Abstract
The lower Pecos River located in the southwest USA, is a naturally saline river system that has been significantly altered in relatively recent years. Climate change, coupled with anthropogenic disturbances such as dam construction have led to portions of the river becoming more susceptible to increased salinization and declines in water quality. These alterations have been documented to be detrimental to multiple freshwater communities; however, there is a lack of knowledge on how these alterations influence long-lived species in the river, such as freshwater turtles, where the effects can appear over dramatically different temporal scales. The Rio Grande Cooter (Pseudemys gorzugi) is a species of concern known to occur in the Pecos River. To understand the current distribution and habitat requirements for P. gorzugi in the Pecos River, we used a single-season, single-species occupancy modeling framework to estimate occurrence while accounting for the sampling process. Day of year, water surface area, and water visibility had the greatest influence on the ability to detect the species given a sampling unit is occupied. Conductivity (a measure of salinity) had the greatest influence on the occupancy probability for the species, where sites with higher conductivity coincided with lower occupancy probabilities. This study indicates that increased salinization on the lower Pecos River is a cause for concern regarding freshwater turtle populations within the Chihuahuan Desert.
Similar content being viewed by others
Introduction
The Pecos River, USA, is the largest tributary of the Rio Grande River, extending approximately 1500 river km from the southern Sangre de Cristo mountains in northeastern New Mexico and flowing southward through eastern New Mexico and west Texas where it joins the Rio Grande at the Mexico-USA border1,2,3. Geographically, the Pecos River is divided into three sections: the upper-, middle-, and lower-Pecos1,2. The hydrological regime varies greatly throughout the river system: (1) the upper Pecos is located within the alpine tundra of northeastern New Mexico, and water is mainly derived from mountain snowmelt; (2) the middle Pecos River stretches from the city of Santa Rosa to the city of Artesia, New Mexico, where water is mainly derived from springs; and (3) the lower Pecos River flows through the Chihuahuan Desert including the Permian Basin, from southeastern New Mexico to southwestern Texas, and southward until it joins the Rio Grande1. Water in the lower Pecos River is mainly derived from occasional thunderstorm runoff and it is regulated by several dams and reservoirs constructed in the middle and lower sections of the river1,2.
Historically, the Pecos River has been a vital water source to settlers for the irrigation of crops and livestock management, allowing domesticated livestock to survive the arid and otherwise harsh conditions of the Chihuahuan Desert and Permian Basin3,4. Native Americans, Spanish explorers, and frontier cattlemen used the river for drinking water for both humans and animals, even though portions were notably salty and foul-tasting4,5. The natural salinity of the middle to lower sections of the Pecos River is derived from salts entering the system through dissolved rock deposits (e.g., halite and gypsum) and underground brine2,4,5. Prior to development, the river was not too saline for freshwater organisms due to higher flood frequency and stronger streamflow5. However, since the late 1800s climate change has, in part, induced a reduction in flood frequency, decrease in streamflow, and increase of evapotranspiration, allowing the persistence of saline groundwater, and subsequently rising salinity levels in the lower Pecos River system3,4,5. Additionally, throughout the Chihuahuan Desert, most years have seen evapotranspiration rates greatly exceeding precipitation rates, resulting in frequent droughts and flash floods when precipitation does occur4. The southwestern USA is currently in a megadrought, the cause of which is attributed 19% to anthropogenically induced climate change6. Anthropogenic disturbances including oil production, agricultural irrigation practices, and dam construction have further allowed areas of the Pecos River to become more susceptible to excess salt buildup, reduction in historical flows, and an overall decline in water quality (e.g., low flows and accumulation of hazardous chemicals)5,7. Dissolved salts within the river system can be stored in lands used for irrigation during low flow events, and subsequently returned highly concentrated through return-flows and in periods of high flow events1. Non-native introductions of Saltcedar (Tamarix sp.) have proven to be detrimental to Pecos River aquatic habitats, as the trees consume vast quantities of river water and its underlying salts and subsequently deposit the salt back to the water’s surface through leaves3,4. From 1890 to 1980, dams and reservoirs were constructed in the Pecos River for flood control and irrigation storage, significantly altering the streamflow: Santa Rosa Dam and Sumner Dam in the middle Pecos, Brantley Dam (took place of McMillan Dam), Avalon Dam, and Red Bluff Dam2,3 in the lower Pecos.
The combination of increased salinity, droughts, and diminished flow may have long-term adverse effects on aquatic organisms, and potentially cause a permanent reduction of biodiversity in the Pecos River system2. Significant alterations of the Pecos River have negatively altered food webs8, fish diversity5,9,10, and mussel populations11, with some species found to be extirpated or greatly reduced5,9,10. However, the consequences of prolonged alterations of the Pecos River have not yet been investigated for long-lived species such as freshwater turtles. Turtles are valuable to their respective ecosystems through involvement in seed dispersal and germination12, bioturbation of soils13, and biomass contribution14. Freshwater turtles serve as bioindicators of environmental quality, as they are known to accumulate chemicals that reside in their respective water systems (e.g., pesticides, polybrominated diphenyl ethers [PBDEs], and polychlorinated biphenyls [PCBs])15,16,17. Despite their importance, turtles are amongst the most threatened groups of vertebrates, and their decline may consequently have detrimental impacts on the ecosystems in which they reside14.
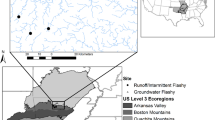
Pseudemys gorzugi is a large riverine turtle belonging to the pond turtle family Emydidae. The species is currently threatened in New Mexico and Mexico and is a species of greatest conservation need in Texas18,19,20,21. The species was evaluated for potential federal listing under the USA Endangered Species Act22, with a final decision in 2022 made to not list the species23. Current known threats to P. gorzugi include habitat degradation through anthropogenic modification of river flow, historical over-exploitation, recreational fishing, and recreational shooting22,24,25. As the westernmost species of its genus, P. gorzugi is native to southeast New Mexico and southwest Texas, USA, extending to Tamaulipas, Nuevo León and Coahuila in northeastern Mexico26,27,28. In the USA, the species is found along the lower Rio Grande River watershed and its tributaries from the city of Brownsville, Texas to the Big Bend region of west Texas and extending into the Devils and Pecos Rivers (Fig. 1)27. In New Mexico, P. gorzugi occurs in the lower Pecos River (i.e., downstream of Brantley Dam) including its tributaries, the Black and Delaware River22,24,26. Recently, the species was documented 80 km north of Brantley Dam in Berrendo Creek29. Primary studies on the species have been conducted in the tributaries of the Pecos and Rio Grande Rivers, especially the Black River in New Mexico and the Devils River in Texas, respectively. The Pecos River represents an important component of the range for P. gorzugi, acting as a corridor between the populations in the Pecos River and Rio Grande tributaries throughout New Mexico and Texas. However, the Pecos River itself has never been surveyed across the entirety of the species assumed range30,31. A study in New Mexico30 reported only four localities where P. gorzugi were captured on the main stretch of the Pecos River (with an additional locality at the Black-Pecos River confluence) while a study in Texas31 found the species at both of their surveyed sites. Neither of these studies, however, assessed relationships between habitat characteristics or environmental variables and the occurrence of the species while accounting for imperfect detection (i.e., failing to detect a species, given it is present).
As the Pecos River has been subjected to heavy anthropogenic modification in recent years, it is important to assess the occurrence of long-lived organisms such as P. gorzugi that may lack the ability to withstand increases in adult mortality32. Chelonians are especially vulnerable to sources of additive mortality due to their delayed sexual maturity and low annual recruitment32,33,34. Though P. gorzugi may appear abundant in the tributaries of the Pecos and Rio Grande Rivers, estimating the current distribution of the species along the Pecos River itself provides insight to potential changes from historical distribution, and environmental factors that influence the presence or absence of the species. We sought to conduct large-scale monitoring across the entirety of the potential distribution of P. gorzugi in the Pecos River and estimate the species’ occurrence by using a single-season, single-species occupancy modeling framework. We aimed to determine which environmental characteristics most influence the occurrence of P. gorzugi and use the results to better understand habitat requirements and aid in the potential conservation for the species.
Results
During 2 years of sampling (2020–2021), 32 sites were surveyed over three survey occasions each, for a total of 96 site visits. We captured 60 unique P. gorzugi from 14 of the 32 sampling sites. The estimated proportion of occupied sites was 48.08% (95% ci = 43.75–59.38%). Overall, male:female sex ratio was 2:1 and only two turtles were considered juveniles (< 110 mm straight line carapace length)35. The best-fit model (Table 1) indicated that the detection probability was most influenced by water visibility, water surface area of a survey site, and day of year. The odds of detecting P. gorzugi given a sampling unit was occupied decreased by 1.75 for every 0.41 m increase in visibility (Fig. 2a), and 2.79 for every 31,768 m2 increase in water surface area (Fig. 2b). In addition, the odds of detecting P. gorzugi given a site was occupied increased by a factor of 1.54 for every 30 d increase in day of year (Fig. 2c). Additionally, according to the third-ranked model (Table 2), the odds of detection increased by a factor of 1.16 for every 3.33 °C increase in water temperature. Using our survey protocol, the cumulative detection probability for P. gorzugi ranged from 0.63 (95% ci = 0.59–0.67) to 0.86 (95% ci = 0.84–0.88) after one to two surveys, respectively (Fig. 3). Detection probabilities reached 0.95 (95% ci = 0.94–0.96) to 0.99 (95% ci = 0.99–0.99) after three to five surveys, respectively (Fig. 3).
The cumulative detection probability of Rio Grande Cooter (Pseudemys gorzugi) along the lower Pecos River, USA, based on covariates (e.g., visibility, day of year, surface area) from the best-fit model using survey data from the summer months (May–August) 2020 and 2021. Note that 45 hoop-net traps were deployed for each survey occasion.
The probability a site was occupied by P. gorzugi was most influenced by conductivity (Table 1), with the odds a sampling unit being occupied decreased by a factor of 146 for every 8719 µS/cm increase in conductivity (Fig. 4). Of all recorded water parameters, conductivity varied the most, with a range of 1424–37,397 µS/cm. Conductivity levels gradually increased from the upstream-most sites to downstream-most sites, with relatively sharp spikes downstream from a dam. Conductivity levels decreased where the Pecos River reached confluences with rivers and creeks. Estimated probabilities of occurrence for P. gorzugi were the highest at sites above the Black River confluence in New Mexico, and near the Independence Creek and Rio Grande confluences in Texas, where conductivity levels were generally lower (Fig. 5).
Discussion
This study assesses the distribution of a desert freshwater turtle in one of the most anthropogenically altered river systems of the American southwest. This is the most extensive study on P. gorzugi on the lower Pecos River, and the only one to do so while accounting for the sampling process. Pseudemys gorzugi is of particular interest given its relatively small distribution and imperiled conservation status in comparison to other species found in this river system. The decision to not list P. gorzugi was based on assessments from detection-only data23, though the current detection/non-detection data along with model-based estimates of the species occurrence along the Pecos River itself would have been beneficial in evaluating the conservation status of the species along a major portion of its range. Pseudemys gorzugi is less tolerant of brackish waters, as lower occupancy probabilities were associated with higher conductivity levels (> 10,000 µS/cm). A distributional gap for P. gorzugi in the Pecos River has been speculated for many years but the extensive study design herein has revealed that higher conductivity levels may contribute to the now documented distributional hiatus of at least ~ 390 km of the Pecos River from Loving and Reeves Counties to Crockett County, Texas, USA (portions of the river with the lowest occupancy probabilities; Fig. 5). Crucially, this area may act as a connectivity barrier to the once continuous population of this turtle from New Mexico into Texas. A population genetic study found that New Mexico and Texas populations of P. gorzugi are relatively homogenous, indicating that the distribution was once continuous36. Therefore, we suggest that the distributional gap found in this study is anthropogenic, and that the populations may no longer be connected, which has implications for the resiliency of P. gorzugi along the Pecos River. Additionally, this may imply isolation of the Black River population from the rest of the species range. This suspected gap, along with rapid changes in environmental conditions and additive anthropogenic barriers have potential to influence the representation of P. gorzugi in the Pecos River system.
While P. gorzugi was detected at 43.75% of the sites, the estimated proportion of sites occupied is only slightly higher, at 48.08% (95% ci = 43.75–59.38%). Day of year, surface area, and water visibility at the time of survey most influenced detection probabilities, which provides insight to survey efficiency for the species. Detection probabilities were higher later in the trapping season around July and August (Fig. 2c). Pseudemys gorzugi was more likely to be detected if water visibility was low, suggesting that the turtles are more likely to enter the traps if they cannot visualize them in the water. Probabilities for detection were higher with lower water surface area, a proxy for trap density, which indicates that useful trapping methods for the species on the Pecos River could include high density hoop-net trap surveys37. Although the correlation between surface area and conductivity for each survey occasion was not strong (|r|< 0.7), some sites with lower conductivity levels had both high and low surface area, and sites with higher conductivity had lower surface area surveyed. With our survey design (45 traps for two consecutive days per survey occasion), cumulative detection probabilities were relatively high, with a probability of detection of 0.63 after only one survey (Fig. 3).
River salinization can contribute to physiological stress and mortality of organisms by altering the osmotic balance in tissues and cells8. Historical values of conductivity (proxy for salinity) on the lower Pecos River are not known. However, in the late 1990s and early 2000s, a study recorded conductivity values in select portions of the river from Red Bluff Dam to Girvin, Texas, as a part of the Pecos River Ecosystem Monitoring Project38. Recorded values in these portions of the river overlap seven of our sampling sites. By comparing mean conductivity levels recorded from 1999 to 200538, it is evident that the measurements taken more than two decades ago are lower than the values reported herein (~ 194–16,000 μS/cm increase). For example, mean conductivity levels increased from ~ 9900 to ~ 10,094 μS/cm near Red Bluff Dam and from ~ 8000 to ~ 20,467 μS/cm approximately 152 river km downstream from Red Bluff Dam. Freshwater organisms are typically not adapted to such high salinity levels, and high concentrations of salts can be toxic8,39. Studies have shown that excess salinity can alter food webs in freshwater systems8,40, and can trigger trophic cascades in ecological communities40. Specifically, declines in zooplankton and increases in phytoplankton have been reported in systems with excess salinity and the presence of fish40. In systems with high salinity and the absence of fish, decreases in filamentous algae were reported40. In the lower Pecos River, the food web composition varies greatly8. Trophic diversity is highest near the spring fed tributaries, where the conductivity is lowest8, which is concurrent with higher occupancy probabilities for P. gorzugi reported herein. Throughout the main stem of the river, however, terrestrial organic matter input is low and there is a lower functional diversity, with the predominant species being salt-tolerant benthic macroinvertebrates and non-native euryhaline fishes8. Pseudemys gorzugi diet consists of algae, dicot vegetation, and arthropods41,42,43. Therefore, in addition to the apparent low tolerance of P. gorzugi to high salinity levels, it is also plausible that salinity along with the reduction in trophic diversity and potential lack of food source items has collectively contributed to the absence of the species in the main stem of the river (i.e., where occupancy probabilities are lowest). Worth noting is that no turtles of any species were captured at the five sites with the highest conductivity levels (19,500–37,397 µS/cm), indicating negative impacts on even generalist turtle species, such as T. s. elegans and A. s. emoryi. Furthermore, at one of the five sites, salt crystal formations were present on the vascular plants that grew along the riverbank (i.e., Saltgrass [Distichlis stricta]). There were also three sites where crude oil was present on the vegetation (e.g., Saltcedar [Tamarix sp.]) along the riverbank and on the surface of the water. This provides evidence that pollution and high salinity in the Pecos River may have negative implications on the entire freshwater turtle community, which warrants further investigation.
Dams can have profound effects on freshwater biodiversity; however, these effects have not been extensively studied in freshwater turtle populations, especially threatened species. A recent literature review44 on the effects of dams in turtle populations found only 43 published studies and emphasized the need for more evaluations for these long-lived organisms. Most of these studies focused on flow changes, barrier effects, and nesting success influenced by dams44. Previous reports on the lower Pecos River indicate climate change and anthropogenic processes including dam construction have significantly altered stream flow in relatively recent years2,3,5, subsequently increasing salinity levels throughout the river. Our study is a significant contribution to this important conservation topic, as it is the first to demonstrate how these riverine changes can in turn affect the distribution of a near threatened freshwater turtle. Turtle conservation in the Pecos River system would benefit from future studies focused on heavy metal contamination of turtles and an evaluation of whether the apparent gap in distribution found in this study has any consequences on species genetic diversity.
Methods
This study was conducted on the lower Pecos River in New Mexico and Texas, USA, between 2020 and 2021. A total of 32 sites were surveyed. Upstream of Red Bluff Dam (Texas-New Mexico border), 17 sites were selected based on available public land access and lands managed by the Bureau of Land Management (BLM). Distances between each site ranged from ~ 3 to 26 river km. Downstream of Red Bluff Dam, site selection was limited to bridge crossings (n = 13) and river access permission granted by the National Park Service (n = 2) and a private landowner (n = 1) as 95% of Texas is privately owned. Distances between downstream sites ranged from ~ 4 to 121 river km. Each site was sampled for three survey occasions within a season.
Reliable methods to capture P. gorzugi involves high intensity surveys using baited hoop-net traps37. To capture turtles, we deployed 45 standard hoop-net traps (50.8 cm diameter and 2.54 cm mesh size; Memphis Net & Twine Co. Memphis, Tennessee, USA), baited with sardines, for 48 h per site, per survey occasion. Additionally, to conserve and evenly distribute resources, every third trap contained a single leaf of romaine lettuce. Traps were checked within the first 24 h, and subsequently pulled at 48 h. Trap theft occurred at two sites, and personnel limitations at another site resulted in the traps remaining in the water for an additional 24 h. The overall trap effort included a range of 269–315 trap days per site.
Environmental and habitat conditions that were hypothesized to influence the occurrence and detection of P. gorzugi were recorded (Table 3). Water quality parameters measured at each site included pH, conductivity (µS/cm), nitrates (mg/L), dissolved oxygen (mg/L), flow (m/s), and temperature (°C). Conductivity, pH, nitrates, and dissolved oxygen were measured using YSI Pro Plus Multiparameter instruments (YSI Incorporated, Yellow Springs, OH, USA). Conductivity is used as a proxy for salinity, as conductance refers to the water’s ability to create an electrical current through ions (i.e., dissolved salt ions)45. Salinity in the lower Pecos River is mainly attributed to NaCl ions derived from underground brine and halite crystal formations2. Conductivity and pH were recorded once per site, per survey occasion. Due to probe availability and limited access to the previous year’s sites (e.g., flood damage to dirt roads in 2021), nitrates and dissolved oxygen were measured only in 2021. To accommodate for missing nitrate and dissolved oxygen measurements for the 2020 sites, values were estimated using the measurements of the nearest 2021 site (closest and furthest distances between the sites were ~ 3 river km and ~ 94 river km, respectively), or the mean values of two 2021 sites of similar distance located on either side of the 2020 site (< 1 river km difference in distances).
Flow was recorded three times per site, per survey occasion. For survey sites in New Mexico, flow was measured by inserting a Flowatch® Flowmeter (JDC Instruments, Switzerland) into the river and recording the maximum flow after 15 s. Flow in Texas was measured by inserting an OTT MF pro meter (OTT HydroMet, Germany) and recording the minimum and maximum flow for 15 s. Water temperature was recorded every 4 h by attaching a HOBO Pendant® MX Temperature/Light Data Logger (ONSET, Bourne, MA, USA) to the third hoop of the hoop-net trap. There were malfunctions of HOBO loggers at six sites, during one survey occasion each. For the sites and survey occasions where temperature recordings failed, linear regression equations were created using recorded water temperatures of the other survey occasions at each site of interest and the corresponding air temperatures from the nearest approximate time and location of survey46. Air temperatures were accessed from timeanddate.com, which includes hourly weather recordings (temperature, precipitation, pressure, cloud cover etc.) of the nearest city derived from forecasting models by CustomWeather©. Additionally, measurements are retrieved by CustomWeather© from stations run by the Meteorological Assimilation Data Ingest System (MADIS) and the World Meteorological Association (WMO). Missing values were then estimated using the linear equations. Additionally, we documented the daily atmospheric conditions of each site, per survey (cloudy or rain or sunny and partly cloudy).
The species is thought to prefer deep, slow moving pools19, therefore depth was measured three times per site, per survey occasion approximately in the center of the river at the beginning, middle, and end of the surveyed stretch by submerging a 10-pound dumbbell weight with a nylon rope marked every 0.5 m. Surface area (m2), a proxy for trap density given the consistency in traps deployed each survey occasion, was calculated per site, per survey occasion using the polygon tool in Google Earth Pro. Polygons were created by outlining the length of each surveyed section of the river using Google Earth Pro satellite imagery (2016–2019) in conjunction with the GPS coordinates of the first, middle, and last traps as reference points. To measure river width (m), 15 evenly spaced lines were created within each polygon and the minimum, maximum, and mean measurements were documented once per survey occasion. Water visibility (i.e., turbidity) was measured three times per site, per survey occasion to the nearest 0.25 m by submerging a Secchi disk and recording the depth at which visibility was lost and regained.
As P. gorzugi rely on basking for thermoregulation year-round47, the presence/absence of basking structures (i.e., fallen logs) was recorded categorically. Pseudemys goruzgi is also thought to prefer areas abundant with aquatic vegetation for cover, foraging, and basking26,27. Therefore, vegetation was documented categorically based on the presence or absence of emergent aquatic vegetation at each site (cattails [Typha sp.] and giant reed [Arundo donax]). We recorded the visual observation of any turtle species (i.e., turtle heads above water) categorically, as a proxy for turtle activity (e.g., foraging activity). Although P. gorzugi are known to exit the water for nesting, there are no specific studies on nest distances from water for the species. A preliminary study48 reported nest distances of approximately 2–36 m from water, indicating that surrounding landscape use may be of importance. To account for surrounding landscape condition of each site, we used the NatureServe Landscape Condition Model (LCM). The LCM uses spatial data from LANDFIRE, USGS ReGap, and National Land Cover Data in the USA to rank the landscape according to the ecological conditions (from 0 [poor] to 1 [excellent]) as a metric of habitat integrity49. Values were calculated for 100 m buffers around each site (i.e., water surface area polygon) created in ArcMap50.
Occupancy models are a statistical approach used to estimate relationships between ecosystem conditions and the occurrence of species while accounting for imperfect detection51,52. Occupancy modeling uses coupled Bernoulli processes and replicate detection/non-detection survey data to estimate occupancy (ψ) and detection (p) probabilities of a species, respectively53. Occupancy probability refers to the probability that a sample unit within the larger study area selected at random is occupied by a species of interest, and detection probability refers to the probability of detecting the species on a survey occasion, given that the sample unit is occupied by the species of interest53,54. The probabilities are explained through explanatory variables using logistic regression53. This sampling design was created to meet the assumptions of occupancy models53: (1) the occupancy state does not change within a single-season (May–August) at a sampling unit; (2) detection probability was independent across sites and survey occasions, using a minimum of 3 km distance between sites and 27 days between survey occasions; (3) the detection and occupancy probabilities were explained through the site-specific and survey-specific covariates chosen based on the study objectives and natural history of the species (Table 3); and (4) false positives do not occur due to proper species identification. Traditionally, P. gorzugi was considered a sedentary species making short distance movements (~ 300 m) within their habitat22,26. More recently, movements of ~ 1.2 km were reported over short-term periods55, while long-distance movements can also occur over longer time periods56. We used over double the maximum recorded short-term distance moved as our minimum distance between sites to maximize our ability to meet the closure and independence assumptions of occupancy models.
We fit single-season, single-species occupancy models using detection/non-detection P. gorzugi data collected from the 32 sites using the unmarked package in program R57,58 to estimate detection and occupancy probabilities. As turtles are long-lived, we used site-specific covariates assuming they are representative of long-term habitat conditions (Table 3): mean pH, mean conductivity, nitrates, dissolved oxygen, mean water visibility, maximum flow, maximum depth, vegetation type (0 = no aquatic vegetation, 1 = aquatic vegetation), the presence/absence of basking structures, and the mean landscape condition value. Survey-specific covariates that could vary across survey occasions included the day of year (ordinal date), observable atmospheric conditions, visual presence/absence of turtles, water temperature, water surface area, and water visibility at time of survey (Table 3). We compared the mean water temperatures of each site and found the coefficient of variation to be 0.06. Therefore, we felt that there was too little variation in temperatures at the site level to include as a site-specific covariate.
Prior to fitting models, all continuous covariates were standardized to have a mean of zero and a standard deviation of one for comparison across a common scale. Covariates were then checked for collinearity using Pearson’s correlation coefficient. Nitrates and pH were excluded from the model as they were highly correlated with other parameters of interest (|r| > 0.7). Models were then constructed using a sequential-by-submodel strategy59. First, we compared all possible models for detection while holding occupancy constant. The detection models were compared using Akaike Information Criterion values corrected for a small sample size (AICc)60, where we considered submodels important if they had a ΔAICc < 5. We then carried important detection probability submodels forward and fit them with all possible submodel combinations for occupancy, while removing any combination of covariates that resulted in convergence issues. Our final model set was limited to the submodels that had a ΔAICc < 5 in each step of this process, however we only consider the models with ΔAICc < 2 as our top ranked models with similar support (i.e., competing models). This approach was used because it has been shown to recover the top-ranked model, recover a substantial portion of the total AICc model weight, and reduce the number of models fit when compared to fitting every combination of covariates for a simple occupancy analyses59. Odds ratio was used to interpret the effects of the top covariates, as effect sizes remain constant across changes in the predictor variables (i.e., covariates)61. Using the best-fit model, we examined the predicted occupancy probabilities at each survey site. We calculated the mean occupancy probability of two sites (i.e., site 1 vs. site 2, and site 2 vs. site 3, etc.) to approximately visualize occupancy probabilities between each survey stretch (Fig. 5).
With the best-fit model we also estimated the number of surveys needed to achieve different levels of cumulative detection probability (p*), given the species was present at a survey site (Fig. 3). In particular, we used the best fit detection probability model to estimate the detection probability (mean and variance) for each survey occasion in our study. Using the package mvmeta62 in program R58, a method-of-moments estimator for multivariate random-effects meta-analysis63 was used to combine these survey occasion specific estimates and calculate an overall mean (and variance) detection probability per survey occasion. The cumulative detection probability was then calculated as p* = 1 – (1 – p)n, where n is the number of surveys. We incorporated uncertainty in our estimates of p* using a parametric bootstrap simulation approach where we randomly sampled p from a beta distribution using the overall mean and variance estimates for detection probability. Scenarios were run for a number of surveys ranging from one to ten, in increments of one. Combinations were then run for 10,000 iterations, and subsequently summarized by their means and 0.025th and 0.975th percentile (i.e., a 95% confidence interval).
Ethics declarations
Research was approved with permits from the New Mexico Department of Game and Fish (3621), Texas Parks and Wildlife Department (SPR-0615-085/SPR-0102-191), and National Park Service (AMIS-2021-SCI-0005), as well as Eastern New Mexico University and Texas State University IACUC (2019-0226-01A1 and 2015-004, respectively). All procedures were performed in accordance with the relevant guidelines and regulations of each institution. ARRIVE guidelines: not applicable.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to sensitive conservation status of the species of interest. Data may be available from the corresponding author on reasonable request.
References
Yuan, F. & Miyamoto, S. Dominant processes controlling water chemistry of the Pecos River in American Southwest. Geophys. Res. Lett. 32(17), L17406. https://doi.org/10.1029/2005GL023359 (2005).
Yuan, F., Miyamoto, S. & Anand, S. Changes in major element hydrochemistry of the Pecos River in the American Southwest since 1935. Appl. Geochem. 22(8), 1798–1813. https://doi.org/10.1016/j.apgeochem.2007.03.036 (2007).
Harley, G. L. & Maxwell, J. T. Current declines of Pecos River (New Mexico, USA) streamflow in a 700-year context. Holocene 28(5), 766–777. https://doi.org/10.1177/0959683617744263 (2018).
Jensen, R., Hatler, W., Mecke, M. & Hart, C. The Influences of Human Activities on the Water of the Pecos River Basin of Texas: A Brief Overview. Technical Report. SR-2006-03. Texas Water Resources Institute (2006).
Hoagstrom, C. W. Causes and impacts of salinization in the lower Pecos River. Gt. Plains Res. 19(1), 27–44 (2009).
Williams, A. P., Cook, B. I. & Smerdon, J. E. Rapid intensification of the emerging North American megadrought in 2020–2021. Nat. Clim. Change 12(3), 232–234. https://doi.org/10.1038/s41558-022-01290-z (2022).
Cheek, C. A. & Taylor, C. M. Salinity and geomorphology drive long-term changes to local and regional fish assemblage attributes in the lower Pecos River, Texas. Ecol. Freshw. Fish 25(3), 340–351. https://doi.org/10.1111/eff.12214 (2015).
Pease, A. A. & Delaune, K. D. Dried and salted: cumulative impacts of diminished flows and salinization on the lower Pecos River food webs. In Proceedings of the Desert Fishes Council Special Publication. Vol. 2021, 2–19. https://doi.org/10.26153/tsw/12364 (2021)
Linam, G. W. & Kleinsasser, L. J. Relationships Between Fishes and Water Quality in the Pecos River, Texas. River Studies Report. No. 9. Texas Parks and Wildlife Department (1996).
Hoagstrom, C. W., Zymonas, N. D., Davenport, S. R., Propst, D. L. & Brooks, J. E. Rapid species replacements between fishes of the North American plains: A case history from the Pecos River. Aquat. Invasions 5(2), 141–153. https://doi.org/10.3391/ai.2010.5.2.03 (2010).
Randklev, C. R. et al. A semi-arid river in distress: Contributing factors and recovery solutions for three imperiled freshwater mussels (Family Unionidae) endemic to the Rio Grande Basin in North America. Sci. Total Environ. 631–632, 733–744. https://doi.org/10.1016/j.scitotenv.2018.03.032 (2018).
Kimmons, J. B. & Moll, D. Seed dispersal by Red-eared sliders (Trachemys scripta elegans) and Common snapping turtles (Chelydra serpentina). Chelonian Conserv. Biol. 9(2), 289–294. https://doi.org/10.2744/CCB-0797.1 (2010).
Lazar, B. et al. Loggerhead sea turtles (Caretta caretta) as bioturbators in neritic habitats: An insight through the analysis of benthic molluscs in the diet. Mar. Ecol. 32(1), 65–74. https://doi.org/10.1111/j.1439-0485.2010.00402.x (2011).
Lovich, J. E., Ennen, J. R., Agha, M. & Gibbons, J. W. Where have all the turtles gone, and why does it matter?. Bioscience 68(10), 771–781. https://doi.org/10.1093/biosci/biy095 (2018).
de Solla, S. R., Fernie, K. J. & Ashpole, S. Snapping turtles (Chelydra serpentina) as bioindicators in Canadian areas of concern in the Great Lakes Basin. II. Changes in hatching success and hatchling deformities in relation to persistent organic pollutants. Environ. Pollut. 153(3), 529–536. https://doi.org/10.1016/j.envpol.2007.09.017 (2008).
Adams, C. I. M., Baker, J. E. & Kjellerup, B. V. Toxicological effects of polychlorinated biphenyls (PCBs) on freshwater turtles in the United States. Chemosphere 154, 148–154. https://doi.org/10.1016/j.chemosphere.2016.03.102 (2016).
Beau, F., Bustamante, P., Michaud, B. & Brischoux, F. Environmental causes and reproductive correlates of mercury contamination in European pond turtles (Emys orbicularis). Environ. Res. 172(4), 338–344. https://doi.org/10.1016/j.envres.2019.01.043 (2019).
van Dijk, P. P. Pseudemys gorzugi (errata version published in 2016). The IUCN Red List of Threatened Species Vol. 2011, e.T18459A97. (2011).
NMDGF [New Mexico Department of Game and Fish]. Threatened and Endangered Species of New Mexico, 2020 Biennial Review. Management and Fisheries Management Divisions (2020).
SEMARNAT [Secretaríade Medio Ambiente y Recursos Naturales]. NORMA Oficial Mexicana NOM-059-SEMARNAT-2010, Protección ambiental–Especies nativas de México de flora y fauna silvestres–Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio–Lista de especies en riesgo. Diario Oficial de la Federación Vol. 2 (2010).
TPWD [Texas Parks & Wildlife Department]. Species Account: the Rio Grande River Cooter (Pseudemys gorzugi). In Texas Comprehensive Wildlife Conservation Strategy 2005–2010 (eds Bender, S., Shelton, S., Bender, K. & Kalmbach, A.). Nongame Division, 1075–7076 (2012).
Pierce, L. J. S., Stuart, J. N., Ward, J. P. & Painter, C. W. Pseudemys gorzugi Ward 1984–Rio Grande Cooter, Western River Cooter, Tortuga de Oreja Amarilla, Jicotéa del Rio Bravo In Conservation Biology of Freshwater Turtles and Tortoises: A Compilation Project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group (eds. Rhodin, A. G. J. et al.). Chelonian Res. Monog. Vol. 5, No. 9, 100.1–100.12. https://doi.org/10.3854/crm.5.100.gorzugi.v1.2016 (2016).
Endangered and Threatened Wildlife and Plants. Endangered and Threatened Wildlife and Plants; three species not warranted for listing as endangered or threatened species. Fed. Reg. 87(49), 14227–14228 (2022).
Bailey, L. A., Forstner, M. R. J., Dixon, J. R. & Hudson, R. Contemporary status of the Rio Grande Cooter (Testudines: Emydidae: Pseudemys gorzugi) in Texas: phylogenetic, ecological and conservation consideration. In Proceedings of the Sixth Symposium on the Natural Resources of the Chihuahuan Desert Region (eds. Hoyt, C. A. & Karges, J.) 320–324. (Chihuahuan Desert Research Institute, 2014).
Suriyamongkol, T., Waldon, K. J. & Mali, I. Trachemys scripta (Red-eared Slider) and Pseudemys gorzugi (Rio Grande Cooter). Fish hook ingestion and shooting. Herpetol. Rev. 50(4), 776–777 (2019).
Degenhardt, W. G., Painter, C. W. & Price, A. H. Amphibians and Reptiles of New Mexico (University of New Mexico Press, 1996).
Ernst, C. H. Turtles of the United States and Canada 2nd edn. (Johns Hopkins University Press, 2009).
Dixon, J. R. Amphibians and Reptiles of Texas: With Keys, Taxonomic Synopses, Bibliography, and Distribution Maps 3rd edn. (Texas A&M University Press, 2013).
Suriyamongkol, T. et al. Geographic distribution. Pseudemys gorzugi (Rio Grande Cooter). Herpetol. Rev. 51(3), 536–537 (2020).
Christman, B. L. & Kamees, L. K. Current Distribution of the Blotched Watersnake (Nerodia erythrogaster) and the Rio Grande Cooter (Pseudemys gorzugi) in the Lower Pecos River System Eddy County, New Mexico 2006–2007. Final Report. New Mexico Department of Game and Fish (2007).
Bogolin, A. P., Davis, D. R., Ruppert, K. M., Kline, R. J. & Rahmann, A. F. Geographic distribution. Pseudemys gorzugi (Rio Grande Cooter). Herpetol. Rev. 50(4), 745 (2019).
Congdon, J. D., Dunham, A. E. & Van Loben Sels, R. C. Demographics of common snapping turtles (Chelydra serpentina): Implications for conservation and management of long-lived organisms. Am. Zool. 34, 397–408. https://doi.org/10.1093/icb/34.3.397 (1994).
Brooks, R. J., Brown, G. P. & Galbraith, D. A. Effects of a sudden increase in natural mortality of adults on a population of the common snapping turtle (Chelydra serpentina). Can. J. Zool. 69, 1314–1320. https://doi.org/10.1139/z91-185 (1991).
Congdon, J. D., Dunham, A. E. & Van Loben Sels, R. C. Delayed sexual maturity and demographics of Blanding’s turtles (Emydoidea blandingii): Implications for conservation and management of long-lived organisms. Conserv. Biol. 7(4), 826–833. https://doi.org/10.1046/j.1523-1739.1993.740826.x (1993).
Suriyamongkol, M. & Mali, I. Aspects of the reproductive biology of the Rio Grande Cooter (Pseudemys gorzugi) on the Black River, New Mexico. Chelonian Conserv. Biol. https://doi.org/10.2744/CCB-1385.1 (2019).
Bailey, L. A., Dixon, J. R., Hudson, R. & Forstner, M. R. J. Minimal genetic structure of the Rio Grande Cooter (Pseudemys gorzugi). Southwest. Nat. 53(3), 406–411. https://doi.org/10.1894/GC-179.1 (2008).
Mali, I., Duarte, A. & Forstner, M. R. J. Comparison of hoop-net trapping and visual surveys to monitor abundance of the Rio Grande Cooter (Pseudemys gorzugi). PeerJ 6, e4677:1-16. https://doi.org/10.7717/peerj.4677 (2018).
Hart, C. R., McDonald, A. & Hatler, W. Pecos River Ecosystem Monitoring Project. Technical Report. Texas Cooperative Extension: The Texas A&M University System. (2005).
Hong, M., Zhang, K., Shu, C., Xie, D. & Shi, H. Effect of salinity on the survival, ions, and urea modulation in Red-eared Slider (Trachemys scripta elegans). Asian Herpetol. Res. 5(2), 128–136. https://doi.org/10.3724/SP.J.1245.2014.00128 (2014).
Hintz, W. D. et al. Salinization triggers a trophic cascade in experimental freshwater communities with varying food-chain length. Ecol. Appl. 27(3), 833–844. https://doi.org/10.1002/eap.1487 (2017).
Letter, A. W., Waldon, K. J., Pollock, D. A. & Mali, I. Dietary habits of Rio Grande Cooters (Pseudemys gorzugi) from two sites within the Black River, Eddy County, New Mexico, USA. J. Herpetol. 53(3), 204–208. https://doi.org/10.1670/18-057 (2019).
Suriyamongkol, T., Ortega-Berno, V., Mahan, L. B. & Mali, I. Using stable isotopes to study resource partitioning between Red-eared Slider and Rio Grande Cooter in the Pecos River watershed. Ichthyol. Herpetol. 110(1), 96–105. https://doi.org/10.1643/h2021023 (2022).
Bassett, L. G., Mali, I., Nowlin, W. H., Foley, D. H. & Forstner, M. R. J. Diet and isotopic niche of the Rio Grande Cooter (Pseudemys gorzugi) and syntopic Red-eared Slider (Trachemys scripta elegans) in San Felipe Creek, Texas, USA. Chelonian Conserv. Biol. (in Press).
Bárcenas-García, A. et al. Impacts of dams on freshwater turtles: A global review to identify conservation solutions. Trop. Conserv. Sci. 15(4), 1–21. https://doi.org/10.1177/194008292211037098 (2021).
Smith, M. J. et al. Association between anuran tadpoles and salinity in a landscape mosaic of wetlands impacted by secondary salinisation. Freshw. Biol. 52(1), 75–84. https://doi.org/10.1111/j.1365-2427.2006.01672.x (2007).
Wohner, P. J. et al. Integrating monitoring and optimization modeling to inform flow decisions for Chinook salmon smolts. Ecol. Model. 471(2022), 110058. https://doi.org/10.1016/j.ecolmodel.2022.110058 (2022).
Suriyamongkol, T., Tian, W. & Mali, I. Monitoring the basking behavior of Rio Grande Cooter (Pseudemys gorzugi) through game camerias in southeastern New Mexico, USA. West. N. Am. Nat. 81(3), 361–371. https://doi.org/10.3398/064.081.0305 (2021).
Painter, C. W. Preliminary Investigations of the Distribution and Natural History of the Rio Grande River Cooter (Pseudemys gorzugi) in New Mexico. Preliminary Report. (United States Department of the Interior–Bureau of Land Management, 1993).
Hak, J. C. & Comer, P. J. Modeling landscape condition for biodiversity assessment—Application in temperate North America. Ecol. Indic. 82, 206–216. https://doi.org/10.1016/j.ecolind.2017.06.049 (2017).
ESRI. ArcGIS Desktop. Ver. 10.8 (Environmental System Research Institute, 2020).
MacKenzie, D. I. et al. Estimating site occupancy rates when detection probabilities are less than one. Ecology 83(8), 2248–2255. https://doi.org/10.1890/0012-9658(2002)083[2248:ESORWD]2.0.CO;2 (2002).
Tyre, A. J. et al. Improving precision and reducing bias in biological surveys: Estimating false-negative error rates. Ecol. Appl. 13(6), 1790–1801. https://doi.org/10.1890/02-5078 (2003).
Mackenzie, D. I. et al. Occupancy Estimation and Modeling: Inferring Dynamics of Species Occurrence 2nd edn. (Elsevier, 2017).
Duarte, A., Whitlock, S. L. & Peterson, J. T. Species distribution modeling. In Encyclopedia of Ecology 2nd edn (ed. Fath, B. D.) (Elsevier, 2019).
MacLaren, A. R., Foley, D. H., Sirsi, S. & Forstner, M. R. J. Updating methods of satellite transmitter attachment for long-term monitoring of the Rio Grande Cooter (Pseudemys gorzugi). Herpetol. Rev. 48(1), 48–52 (2017).
MacLaren, A. R., Sirsi, S., Foley, D. H. & Forstner, M. R. J. Pseudemys gorzugi (Rio Grande Cooter). Long distance dispersal. Herpetol. Rev. 48(1), 180–181 (2017).
Fiske, I. & Chandler, R. unmarked: An R package for fitting hierarchical models of wildlife occurrence and abundance. J. Stat. Softw. 43(10), 1–23. https://doi.org/10.18637/jss.v043.i10 (2011).
R Core Team. R: A Language and Environment for Statistical Computing (Foundation For Statistical Computing, 2021).
Morin, D. J. et al. Is your ad hoc model selection strategy affecting your multimodel inference?. Ecosphere 11(1), e02997. https://doi.org/10.1002/ecs2.2997 (2020).
Burnham, K. P. & Anderson, D. R. Model Selection and Inference: A Practical Information-Theoretic Approach 1st edn. (Springer, XXX, 1998).
Hosmer, D. W., Lemeshow, S. & Sturdivant, R. X. Applied Logistic Regression 3rd edn. (Wiley, 2013).
Gasparrini, A., Armstrong, B. & Kenward, M. G. Multivariate meta-analysis for non-linear and other multi-parameter associations. Stat. Med. 31(29), 3821–3839. https://doi.org/10.1002/sim.5471 (2012).
Jackson, D., White, I. R. & Riley, R. D. A matrix-based method of moments for fitting the multivariate random effects model for meta-analysis and meta-regression. Biom. J. 55(2), 231–245. https://doi.org/10.1002/bimj.201200152 (2013).
Acknowledgements
We thank private landowners, the Bureau of Land Management, and the National Park Service for permission and assistance in accessing sampling sites. This work was funded by the National Fish and Wildlife Foundation (project #63348) and the New Mexico Water Resources Research Institute. A. Duarte would also like to acknowledge support from the U.S.D.A Forest Service, Pacific Northwest Research Station. We also thank representatives from Eastern New Mexico University, Sul Ross State University, and Texas State University for assistance in the field. The use of trade or firm names in this publication is for reader information and does not imply endorsement by the U.S. Government of any product or service.
Funding
This project was funded by the National Fish and Wildlife Foundation (project #63348) and the New Mexico Water Resources Research Institute. A. Duarte would also like to acknowledge support from the U.S.D.A Forest Service, Pacific Northwest Research Station.
Author information
Authors and Affiliations
Contributions
I. M. conceived the initial idea for the project and acquired funding. I. M. and M. R. J. provided equipment for surveys and assisted with data collection. L. B. M. and L. G. B. performed the majority of data collection. I. M. and A. D. assisted in statistical analyses. L. B. M. conducted statistical analyses and wrote the initial draft. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mahan, L.B., Bassett, L.G., Duarte, A. et al. Effects of salinization on the occurrence of a long-lived vertebrate in a desert river. Sci Rep 12, 15907 (2022). https://doi.org/10.1038/s41598-022-20199-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-20199-3
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.