Abstract
An ovipositing insect evaluates the benefits and risks associated with the selection of an oviposition site for optimizing the fitness and survival of its offspring. The greater wax moth, Galleria mellonella (L.), uses beehives as an oviposition site. During egg-laying, the gravid wax moth confronts two kinds of risks, namely, bees and conspecific larvae. While bees are known to attack the moth’s offspring and remove them from the hive, the conspecific larvae compete for resources with the new offspring. To date, little is known about the mechanisms involved in the assessment of oviposition site by the greater wax moth, G. mellonella (L.). Here, we demonstrate that the wax moth uses two different sensory modalities to detect risks to its offspring in the hives of Apis cerena. Bees appear to be detected by the contact-chemoreception system of the gravid wax moth, while detection of conspecifics relies on the olfactory system. Hence, our findings suggest that two different sensory modalities are used to detect two different risks to the offspring and that the selection of oviposition sites by G. mellonella (L.) relies on the integration of inputs from both the olfactory and contact-chemoreception systems.
Similar content being viewed by others
Introduction
Risk evaluation of an oviposition site is crucial for the fitness of an organism, as choosing inappropriate sites can place the offspring at risk or reduce the offspring’s performance1,2,3,4. An ovipositing organism is faced with a plethora of challenges and may choose an appropriate oviposition site to avoid predators, competition, or other risks5,6,7. However, oviposition site selection requires an animal to evaluate multiple, possibly, conflicting sensory signals associated with risks and benefits. For example, when a gravid female of Plutella xylostella (L.) detects natural enemies (risk) at an oviposition site in their preferred host, the moth tends to chooses an alternate host for oviposition despite knowing that its offspring may develop poorly in the alternate host. The study reveals that herbivorous insects must evaluate both, the risk of natural enemies and the quality of an oviposition site and for a gravid female, the survival of its offspring is crucial than the nutritional quality of the oviposition site8. Hence, oviposition site selection behavior provides an excellent means to evaluate how animals perceive and rank various risks.
The greater wax moth, Galleria mellonella (L.) (Lepidoptera: Pyralidae) is a noxious pest of honeybees9.The moth prefers bee colonies and uses beehive volatiles as cues to locate suitable oviposition sites9,10,11. After locating a suitable beehive from a distance, the gravid female approaches the beehive at night, when the bees are less active, and lays eggs into crevices in the beehive12. Hatched neonate larvae of G. mellonella tunnel into the honeycomb and feed on pollen, honey, wax, and occasionally brood9,13,14. However, the bees do not tolerate the intrusion and eliminate the larvae from the hives15. This places the wax moth’s offspring in jeopardy thus making live beehives a less likely site for oviposition. But previous studies have shown that the wax moths are attracted to live beehives and lay eggs in them10. A recent study has shown that during oviposition, G. mellonella are aware and can detect bee alarm pheromones (isopentyl acetate, benzyl acetate, octyl acetate, and 2-heptanone) but ignores them in favor of an appropriate oviposition site15. However, with imminent risk to their offspring does the greater wax moth choose live beehives as a suitable oviposition site when given a choice?
Here, using the interaction between G. mellonella (pest) and Apis cerana (host), we investigated the risk evaluating behavior during oviposition site selection. We hypothesized that the greater wax moths consider bees and conspecifics as an imminent risk to their offspring and reduce egg-laying in beehives with bees or conspecifics. First, using natural infestation of wax moths to beehives with bees (BB; a known risk to the gravid moth and its offspring), beehives without bees (BW; not a risk to the gravid moth or to its offspring) and beehives with conspecifics (BC; a risk to its offspring), we wanted to see if the wax moths could distinguish the presented risks. Next, we measured the ability of G. mellonella’s olfactory system in distinguishing oviposition sites with possible risks using volatile cues from BB, BW and BC. Lastly, we measured the ability of G. mellonella’s contact-chemoreception system in distinguishing volatile cues from BB, BW and BC and making egg-laying choice. This interaction of bees and the greater wax moth provides us with an opportunity to understand how organisms integrate inputs from the olfactory and contact-chemoreception systems to detect risk during oviposition site selection.
Results
Gravid female wax moths avoid ovipositing in beehives with bees or conspecifics
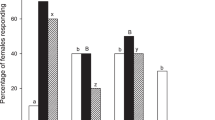
To test gravid female wax moths’ ability to detect bees and conspecific larvae, their attraction and oviposition preferences for beehive with bees (BB), beehive without bees (BW) or beehive with conspecifics (BC) were assessed in the field. Significantly greater moth emergence from BW combs (37.15 ± 2.379 moths; mean ± s.e.m, Tukey’s post hoc P < 0.0001) over BB (9.80 ± 0.578 moths) and BC (8.55 ± 0.799 moths) demonstrate the wax moth’s preference for BW (One-way ANOVA; F (2,57) = 79.70; df = 19; P < 0.0001; Fig. 1). This shows the wax moths’ ability to detect and avoid possible risks to their offspring during oviposition site selection.
Field preference of gravid wax moths to beehives. Gravid female wax moths preferred and laid significantly more eggs in the beehive without bees (BW) than onto beehives with bees (BB) or with conspecifics. Error bars represent s.e.m. Statistical difference was analyzed by one-way ANOVA. Letters indicate statistical differences derived from One-way ANOVA analysis and Tukey’s multiple comparison post hoc tests.
Antennal and tarsus responses to chemical cues from BB, BW and BC
Insects rely on olfaction to locate oviposition sites from a distance, but upon landing on an oviposition site, they use contact-chemoreception to evaluate the suitability of the site for egg-laying16. As shown in the field preference assays, wax moths clearly chose BW as the choice of oviposition site. Therefore, we asked if the moth’s olfactory and contact-chemoreception system, represented by the antenna and tarsus respectively, could detect chemical cues from BB, BW and BC. In electrophysiology experiments, the antenna responded with a higher amplitude to chemical cues from BB = 0.146 ± 0.008 mV (mean ± s.e.m), BW = 0.159 ± 0.011 mV and BC = 0.127 ± 0.002 mV that were significantly different from controls (Air = 0.005 ± 0.001 mV; Solvent = 0.013 ± 0.002 mV). However, the tarsus responded with a higher amplitude to chemical cues of BB (0.153 ± 0.007 mV) and BW (0.158 ± 0.009 mV) and were significantly different from the amplitude of BC (0.028 ± 0.004 mV) and controls (Filter paper = 0.022 ± 0.006; Solvent = 0.032 ± 0.006 mV) (One-way ANOVA; F (4,45) = 97.64; df = 9; P < 0.0001; Fig. 2A,B; Fig. S2.A and B). These results suggest that the wax moth used their olfactory system to detect chemical cues from BB, BW and BC, whereas the contact-chemoreception system could detect cues from BB and BW only.
Electroantennogram (EAG) and Electrotarsogram (ETG) of chemical cues from beehives. Wax moths’ antenna and tarsus were subjected to electrophysiological study. (A) We found that the olfactory system (represented by antenna) responded significantly to chemical cues from beehive with bees (BB), beehive without bees (BW) and beehive with conspecifics (BC). (B) However, the contact-chemoreception system (represented by tarsi) responded significantly to chemical cues from beehive with bees (BB) and beehive without bees (BW). Air (AR), filter paper (FP) and solvent (SL) were used as control. Error bars represent s.e.m. Date were analyzed using One-way ANOVA. Similar letters indicate the absence of significant differences.
Olfactory preference of gravid wax moth to chemical cues from BB, BW and BC
In field preference assays, wax moths clearly preferred BW as an appropriate oviposition site, suggesting that wax moths were capable of sensing risk to their offspring. Further, electrophysiology studies revealed that the wax moth’s antenna and tarsus responded to chemical cues from BB, BW and BC. However, we wanted to understand the wax moth’s behavior towards these chemical cues. The attraction of gravid female wax moths was measured in Y-tube olfactometer assays to determine their ability to detect the presence of bees or conspecifics from distance using their sense of smell. Our results indicate that female moths were equally attracted (Tukey’s post hoc, P = 0.8659) to chemical cues from BB (Attraction index (AI) = + 0.69 ± 0.049,) and BW (AI = + 0.68 ± 0.018,), but were less attracted to chemical cues from BC (AI = -0.26 ± 0.047). ANOVA followed by Tukey’s multiple comparison test showed that there was no significant difference between the AI of BB and BW (P = 0.8659), but AI of BC was significantly different from AI of BB (P < 0.0001) and BW (P < 0.0001) (One-way ANOVA; F (2,12) = 179.4; df = 4; P < 0.0001; Fig. 3A). A one sample t-test proved that the mean of BB (t = 9.487, df = 4, P = 0.001) and BW (t = 8.552, df = 4, P = 0.001) were significantly different from the theoretical mean of 0, whereas mean of BC (t = 2.064, df = 4, P = 0.108) was not significantly different from 0. From the results, we infer that although the wax moths sense the presence of bees in the volatiles of BB15, they still showed equal attraction to the chemical cues of both BB and BW using their olfactory system. However, the moths could detect conspecifics as they avoided the olfactometer arm with chemical cues from BC.
Attraction and oviposition behavior of wax moths to chemical cues of beehives. (A) In y-tube olfactometer assays, we showed that gravid female wax moths were significantly attracted to chemical cues of beehive with bees (BB) and beehive without bees (BW). However, the chemical cues of beehive with conspecifics (BC) were less attractive to moths. (B) Similarly, in oviposition assays, gravid moths preferred to lay significantly more eggs on filter paper with chemical cues of beehive without bees (BW) than on filter paper with chemical cues of beehive with bees (BB) or beehive with conspecifics (BC). Error bars represent s.e.m. Significant difference was analyzed by One-way ANOVA. Similar letter significs that the means are not significantly different.
Oviposition preference of gravid wax moth to headspace volatiles of BB, BW and BC
Next, we assessed the oviposition preference of G. mellonella to headspace volatiles of BB, BW and BC using oviposition assays. The moths had access to the filter papers with chemical cues and had no restriction in choosing either the filter paper containing the test samples or control to lay eggs. Gravid moths deposited more eggs on filter paper containing chemical cues of BW with an oviposition index (OI) of 0.77 ± 0.023 (mean ± s.e.m) but deposited less eggs on filter paper with BB (OI = 0.22 ± 0.022) and BC (OI = -0.04 ± 0.059). A one sample t-test proved that the mean of BB (t = 9.818, df = 14, P < 0.0001) and BW (t = 32.81, df = 14, P < 0.0001) were significantly different from the theoretical mean of 0, whereas mean of BC (t = 0.777, df = 14, P = 0.450) was not significantly different from 0. One sample t-test along with ANOVA followed by Tukey’s multiple comparison test (One-way ANOVA; F (2,42) = 127.3; df = 14; P < 0.0001; Fig. 3B) suggested that G. mellonella significantly preferred to lay more eggs onto filter paper with chemical cues of BW than with BB or BC.
GC–MS analysis of chemical cues from BB, BW and BC
GC–MS analysis revealed that the headspace samples from BB, BW and BC contained acids, esters, alkanes, alcohols, aldehydes, and terpenes (Table 1). The similarity of the chemical cues emanating from each source (BB, BW and BC), was examined using a multivariant correlation analysis based on the presence and concentration of compounds. The results suggest that the compounds present in BW and BC and their concentrations were significantly similar (Pearson r = 0.76) (Fig. 4), whereas compounds present in BB and their concentrations was different from that of BW (Pearson r = − 0.02) and BC (Pearson r = − 0.11).
Multivariant correlation analysis of chemical compounds and their concentrations from the air-entrained chemical cues of beehive with bees (BB), beehive without bees (BW) and beehive with conspecifics (BC). The analysis found that the chemical compounds and their concentrations from BW and BC were significantly similar.
Discussion
The oviposition strategy of an insect is a complex process with trade-off between many factors17,18,19. Risk to an insect’s offspring is one factor that can have a strong influence on its oviposition site selection1,2,3,4,5,20,21,22. A mated female insect may select a specific oviposition site to avoid predators or competitors, or because the site has advantageous physical characteristics2,7,23,24. Discriminating risks at an oviposition site is challenging and a costly affair, it is therefore crucial for a gravid insect to detect specific chemical information from a distance to distinguish suitable from unsuitable oviposition sites6,25,26,27,28,29,30. Here, we demonstrate that the gravid wax moths could detect risks at the oviposition sites by integrating inputs from two separate sensory modalities.
Previous studies revealed that the G. mellonella communicates through different sensory modalities, including auditory and pheromone signalling31. Wax moths are known to utilize their ears to detect risk auditory signals from echolocating insectivorous bats and decide to tradeoff the signals to find mate and an appropriate oviposition site32,33. But as they attack the beehives at night, when the bees are less active, detecting auditory signals from beehives is less likely. Therefore, we considered the olfactory and contact-chemoreception for our studies. In field preference assays, gravid wax moths considered bees and conspecifics as risks, preferring BW over BB and BC as oviposition site. Interestingly, in olfactometer assays, where gravid wax moths were allowed to use only their olfactory system to make choices, gravid moths preferred and were significantly attracted to chemical cues of BB and BW but were not attracted to chemical cues from BC. This proved that the wax moth’s olfactory system could only sense the risk posed by conspecifics and not by bees15. In oviposition assays, where the moths are allowed to use both their olfactory and contact-chemoreception system to make choices, gravid moths laid significantly less eggs on filter paper with chemical cues from BB and BC. This clearly proved that the moth’s contact-chemoreception system is required to detect bees as a risk. In addition, electrophysiology studies also revealed that the wax moth’s response was higher to BW rather than BB and BC, which gives us a clue that the contact-chemoreceptors situated on the tarsus are tuned to some of the volatile compounds from BW which evoked the oviposition in greater wax moth. Further studies are being conducted to identify these oviposition cues. In a recent study, Kwadha et al.34 detected terpenes (sylvestrene), aldehydes (nonanal and decanal), and esters (ethyl propanoate, 2-methyl ethyl propanoate, 2-methyl ethyl butanoate, and 3-methyl butyl acetate) as some of the cues that attract G. melonella to honeycomb volatiles. These cues may be significant in mediating oviposition behaviour in the greater wax moth.
Another interesting aspect in this study that requires an in-depth analysis was the wax moth’s choice of oviposition site based on chemical cues. Upon multivariant correlation analysis of the chemical compounds from BB, BW and BC, we found BW and BC to be significantly similar. This meant that the wax moths should have been attracted to BC as much as they did to BW. However, this was not the case either in the field, olfactometer or oviposition assays. This clearly shows that the chemical cues, although important, is not the only criteria in decision making process in insects. It is imperative that an insect can contextually integrate sensory signals to formulate appropriate behavioral responses35.
Considering the signal integration from olfactory and contact-chemoreception system in the wax moth, we developed three possible neuronal models (Fig. 5). Model 1: When the moth senses a BB, the positive signal from their olfactory system to the motor system instigates flight towards the beehive, upon landing on the beehive their contact-chemoreception system senses the presence of bees and sends a negative signal to the motor system, thus restraining oviposition (Fig. 5A). Model 2: Similarly, when a moth senses a BW, positive signals sent to the motor system from both the olfactory and contact-chemoreception system instigates flight towards BW and stimulates oviposition (Fig. 5B). Model 3: However, when a moth senses a BC, the olfactory system sends a negative signal to the motor system thus causing no flight towards BC (Fig. 5C). Our data clearly supports the neuronal model where central integration of signals from olfactory and contact-chemoreception systems mediate risk detection by female moths. However, further work on the neurobiology of this moth needs to be done to validate these models. We suggest that our paradigm can be used as a simple model for risk detection by ovipositing insects. Apart, our study can also play an important role in improving control methods of this devastating pest of bees.
Neuronal models for the interaction between contact-chemoreception (CC) and olfactory system (OS) of wax moth during oviposition site selection. (A) Model 1: When the moth senses a BB, the positive signal (green arrow) from their olfactory system to the motor system instigates flight towards the beehive, upon landing on the beehive their contact-chemoreception system senses the presence of bees and sends a negative signal (red arrow) to the motor system (MS), thus restraining oviposition (OV). (B) Model 2: Similarly, when a moth senses a BW, positive signals sent to the motor system from both the olfactory and contact-chemoreception system instigates flight towards BW and stimulates oviposition. (C) Model 3: However, when a moth senses a BC, the olfactory system sends a negative signal to the motor system thus causing no flight towards BC.
Materials and methods
Insects
Galleria mellonella larvae (7–8th instar) and Apis cerena colonies were obtained from the Department of Entomology, University of Agricultural Sciences, GKVK, Bengaluru, India and maintained at the Division of Crop Protection, ICAR-Indian Institute of Horticultural Research, Bengaluru, India. Larvae of G. mellonella were reared on honeycombs of A. cerana in a dark plastic container (20 × 20 × 30.5 cm; length × width × height), at ambient conditions (27 ± 1 °C, 75 ± 2% RH and 14L: 10D h photoperiod). Cocoons (n = 150) of G. mellonella were collected and placed in cages (30 × 30 × 30 cm2) for emergence. Emerged adult moths were provided with honey solution (2%) and water moistened on cotton swabs ad libitum. Adults, 3–5 days old, were allowed to mate for 48 h and gravid females were separated into different cages for use in further experiments. A. cerena colonies in bee boxes were placed in the shade in a mango orchard at ICAR-Indian Institute of Horticultural Research, Bengaluru, India (13.1348° N, 77.4960° E) and were provided with water in a container and were allowed to forage on flowers in the orchard.
Preference of wax moth to BB, BW and BC in field conditions
Beehive with bees (BB), beehive without bees (BW) and beehive with conspecific damage (BC) were positioned in beehive boxes. These boxes were placed in the field for a week (during peak infestation season, July–August) for the infestation of G. mellonella to occur. After the field exposure period of one week, the exposed combs were collected and placed separately in cages (30 × 30 × 30 cm) for adult moths to emerge. For combs of BB, the bees were transferred to a new comb as they may detect larvae and remove them from the beehive. The number of adults emerged from BB, BW and BC were enumerated. A total of 20 trials was conducted for each kind of beehive.
Headspace volatile collection from BB, BW and BC
Headspace collections from BB, BW and BC were performed at night (7 pm to 7 am Indian Standard Time) using a customized air entrainment system described by Kamala Jayanthi et al.36. Porapak Q (50 mg; 60/80 mesh; Supelco, Sigma-Aldrich) was packed in a glass tube (L = 5 cm, dia. = 5 mm) and activated at 180 °C under a stream of nitrogen. Activated Porapak Q tubes were used to collect headspace volatiles. A beehive box consisting of a strong healthy bee colony (5–6 months old), a beehive box without bees but with intact honeycombs and a beehive box with honeycombs damaged by wax moth larvae were selected for headspace volatile collection. All connections were made with polytetrafluoroethylene (PTFE) tubing with brass ferrules and fittings (Swagelok, India) and sealed with PTFE tape. The tubes fitted with Porapak Q were gently inserted into an opening in the beehive boxes and air was drawn at the rate of 400 mL/min. Simultaneously, headspace volatiles from an empty bee box were entrained for use as a control. Volatiles was entrained for 12 h and was eluted with 750 µL of redistilled diethyl ether (99.7% pure, Merck, India). The eluted headspace volatile samples were collected in clean glass vials (2 mL; Supelco, India). n-Nonyl acetate, (99.9%, Sigma-Aldrich, India), (5 µL; 500 ng/µL) was added to the headspace volatile samples as an internal standard for quantification37 and were stored at − 20 °C until further use.
Y-tube olfactometer assays
A glass Y-tube olfactometer (Fig. S1. A; I.D. = 3.5 cm; length of main stem and side arms = 30 cm; angle of Y = 90°) was used to test the attraction of mated G. mellonella to headspace volatiles of beehive with bees (BB), beehive without bees (BW), beehive with conspecific damage (BC) and control. Before each experiment, all glassware was washed with a non-ionic detergent, rinsed with acetone, and distilled water, and dried in an oven at 180 °C for 2 h. The experiment was set up in a dark room, at night (7 pm to 9 pm), at ambient conditions (27 ± 2 °C and 60 ± 5% RH). Test sample (50 µL) and control (50 µL solvent) were dispensed onto separate cotton wicks (3–5 inch) and was allowed 1 min for the solvent to evaporate. The cotton wicks were then placed into two gas wash bottles with an inlet and an outlet. The Y-tube setup aided the air passed through an activated charcoal filter to be pushed separately into the gas wash bottles with one holding a cotton wick with test sample and the other holding a cotton wick with control through the inlet. The air from the outlet of the gas wash bottle loaded with headspace volatiles was gently pushed into the treatment arm and the air from the outlet of the gas wash bottle loaded with control was pushed into the control arm38. The flow rate was adjusted to 1.5 mL/min using a flowmeter. Gravid females of G. mellonella were introduced individually through an opening in the main stem of the olfactometer (Fig. S1). In a replicate, each moth was given 2 min to acclimatize in the olfactometer, after which the experiment was run for 5 min. Each set of the Y-tube olfactometer assay was repeated 5 times for a test sample, with each set having 10 replicates. If a moth did not choose any arm, the replicate was discarded and repeated. Attraction index (AI) was calculated using the formula AI = [(no. of moth entry into treatment arm – no. of moth entry into control arm)/(total no. of moth entry into treatment arm + no. of moth entry into control arm)]. After each replicate, the direction of the olfactometer was changed to eliminate any directional bias. Each insect was used only once in the assay.
Oviposition assay
Oviposition assay for G. mellonella was conducted in a transparent plastic box (30 cm × 20 cm × 15 cm) divided into two equal parts with one part containing filter paper disc with solvent and the other part containing the filter paper with headspace volatiles of BB, BW,BC and control (50 µL). Test samples or solvent were applied onto a filter paper disc (Whatman No. 1, 5 cm length, 3 cm breadth) using a micropipette and the solvent was allowed to evaporate before placing the filter paper disc inside the plastic box. Gravid females G. mellonella were individually released into a plastic box closed with a black muslin cloth. Moths were given 24 h to lay eggs. The assays were conducted at night (7 pm onwards). Eggs laid on the filter paper were enumerated using a Leica M205 series stereomicroscope. Oviposition index (OI) was calculated using the formula OI = [(no. of eggs laid on treatment filter paper – no. of eggs laid on control filter paper) / (no. of total eggs laid)]. A single insect was used per trial, and 15 trials were conducted to test moth oviposition preferences between each test samples and a control.
Electrophysiological recording
Electrophysiological recordings were done using the antenna (olfaction) and tarsus (contact-chemoreception) of gravid female G. mellonella (2–3 days old). The test insect was immobilized by chilling on ice. The antenna or tarsus of the immobilized insect was excised using a pair of micro-scissors and placed on a probe holder (Syntech, Germany, Fig. S1. B) containing a small amount of electrode gel (Signa, Parker Laboratories, USA). The base of the antenna or tarsus was placed touching the indifferent ground electrode and the other end touching the recording electrode. For olfactory stimuli, 10 µL of the headspace volatiles (BB, BW or BC) were pipetted onto filter paper strips (Whatman No. 1, 6 cm length × 0.5 cm breadth). The solvent was allowed to evaporate for 30 s before placing the filter paper inside a glass Pasteur Pipette (10 cm length and 6 mm outer dia.). The probed antenna was stimulated by puffing purified air (continuous airflow of 300 mL/min) carrying headspace volatile samples (for 0.5 s) over the antennae. For contact-chemoreception stimuli, 10 µL of the headspace volatiles (BB, BW or BC) were pipetted onto separate filter paper strips (Whatman No. 1, 6 cm length × 0.5 cm breadth). The stimuli for puffs were random and no specific sequence followed. The solvent was allowed to evaporate for 30 s before fixing the filter paper strip to a clip attached to a micro-manipulator. The probed tarsus was stimulated by carefully touching the filter paper containing headspace volatiles to the tarsus for 1–2 s. The responses from the antenna and tarsus were acquired using an Intelligent Data Acquisition Controller (Syntech Model IDAC-4,) and recorded using AutoSpike software (Syntech, Germany). The configuration in the AutoSpike properties tab for the channel with the electrophysiology probe was set at a sampling rate of 100 and a filter of 0–32 Hz. The responses (amplitudes) to the treatments were expressed as the mean of all recorded antennal depolarizations. A total of 10 replicates were carried out for each treatment, and fresh antenna or tarsus was used for each recording. Filter paper with solvent was used as control.
Gas chromatography coupled mass spectrometry analysis (GC–MS)
The chemical composition of headspace samples of BB, BW and BC was analyzed using Gas-chromatography (Agilent 7890B GC system) coupled with a mass spectrometer (Agilent 5977 MSD). A capillary column (HP-5 MS UI column of L = 30 m, Dia. = 0.25 mm & Thickness = 0.25 µm) was used to examine samples. The thermal program was set initially at an oven temperature of 60 °C for 1 min, and then ramped at 7 °C/min to 240 °C, with helium as the carrier gas, with the flow rate 1 mL/min and held for 2 min at pressure 8.3 psi. Mass spectrometer was in full scan mode (70 eV) and atomic mass unit (AMU) ranged from 40 to 450. One microliter of the sample was injected in split-less mode (40 mL/min) with injection temperature at 270 °C. Individual compounds were identified using the Kovats Index, calculated using a homologous series of n-alkanes (C7 to C30; Sigma-Aldrich) as standard39, and comparing the MS spectra with a spectral library, NIST 14. Identified compounds were authenticated by co-injecting standard synthetic compounds along with samples. Quantification of volatiles was performed using the internal standard method37 and the relative abundance of each compound was calculated based on the internal standard of n-nonyl acetate.
Statistics
Statistical analyses were performed using GraphPad Prism version 9.1 for Mac (GraphPad Software Inc, San Diego, CA, USA). Data were subjected to normal distribution tests before any statistical analysis. One-way ANOVA followed by Tukey’s multiple comparison test was used as data conformed to a normal distribution. Error bars in figures were mean ± standard error of the mean (s.e.m). The means of attraction index (AI) and oviposition index (OI) followed normal distribution, the means were subjected to one sample t-test to find if the means were significantly different from 0. The mean of relative abundance of compounds detected from BB, BW and BC were subjected to multivariant correlation analysis. A correlation matrix was constructed to understand the similarity of the volatile sources. The matrix contains the Pearson R value.
Data availability
The datasets generated and analyzed during this current study are available from ResearchGate (https://doi.org/10.13140/RG.2.2.17899.21289).
References
Refsnider, J. M. & Janzen, F. J. Putting eggs in one basket: Ecological and evolutionary hypotheses for variation in oviposition-site choice. Annu. Rev. Ecol. Evol. Syst. 41, 39–57 (2010).
Rudolf, V. H. W. & Rodel, M. O. Oviposition site selection in a complex and variable environment: The role of habitat quality and conspecific cues. Oecologia 142, 316–325 (2005).
Blaustein, L. Oviposition site selection in response to risk of predation: Evidence from aquatic habitats and consequences for population dynamics and community structure. In Evolutionary Theory and Processes: Modern Perspectives (ed. Wasser, S. P.) 441–456 (Springer, 1999).
Elsensohn, J. E., Schal, C. & Burrack, H. J. Plasticity in oviposition site selection behavior in drosophila suzukii (diptera: drosophilidae) in relation to adult density and host distribution and quality. J. Econ. Entomol. 114, 1517–1522 (2021).
Kempraj, V., Park, S. J. & Taylor, P. W. Forewarned is forearmed: Queensland fruit flies detect olfactory cues from predators and respond with predator-specific behaviour. Sci. Rep. 10, 7297 (2020).
Damodaram, K. J. P. et al. Centuries of domestication has not impaired oviposition site-selection function in the silkmoth, Bombyx mori. Sci. Rep. 4, 1–6 (2014).
Hansson, B. S. & Stensmyr, M. C. Evolution of insect olfaction. Neuron 72, 698–711 (2011).
Ghosh, E., Sasidharan, A., Ode, P. J. & Venkatesan, R. Oviposition preference and performance of a specialist herbivore is modulated by natural enemies, larval odors, and immune status. J. Chem. Ecol. 48, 670–682 (2022).
Nielsen, R. A. & Brister, C. D. The greater wax moth: Adult behavior. Ann. Entomol. Soc. Am. 70, 101–103 (1977).
Kwadha, C. A., Ong’Amo, G. O., Ndegwa, P. N., Raina, S. K. & Fombong, A. T. The biology and control of the greater wax moth, Galleria mellonella. Insects 8, 61 (2017).
Kebede, E. Prevalence of wax moth in modern hive with colonies in Kafta Humera. Anim. Vet. Sci. 3, 132–135 (2015).
Ellis, J. D., Graham, J. R. & Mortensen, A. Standard methods for wax moth research. J. Apic. Res. 52, 1–17 (2013).
Hepburn, H. R. & Radloff, S. E. Honeybees of Africa 227–241 (Springer, 1998). https://doi.org/10.1007/978-3-662-03604-4.
Fletcher, D. J. C. The African Bee, Apis mellifera adansonii, Africa. Annu. Rev. Entomol. 23, 151–171 (1978).
Li, Y. et al. Losing the arms race: Greater wax moths sense but ignore bee alarm pheromones. Insects 10, 81 (2019).
Feng, B., Qian, K. & Du, Y. J. Floral volatiles from Vigna unguiculata are olfactory and gustatory stimulants for oviposition by the bean pod borer moth Maruca vitrata. Insects 8, 60 (2017).
Janz, N. Evolutionary ecology of oviposition strategies. In Chemoecology of Insect Eggs and Egg Deposition (eds Hilker, M. & Meiners, T.) 349–376 (Willey, 2008). https://doi.org/10.1002/9780470760253.ch13.
Renwick, J. A. A. & Chew, F. S. Oviposition behavior in lepidoptera. Annu. Rev. Entomol. 39, 377–400 (1994).
Nakajima, Y. & Fujisaki, K. Fitness trade-offs associated with oviposition strategy in the winter cherry bug, Acanthocoris sordidus. Entomol. Exp. Appl. 137, 280–289 (2010).
Murphy, P. J. Context-dependent reproductive site choice in a Neotropical frog. Behav. Ecol. 14, 626–633 (2003).
Geoffrey, G. et al. Larviposition site selection mediated by volatile semiochemicals in Glossina palpalis gambiensis. Ecol. Entomol. 46, 301–309 (2021).
Yao, F. L. et al. Oviposition preference and adult performance of the whitefly predator Serangium japonicum (Coleoptera: Coccinellidae): Effect of leaf microstructure associated with ladybeetle attachment ability. Pest Manag. Sci. 77, 113–125 (2021).
Spieler, M. & Linsenmair, K. E. Choice of optimal oviposition sites by Hoplobatrachus occipitalis (Anura: Ranidae) in an unpredictable and patchy environment. Oecologia 109, 184–199 (1997).
Figiel, C. R. & Semlitsch, R. D. Experimental determination of oviposition site selection in the marbled salamander, Ambystoma opacum. J. Herpetol. 29, 452 (1995).
Kotler, B. P. & Mitchell, W. A. The effect of costly information in diet choice. Evol. Ecol. 9, 18–29 (1995).
Nylin, S. & Janz, N. Oviposition preference and larval performance in Polygonia c-album (Lepidoptera: Nymphalidae): the choice between bad and worse. Ecol. Entomol. 18, 394–398 (1993).
Nagaya, H., Stewart, F. J. & Kinoshita, M. Swallowtail butterflies use multiple visual cues to select oviposition sites. Insects 12, 1047 (2021).
Scolari, F., Valerio, F., Benelli, G., Papadopoulos, N. T. & Vaníčková, L. Tephritid fruit fly semiochemicals: Current knowledge and future perspectives. Insects 12, 408 (2021).
Haverkamp, A., Hansson, B. S. & Knaden, M. Combinatorial codes and labelled lines: How insects use olfactory cues to find and judge food, mates, and oviposition sites in complex environments. Front. Physiol. 9, 49 (2018).
Ichinosé, T., Honda, H. & Honda, H. Ovipositional behavior of papilio protenor demetrius Cramer and the factors involved in its host plants. Appl. Entomol. Zool. 13, 103–114 (1978).
Spangler, H. G. Functional and temporal analysis of sound production in Galleria mellonella L. (Lepidoptera: Pyralidae). J. Comp. Physiol. A 159, 751–756 (1986).
Spangler, H. G. & Takessian, A. Sound perception by two species of wax moths (Lepidoptera: Pyralidae). Ann. Entomol. Soc. Am. 76, 94–97 (1983).
Skals, N. & Surlykke, A. Hearing and evasive behaviour in the greater wax moth, Galleria mellonella (Pyralidae). Physiol. Entomol. 25, 354–362 (2008).
Kwadha, C. A. Determination of Attractant Semio-Chemicals of the Wax Moth, Galleria mellonella L., in Honeybee Colonies. M.Sc. Thesis, University of Nairobi, Kenya (2017).
Pickard, S. C., Quinn, R. D. & Szczecinski, N. C. A dynamical model exploring sensory integration in the insect central complex substructures. Bioinspir. Biomim. 15, 026003. https://doi.org/10.1088/1748-3190/ab57b6 (2020).
Kamala Jayanthi, P. D., Saravan Kumar, P. & Vyas, M. Odour cues from fruit arils of artocarpus heterophyllus attract both sexes of oriental fruit flies. J. Chem. Ecol. 47, 552–563 (2021).
Anfora, G., Tasin, M., de Cristofaro, A., Ioriatti, C. & Lucchi, A. Synthetic grape volatiles attract mated Lobesia botrana females in laboratory and field bioassays. J. Chem. Ecol. 35, 1054–1062 (2009).
Fand, B. B. et al. Bacterial volatiles from mealybug honeydew exhibit kairomonal activity toward solitary endoparasitoid Anagyrus dactylopii. J. Pest Sci. 93, 195–206 (2020).
Kovats, E. Gas chromatographic characterization of organic substances in the retention index system. Adv. Chromotogr. 1, 229–247 (1965).
Acknowledgements
The authors thank the Director, of IIHR for providing research facilities. The financial support from the Indian Council of Agricultural Research through the National Fellow project and DBT- NER Chemical Ecology project is highly acknowledged. Special thanks to Dr. P.V. Rami Reddy for supporting us to conduct field experiments. We thank Sagar J, Mayuri Kashinath Shewale, Varun Y B and Nandini for their technical support with the handling of insects and maintenance of the culture.
Author information
Authors and Affiliations
Contributions
S.K.P, K.J.P.D and V.K. designed the study. S.K.P, D.S.D and G.K.R performed experiments. V.K, S.K.P and K.J.P.D contributed to the analysis and interpretation of the data. S.K.P drafted the manuscript and all authors contributed to the final version of the article and approved the submitted version. All illustrations were made by V.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Parepely, S.K., Kempraj, V., Sanganahalli Dharanesh, D. et al. The greater wax moth, Galleria mellonella (L.) uses two different sensory modalities to evaluate the suitability of potential oviposition sites. Sci Rep 13, 211 (2023). https://doi.org/10.1038/s41598-022-26826-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-26826-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.