores and functional connectivity (all p < 0.05).
Discussion
The aims of this study were threefold: (1) to assess resting-state functional connectivity changes in tinnitus patients compared to control participants, and to investigate resting-state functional connectivity in relation to (2) the patients’ cognitive abilities and to (3) their tinnitus distress. Based on prior work, our expectations were to find disrupted functional resting-state connectivity in tinnitus patients in the following brain regions: auditory cortex, thalamus, limbic, and prefrontal areas9,10,11,12,13, as well as in typical resting-state networks, such as the default mode and dorsal attention networks, along with their connections to the precuneus15,16,17,18. Further, we assumed that tinnitus patients may exhibit deficits in working memory and general cognitive abilities22,23,24,25,27,29,30, which may be reflected in decreased resting-state connectivity between the default mode and dorsal attention networks16,24. Moreover, we hypothesized that tinnitus distress is correlated with decreased functional resting-state connectivity between right middle temporal gyrus and middle frontal gyrus19 along with increased connectivity between middle frontal gyrus and the precuneus20. Indeed, regarding cognitive abilities assessed by the MoCA and tinnitus distress assessed by the TFI, our results showed some positive associations with resting-state functional connectivity in the tinnitus group which were not observed in the control group. We obtained significant positive relations between MoCA scores and resting-state functional connectivity of the default mode network with (1) the left superior parietal lobule and (2) orbitofrontal cortex; and of the precuneus with (3) left and (4) right superior parietal lobule, (5) orbitofrontal cortex and (6) supramarginal gyrus. Further, we were able to demonstrate a significant positive correlation between TFI scores and resting-state functional connectivity of the precuneus and the right lateral occipital cortex in tinnitus patients.
No differences in resting-state functional connectivity between tinnitus and control participants
Our resting-state functional connectivity analysis did not show any significant differences between tinnitus patients and the control group. This is a surprising finding considering the research that has demonstrated disrupted functional resting-state connectivity in auditory cortex, thalamic, limbic, and prefrontal brain regions as well as in default mode and dorsal attention networks9,10,11,12,13,15,16,17,18. Possible reasons for the absence of these effects might be a difference in seed regions10,15, a higher number of participants in other studies10,11,15, groups with unilateral tinnitus (12,16), but also control groups that were not matched for age and hearing loss9,11,13. Another possibility is that different analysis methods [seed-to-voxel, ROI-to-ROI, or independent component analysis] may have yielded differing results. In addition, some studies used an “eyes-open” paradigm and others used an "eyes-closed” paradigm for the resting-state acquisition, which might explain some of the heterogeneous findings32. It has been previously demonstrated that the choice of paradigm significantly impacts resting-state functional connectivity of visual, auditory and sensorimotor networks33. We employed an “eyes-open” approach and our groups were matched in terms of age, sex and hearing loss. However, other patient characteristics, such as extent of hearing loss, tinnitus duration, or severity of tinnitus distress vary across studies32,33. The co-occurrence of hyperacusis might play a relevant role as well34. A recent study on the Human Connectome Project data showed that functional connectivity of the auditory cortex decreases with age and that tinnitus patients presented even less auditory cortex connectivity than their age-matched controls35. Hence, it is essential to carefully match the control groups in future tinnitus research. Another suggestion for future studies might be to include a larger sample size enabling the analysis of specific subtypes of patient groups (for instance, groups with high versus low tinnitus distress). A recent meta-analysis reached a similar conclusion36. Furthermore, using similar acquisition paradigms, seed regions and analysis methods across studies to allow comparability and reproducibility would be beneficial.
General cognitive abilities associated with resting-state functional connectivity of the default mode network and the precuneus
Importantly, we obtained several positive associations between general cognitive abilities assessed by the MoCA and resting-state functional connectivity of the default mode network and the precuneus in tinnitus patients that were not observed in the control participants. The correlations were similar for the default mode network and the precuneus, whose functional connectivity to both orbitofrontal cortex and the superior parietal lobule were associated with general cognitive abilities. Furthermore, resting-state functional connectivity between precuneus and supramarginal gyrus correlated with MoCA values. All of these brain areas have been mentioned in previous tinnitus research. The superior parietal lobule shows less beta activity37, reduced connectivity38, and altered BOLD responses for visual and auditory attentional tasks in tinnitus patients39. Moreover, increased brain activity in the superior parietal lobule has been demonstrated during auditory interference conditions with cognitive conflict in tinnitus patients26. The orbitofrontal cortex is a nexus for sensory integration, modulation of autonomic reactions, as well as emotional and reward-related behaviors40. It functions as part of a network that includes regions of the medial prefrontal cortex, amygdala, insula, and the dopaminergic midbrain projecting to the ventral and dorsal striatum40,41. Thus, this network hub, which is controlled by the orbitofrontal cortex, houses the frontostriatal gating mechanism that has been proposed to reduce the intensity of tinnitus and chronic pain, if it is intact, but enables or exacerbates tinnitus, if it is broken42,43. Other studies have demonstrated increased functional connectivity of the orbitofrontal cortex11,13 and even found an atypical resting-state network involving the orbitofrontal cortex in tinnitus patients44. Moreover, a magnetoencephalographic study revealed differences in global resting-state networks that covered prefrontal, orbitofrontal and parieto-occipital cortex when comparing tinnitus patients to controls45. The supramarginal gyrus has been associated with increased responses in an emotional task in chronic tinnitus compared to tinnitus patients with recent onset46,47 along with increased regional homogeneity in chronic tinnitus patients48,49. Taken together, previous research has suggested that the orbitofrontal cortex, together with the superior parietal lobule and the supramarginal gyrus, might be engaged in compensatory processes attenuating the tinnitus signal. The present study is the first to show a positive relationship between cognitive abilities and changes in resting-state functional connectivity of the default mode network and precuneus to orbitofrontal cortex, superior parietal lobule, and supramarginal gyrus in chronic tinnitus. Decreased coupling was related to lower cognitive scores while tinnitus patients did not show cognitive deficits as a group (mean MoCA score was 27; see “Discussion” below). However, taking a closer look at the correlation between MoCa scores and connectivity, it seems that lower MoCa scores (< 26) are correlated with negative connectivity values (anticorrelation), while higher scores (> 26 indicative of normal cognitive function) are correlated with positive connectivity values. Hence, disruptions of default mode network and precuneus coupling may indeed be related to cognitive dysfunctions in bilateral tinnitus. It is probable, that the constant effort to decrease the tinnitus signal is associated with changes in resting-state functional connectivity. Those resources might no longer be available for other cognitive operations leading to diminished cognitive abilities. Because previous resting-state functional connectivity studies did not assess cognitive abilities, it is unclear whether their findings can be merely attributed to the chronic tinnitus perception or may also be intertwined with cognitive abilities. Further, it was recently shown that there is a bidirectional relationship between tinnitus sensation and cognitive control22. Thus, we want to stress the importance of including assessments of cognitive abilities in tinnitus research in the future.
Contrary to our expectations, tinnitus patients did not exhibit deficits in working memory or general cognitive abilities as a group, although some tinnitus patients showed decreased general cognitive status indicated by a MoCa score < 2623,24,25,27,29,30. Scores from the attention subtests in the MoCa showed a similar pattern (no impairment, no significant differences from control participants). Unfortunately, the abbreviated form of the trail making test which is included in the MoCa does not include the assessment of processing speed. It is probable, that the complete MoCA score or attention subscores as well as the two-back performance that were used in our study were not targeting the affected cognitive processes in our tinnitus sample. Thus, future research should consider including detailed assessments of attention and attention-switching such as the trail making test, specifically subtests 1 and 516, digit span task16, Attention Network Test23, a go/no-go task25, or a Stroop task26. The systematic evaluation of cognitive abilities and clinical characteristics in tinnitus patients along with their association to functional brain changes might also aid in developing or improving treatment options that target their health and wellbeing50. Cognitive training or cognitive behavioral therapy might assist tinnitus patients in switching their attention away from the tinnitus sensation and hence reduce tinnitus distress29,51. In summary, these results provide a first detailed analysis about the relationship between resting-state functional connectivity of default mode network and precuneus with general cognitive abilities in chronic bilateral tinnitus. The mentioned brain areas should also be considered in neuromodulatory therapies that may aid in restoring functional connectivity of the default mode network and specifically the precuneus.
Tinnitus distress related to resting-state functional connectivity of the precuneus
Additionally, our results demonstrate a positive relationship between tinnitus distress assessed by the TFI and resting-state functional connectivity between the precuneus and the lateral occipital cortex. Previous research has suggested that decreased resting-state functional connectivity of the precuneus may interfere with its underlying functioning and anticorrelation with the dorsal attention network18. By contrast, other research has provided evidence of increased resting-state functional coupling of the precuneus in chronic tinnitus patients compared to controls24 and to patients with high compared to low tinnitus distress20. Here we also demonstrate increased resting-state functional connectivity between the precuneus and the lateral occipital cortex (a higher-order visual region) with increased tinnitus distress. Similarly, we showed in a previous study that increased cortical thickness in the precuneus is correlated with increased tinnitus distress52. Hence, neuroanatomical as well as functional changes in the precuneus might be primarily related to increased distress from tinnitus and thereby an increased effort to decrease it. The precuneus, a medial parietal region with widespread cortical and subcortical connections, plays a central role in the modulation of conscious processes and has been found to be activated during various forms of imagery53. Enhanced coupling could be a sign of cross-modal plasticity (similar to that seen in deaf or blind patients) attempting to reduce the gain of the tinnitus sensation (as part of a ‘noise cancellation system’ suggested previously42). Visual brain regions might be responding to the internal sensation of the tinnitus signal15 by trying to reduce involuntary attention to it21,54. Alterations in the visual network are, therefore, mostly thought of as effects of the tinnitus rather than a cause of it33. Indeed, various other studies have shown cross-modal effects with involvement of visual brain areas in chronic tinnitus13,15,19,21,38,45,54. Some studies even suggest that tinnitus can be triggered or modulated by inputs from other sensory modalities or sensorimotor systems such as the visuo-motor system55,56. Hence, visual areas may also serve as potential target areas for interventions, for instance neuromodulatory therapies. Possible intervention strategies may also involve attention-demanding visual tasks such as video games since those may aid in alleviating concentration difficulties and reducing tinnitus distress39. However, additional research is needed to clarify the role of visual areas in chronic tinnitus.
Limitations of the study
There are some limitations of the study. First of all, it is relevant to note that in resting-state fMRI studies, the data are collected during continuous scanning, i.e. there is constant scanner noise. This scanner noise might have interfered with the tinnitus perception, as it could have either masked or exacerbated the tinnitus sensation. In addition, stress and anxiety are known to modulate tinnitus loudness and distress. However, we did not assess these possible effects, which could start well in advance of the actual study. To further assess stress and distress levels of the patients, measuring physiological data such as heart rate variability, blood pressure, or respiratory indicators might provide additional information. Moreover, it is unclear whether the observed alterations reflect underlying changes contributing to the cause of tinnitus or whether they arise as a consequence of tinnitus33. An answer to this question is difficult to determine solely on the basis of correlational analyses. Further, tinnitus involves a complex interaction of hearing abilities, age, distress, and cognitive components22. Longitudinal studies may aid in disentangling this complexity as well as making inferences about causality of neural alterations in chronic tinnitus.
Conclusion
This is the first study providing evidence of associations between general cognitive abilities assessed by the MoCa and resting-state functional connectivity of the default mode network and the precuneus in tinnitus patients. Decreased coupling of the default mode network and precuneus (a medial parietal region) was observed with the superior parietal lobule, the supramarginal gyrus (in the inferior parietal lobule), and the orbitofrontal cortex, with decreasing MoCa values. Further, tinnitus distress correlated positively with resting-state functional connectivity between precuneus and lateral occipital complex. The orbitofrontal cortex, together with the superior parietal lobule and the supramarginal gyrus, is thought to be engaged in compensatory processes attenuating the tinnitus signal. We argue that disruptions of its resting-state connectivity are related to cognitive abilities in chronic tinnitus, because the affected areas are involved in decreasing the intensity of the tinnitus sensation. Hence, those resources are occupied and no longer available for concurrent cognitive operations. Further, the increased coupling of the precuneus with increased tinnitus distress might be a sign of compensatory mechanisms attempting to decrease the tinnitus sensation (as part of a ‘noise cancellation system’). Based on our results, we want to stress the importance of including assessments of cognitive abilities in tinnitus research in the future. Furthermore, future studies should aim at replicating previous findings by using similar acquisition paradigms, seed regions and analysis methods across studies, which might help in identifying possible biomarkers for tinnitus. This is of crucial importance in order to advance treatment options for tinnitus or evaluating the efficacy of tinnitus interventions.
Methods
Participants
20 tinnitus patients and 20 control participants volunteered in the study. The tinnitus patients and control participants were matched in terms of age and sex. Each group comprised 7 female and 13 male participants. The mean age in tinnitus patients was 58.5 (± 9.82) years and the mean age in the control group was 57.7 (± 10.7) years.
The participants were recruited from previous studies in our lab as well as through social networks and local advertisements. The following groups were excluded: pediatric populations, individuals with HIV, individuals with history of seizures or other neurological disorders, with MRI-incompatible implants, with significant ear asymmetries, those with exposure to loud noise 24 h prior to testing, and pregnant women.
Ethics declarations
The approval for the study was obtained by the Institutional Review Board at Georgetown University, and the study was conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). All participants gave written and informed consent and were paid for participating in this study.
Audiometric assessment
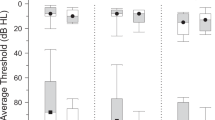
Pure-tone thresholds of the frequencies ranging from 250 Hz to 16 kHz were assessed in a soundproof chamber at the Division of Audiology and Hearing Research at Medstar Georgetown University Medical Center. Pure-tone audiograms averaged over both ears for the two groups are depicted in Fig. 4. Hearing thresholds at 3 kHz and 4 kHz as well as the mean hearing loss between 250 Hz and 8 kHz (T(38) = 2.194, p = 0.034) differed significantly between tinnitus patients and control subjects. Hearing thresholds were therefore included in the between-group analysis (see “Data analysis”).
Behavioral assessment
All participants filled in questionnaires assessing anxiety, depression and emotional distress: The Patient Health Questionnaire 9 [PHQ9]57, the Generalized Anxiety Disorder Questionnaire [GAD7]58, and the Hospital Anxiety and Depression Scale [HADS]59. Every participant conducted the Modified Khalfa Hyperacusis Questionnaire as well60. Moreover, tinnitus patients completed the Tinnitus Handicap Inventory [THI]61, the Tinnitus Sample Case History Questionnaire [TSCHQ]62, and the Tinnitus Functional Index [TFI]63. In addition, general cognitive abilities were assessed with the Montreal Cognitive Assessment [MoCA]64, and a two-back task was conducted as a measure of working memory (including numbers, duration: 10 min).
Data acquisition
MRI data were acquired by a 3 T whole-body Siemens Magnetom Prisma MRI machine with a 64-channel head coil. Resting-state data were recorded from all participants while fixating a black dot presented centrally on a grey screen. T2*-weighted images were acquired with a gradient echo planar imaging (EPI) sequence (530 volumes, TR = 1430 ms, TE = 35 ms, voxel size = 2.0 × 2.0 × 1.8 mm, flip angle 30 degrees, 68 slices). Structural images were acquired with a 3-D T1-weighted sequence (MP-RAGE, TR = 1900 ms, TE = 2.52 ms, voxel size = 1.0 × 1.0 × 1.0 mm3, flip angle 9 degrees, 160 sagittal slices).
Data analysis
Resting-state functional connectivity analyses were done in SPM12 and CONN version 20b31 based on Matlab 2020b. Preprocessing in SPM included spatial realignment estimation, slice-time correction, co-registration, normalization to the Montreal Neurological Institute space using parameters obtained from segmentation of the anatomical T1-weighted image and smoothing (full-width-at-half-maximum = 8 mm). This was followed by preprocessing in CONN including detrending, band-pass filtering (0.008–0.09 Hz), functional outlier detection (Artifact detection tools-based scrubbing65) and nuisance regression (motion, mean white matter and cerebrospinal fluid). To ensure data quality, a threshold of 30% of invalid/outlier scans detected with ART was set as exclusion criterion. No subject was excluded (mean 0.009 ± 0.015% outlier scans).
For the group analysis, each subject’s seed-to-voxel connectivity maps of a specific seed to the whole brain (Fisher-transformed correlation coefficients) were entered into a second-level analysis. On the group level, between-subject comparisons (tinnitus patients versus control participants) were performed (corrected for hearing loss). Further, a multiple linear regression analysis with values from the cognitive tasks (MOCA and two-back task) and for tinnitus distress (THI and TFI) within the tinnitus group was conducted. This analysis was controlled for age and sex because of the inhomogeneous tinnitus group (age range 29–71 years; 7 female and 13 male).
Seed regions included the auditory cortex and the precuneus as well as the default mode network, salience network, and dorsal attention network. For the correlation analysis with tinnitus distress, we further used the right middle temporal gyrus as a seed. The seed areas for the auditory cortex (left and right Brodmann areas 41 and 42) and the right middle temporal gyrus were defined using the Automated Anatomical Labeling (AAL) ROI-Library within the Wake Forest University (WFU) PickAtlas66,67,68. Masks of the default mode, salience and dorsal attention network seeds as well as the precuneus were provided by the atlas implemented in CONN (the FSL Harvard–Oxford atlas was used for cortical and subcortical areas).
All nodes of a network were equally weighted, contributing jointly to the seed network's connectivity. Effects were determined to be significant when passing a threshold of p < 0.05 (FWE cluster size inference with p < 0.001 cluster-forming threshold). Bonferroni-correction was applied to correct for multiple comparisons (i.e. five seeds; for tinnitus distress six seeds). Peak coordinates are reported in MNI space.
Data availability
The datasets for this study can be found in the OSF project “Tinnitus as a network problem – plasticity in anatomical and functional connectivity”: https://osf.io/2nse8/. Data analysis was preregistered on OSF on July 20, 2021 and can be found at https://osf.io/xhsm5. A preprint version of this manuscript was published on OSF on https://osf.io/r83nw.
References
Bauer, C. A. Tinnitus. N. Engl. J. Med. 378, 1224–1231 (2018).
Chang, N. C. et al. Prevalence of persistent tinnitus and dizziness in an elderly population in southern Taiwan. J. Int. Adv. Otol. 15, 99–105 (2019).
Chen, Q., Lv, H., Chen, Y.-C., Song, J.-J. & Wang, Z. Editorial: Neuroimaging approaches to the study of tinnitus and hyperacusis. Front. Neurosci. 15, 700670 (2021).
Knipper, M. et al. The neural bases of tinnitus: Lessons from deafness and cochlear implants. J. Neurosci. 40, 7190–7202 (2020).
Brueggemann, P. et al. Dimensions of tinnitus-related distress. Brain Sci. 12, 275 (2022).
Noreña, A. J. An integrative model of tinnitus based on a central gain controlling neural sensitivity. Neurosci. Biobehav. Rev. 35, 1089–1109 (2011).
Lee, M. H., Smyser, C. D. & Shimony, J. S. Resting-state fMRI: A review of methods and clinical applications. AJNR Am. J. Neuroradiol. 34, 1866–1872 (2013).
Schecklmann, M., Landgrebe, M. & Langguth, B. Phenotypic characteristics of hyperacusis in tinnitus. PLoS ONE 9, e86944 (2014).
Leaver, A. M., Seydell-Greenwald, A. & Rauschecker, J. P. Auditory–limbic interactions in chronic tinnitus: Challenges for neuroimaging research. Hear. Res. 334, 49–57 (2016).
Feng, Y. et al. Increased resting-state cerebellar-cerebral functional connectivity underlying chronic tinnitus. Front. Aging Neurosci. 10, 59 (2018).
Hullfish, J., Abenes, I., Yoo, H. B., De Ridder, D. & Vanneste, S. Frontostriatal network dysfunction as a domain-general mechanism underlying phantom perception. Hum. Brain Mapp. 40, 2241–2251 (2019).
Lanting, C. P., de Kleine, E., Langers, D. R. M. & van Dijk, P. Unilateral tinnitus: Changes in connectivity and response lateralization measured with FMRI. PLoS ONE 9, e110704 (2014).
Maudoux, A. et al. Auditory resting-state network connectivity in tinnitus: A functional MRI study. PLoS ONE 7, e36222 (2012).
Xu, J.-J. et al. Chronic tinnitus exhibits bidirectional functional dysconnectivity in frontostriatal circuit. Front. Neurosci. 13, 1299 (2019).
Chen, Y.-C. et al. Abnormal resting-state functional connectivity of the anterior cingulate cortex in unilateral chronic tinnitus patients. Front. Neurosci. 12, 9 (2018).
Chen, Y.-C. et al. Alterations of the default mode network and cognitive impairment in patients with unilateral chronic tinnitus. Quant. Imaging Med. Surg. 8, 1020–1029 (2018).
Husain, F. T. & Schmidt, S. A. Using resting state functional connectivity to unravel networks of tinnitus. Hear. Res. 307, 153–162 (2014).
Schmidt, S. A., Carpenter-Thompson, J. R. & Husain, F. Connectivity of precuneus to the default mode and dorsal attention networks: A possible invariant marker of long-term tinnitus. NeuroImage Clin. https://doi.org/10.1016/j.nicl.2017.07.015 (2017).
Han, Q. et al. Disrupted local neural activity and functional connectivity in subjective tinnitus patients: Evidence from resting-state fMRI study. Neuroradiology 60, 1193–1201 (2018).
Golm, D., Schmidt-Samoa, C., Dechent, P. & Kröner-Herwig, B. Neural correlates of tinnitus related distress: An fMRI-study. Hear. Res. 295, 87–99 (2013).
Chen, Y.-C. et al. Resting-state brain abnormalities in chronic subjective tinnitus: A meta-analysis. Front. Hum. Neurosci. 11, 22 (2017).
Brueggemann, P. et al. On the relationship between tinnitus distress, cognitive performance and aging. Prog. Brain Res 262, 263–285 (2021).
Heeren, A. et al. Tinnitus specifically alters the top-down executive control sub-component of attention: Evidence from the attention network task. Behav. Brain Res. 269, 147–154 (2014).
Trevis, K. J., McLachlan, N. M. & Wilson, S. J. Cognitive mechanisms in chronic tinnitus: Psychological markers of a failure to switch attention. Front. Psychol. 7, 1262 (2016).
Araneda, R. et al. Altered top-down cognitive control and auditory processing in tinnitus: Evidences from auditory and visual spatial stroop. Restor. Neurol. Neurosci. 33, 67–80 (2015).
Araneda, R. et al. A key role of the prefrontal cortex in the maintenance of chronic tinnitus: An fMRI study using a Stroop task. Neuroimage Clin. 17, 325–334 (2018).
Wang, Y. et al. The characteristics of cognitive impairment in subjective chronic tinnitus. Brain Behav. 8, e00918 (2018).
Mohamad, N., Hoare, D. J. & Hall, D. A. The consequences of tinnitus and tinnitus severity on cognition: A review of the behavioural evidence. Hear. Res. 332, 199–209 (2016).
Tegg-Quinn, S., Bennett, R. J., Eikelboom, R. H. & Baguley, D. M. The impact of tinnitus upon cognition in adults: A systematic review. Int. J. Audiol. 55, 533–540 (2016).
Araneda, R., De Volder, A. G., Deggouj, N. & Renier, L. Altered inhibitory control and increased sensitivity to cross-modal interference in tinnitus during auditory and visual tasks. PLoS ONE 10, e0120387 (2015).
Whitfield-Gabrieli, S. & Nieto-Castanon, A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2, 125–141 (2012).
Agcaoglu, O., Wilson, T. W., Wang, Y.-P., Stephen, J. & Calhoun, V. D. Resting state connectivity differences in eyes open versus eyes closed conditions. Hum. Brain Mapp. 40, 2488–2498 (2019).
Kok, T. E. et al. Resting-state networks in tinnitus: A scoping review. Clin. Neuroradiol. https://doi.org/10.1007/s00062-022-01170-1 (2022).
Kleinjung, T. & Langguth, B. Avenue for future tinnitus treatments. Otolaryngol. Clin. N. Am. 53, 667–683 (2020).
Refat, F. et al. Co-occurrence of hyperacusis accelerates with tinnitus burden over time and requires medical care. Front. Neurol. 12, 627522 (2021).
Minami, S. B., Oishi, N., Watabe, T., Wasano, K. & Ogawa, K. Age-related change of auditory functional connectivity in Human Connectome Project data and tinnitus patients. Laryngoscope Investig. Otolaryngol. 5, 132–136 (2020).
De Ridder, D. et al. In Tinnitus—An Interdisciplinary Approach Towards Individualized Treatment (eds. Schlee, W. et al.) 1–25 (Elsevier B.V., 2021) https://doi.org/10.1016/bs.pbr.2020.12.002.
Song, J.-J., De Ridder, D., Schlee, W., Van de Heyning, P. & Vanneste, S. “Distressed aging”: The differences in brain activity between early- and late-onset tinnitus. Neurobiol. Aging 34, 1853–1863 (2013).
Han, L. et al. Abnormal baseline brain activity in patients with pulsatile tinnitus: A resting-state fMRI study. Neural Plast. 2014, e549162 (2014).
Husain, F. T., Akrofi, K., Carpenter-Thompson, J. R. & Schmidt, S. A. Alterations to the attention system in adults with tinnitus are modality specific. Brain Res. 1620, 81–97 (2015).
Kringelbach, M. L. The human orbitofrontal cortex: Linking reward to hedonic experience. Nat. Rev. Neurosci. 6, 691–702 (2005).
Rolls, E. T. The neuroscience of emotional disorders. Handb. Clin. Neurol. 183, 1–26 (2021).
Rauschecker, J. P., Leaver, A. M. & Mühlau, M. Tuning out the noise: Limbic-auditory interactions in tinnitus. Neuron 66, 819–826 (2010).
Rauschecker, J. P., May, E. S., Maudoux, A. & Ploner, M. Frontostriatal gating of tinnitus and chronic pain. Trends Cogn. Sci. 19, 567–578 (2015).
Leaver, A. M. et al. Intrinsic network activity in tinnitus investigated using functional MRI: Intrinsic Networks in Tinnitus. Hum. Brain Mapp. 37, 2717–2735 (2016).
Schlee, W. et al. Mapping cortical hubs in tinnitus. BMC Biol. 7, 80 (2009).
Carpenter-Thompson, J. R., Schmidt, S. A. & Husain, F. T. Neural plasticity of mild tinnitus: An fMRI investigation comparing those recently diagnosed with tinnitus to those that had tinnitus for a long period of time. Neural Plast. 2015, 161478 (2015).
Carpenter-Thompson, J. R., Schmidt, S., McAuley, E. & Husain, F. T. Increased frontal response may underlie decreased tinnitus severity. PLoS ONE 10, e0144419 (2015).
Chen, Y.-C. et al. Altered intra- and interregional synchronization in resting-state cerebral networks associated with chronic tinnitus. Neural Plast. 2015, 475382 (2015).
Gentil, A. et al. Alterations in regional homogeneity in patients with unilateral chronic tinnitus. Trends Hear. 23, 2331216519830237 (2019).
Knipper, M., Mazurek, B., van Dijk, P. & Schulze, H. Too blind to see the elephant? Why neuroscientists ought to be interested in tinnitus. J. Assoc. Res. Otolaryngol. 22, 609–621 (2021).
Chen, Y.-C. et al. Tinnitus distress is associated with enhanced resting-state functional connectivity within the default mode network. Neuropsychiatr. Dis. Treat. 14, 1919–1927 (2018).
Rosemann, S. & Rauschecker, J. P. Neuroanatomical alterations in middle frontal gyrus and the precuneus related to tinnitus and tinnitus distress. Hear. Res. https://doi.org/10.1016/j.heares.2022.108595 (2022).
Cavanna, A. E. & Trimble, M. R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129, 564–583 (2006).
Burton, H. et al. Altered networks in bothersome tinnitus: A functional connectivity study. BMC Neurosci. 13, 3 (2012).
Cacace, A. T. Expanding the biological basis of tinnitus: Crossmodal origins and the role of neuroplasticity. Hear. Res. 175, 112–132 (2003).
Kroenke, K., Spitzer, R. L. & Williams, J. B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 16, 606–613 (2001).
Spitzer, R. L., Kroenke, K., Williams, J. B. W. & Löwe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 166, 1092–1097 (2006).
Zigmond, A. S. & Snaith, R. P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 67, 361–370 (1983).
Khalfa, S. et al. Psychometric normalization of a hyperacusis questionnaire. ORL 64, 436–442 (2002).
Newman, C. W., Jacobson, G. P. & Spitzer, J. B. Development of the tinnitus handicap inventory. Arch. Otolaryngol. Head Neck Surg. 122, 143–148 (1996).
Langguth, B. et al. Consensus for tinnitus patient assessment and treatment outcome measurement: Tinnitus Research Initiative meeting, Regensburg, July 2006. Prog. Brain Res. 166, 525–536 (2007).
Henry, J. A. et al. Tinnitus Functional Index: Development, validation, outcomes research, and clinical application. Hear. Res. 334, 58–64 (2016).
Nasreddine, Z. S. et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699 (2005).
Whitfield-Gabrieli, S., Nieto-Castanon, A. & Ghosh, S. Artifact detection tools (ART). Cambridge, MA. Release Version vol. 7, 11 (2011).
Maldjian, J. A., Laurienti, P. J. & Burdette, J. H. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage 21, 450–455 (2004).
Maldjian, J. A., Laurienti, P. J., Kraft, R. A. & Burdette, J. H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19, 1233–1239 (2003).
Tzourio-Mazoyer, N. et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289 (2002).
Acknowledgements
The authors wish to thank Anjul Bhangu for assistance in behavioral data curation.
Funding
SR is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation; RO6114/1-1).
Author information
Authors and Affiliations
Contributions
S.R. designed the study, was involved in data acquisition, analyzed the data and wrote the manuscript. J.P.R. designed the study, was involved in interpretation of the results and revised the manuscript. Both authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rosemann, S., Rauschecker, J.P. Disruptions of default mode network and precuneus connectivity associated with cognitive dysfunctions in tinnitus. Sci Rep 13, 5746 (2023). https://doi.org/10.1038/s41598-023-32599-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32599-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.