Abstract
The Axin family of scaffolding proteins control diverse processes, such as facilitating the interactions between cellular components and providing specificity to signaling pathways. While several Axin family members have been discovered in metazoans and shown to play crucial roles, their mechanism of action are not well understood. The Caenorhabditis elegans Axin homolog, pry-1, is a powerful tool for identifying interacting genes and downstream effectors that function in a conserved manner to regulate Axin-mediated signaling. Our lab and others have established pry-1’s essential role in developmental processes that affect the reproductive system, seam cells, and a posterior P lineage cell, P11.p. Additionally, pry-1 is crucial for lipid metabolism, stress responses, and aging. In this study, we expanded on our previous work on pry-1 by reporting a novel interacting gene named picd-1 (pry-1-interacting and Cabin1 domain-containing). PICD-1 protein shares sequence conservation with CABIN1, a component of the HUCA complex. Our findings have revealed that PICD-1 is involved in several pry-1-mediated processes, including stress response and lifespan maintenance. picd-1’s expression overlapped with that of pry-1 in multiple tissues throughout the lifespan. Furthermore, PRY-1 and PICD-1 inhibited CREB-regulated transcriptional coactivator homolog CRTC-1, which promotes longevity in a calcineurin-dependent manner. Overall, our study has demonstrated that picd-1 is necessary for mediating pry-1 function and provides the basis to investigate whether Cabin-1 domain-containing protein plays a similar role in Axin signaling in other systems.
Similar content being viewed by others
Introduction
Signaling pathways enable cells to communicate with each other and their environment. Because of their essential role in cells, these pathway components are tightly regulated via interaction with a host of cellular factors. The Axin family is a group of scaffolding proteins that bring together different proteins to facilitate their interactions and regulate their activity1. There are two Axin homologs in mammals: Axin1 and Axin2. Axin was initially discovered as a negative regulator of the WNT-mediated signaling cascade. However, subsequent studies revealed a much broader role of these proteins in other pathways, including JNK, AMPK, and TGFβ1,2,3,4. Axin regulates several processes, including organogenesis, anterior–posterior axis formation, neuronal development, and metabolic homeostasis. Further, loss of Axin function causes lethality, neuroectodermal defects, abnormal body axis patterning, and reduced adipogenesis1. However, the mechanism by which Axin regulates different biological processes and mediates specific interactions is not well understood.
In the nematode C. elegans, the Axin homolog PRY-1 controls processes such as embryogenesis, neuronal differentiation, vulval development, P11.p cell fate, and seam cell development1,5,6,7. The WNT-dependent function of PRY-1 in vulval cells involves its interactions with several other proteins to form a destruction complex that results in the phosphorylation and degradation of BAR-1 (β-Catenin)5. However, little is known about the factors that interact with PRY-1 in WNT-independent processes. A comprehensive understanding of pry-1 function will require identification of its interacting proteins and downstream effectors. To this end, we previously performed a transcriptome profiling of pry-1 that revealed novel interacting partners and genetic network of pry-1 that regulate post-developmental events6,8. Specifically, we showed that PRY-1 is crucial for lipid metabolism, stress response, and lifespan maintenance, where it interacts with WNT-independent signaling pathway components4,8,9,10,11. These include SBP-1/SREBP and vitellogenin involved in fatty acid synthesis and lipid storage, AAK-2/AMPK in the muscle that non-autonomously activates DAF-16/FOXO in the intestine and delays aging8,10, and the components of the endoplasmic reticulum unfolded protein response (ER-UPR) pathway. Additionally, in a separate study, we reported genes regulated by pry-1 during stress response and lifespan maintenance that include cpz-1 (proteolysis), cdk-1 (cell cycle), rnr-1 (DNA replication), his-7 (gene expression), and ard-1 (mitochondrial oxidation/reduction)7.
This paper reports a novel downstream effector of pry-1 signaling called picd-1 that is critical for regulating multiple developmental and post-developmental processes. PICD-1 shares a domain with the mammalian calcineurin-binding protein 1 (CABIN1), a component of the histone H3.3 chaperone complex HUCA12. cabin1 negatively regulates calcineurin signaling, which in turn affects various cellular functions, including stress response and lifespan13,14,15,16. We show that PICD-1 negatively regulates CREB-regulated transcriptional coactivator (CRTC) homolog, CRTC-1, which promotes longevity mediated by calcineurin signaling17. Consistent with the pry-1’s role in promoting picd-1 expression, pry-1 mutants exhibit nuclear localization of CRTC-1, suggesting that PICD-1 is involved in PRY-1-mediated CRTC-1 regulation. These results demonstrate the critical role of PICD-1 in C. elegans and prompt future studies to investigate the involvement of Cabin1 and calcineurin signaling in Axin-mediated processes in eukaryotes.
Results
picd-1 encodes a CABIN1 domain-containing protein
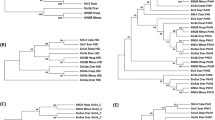
A CRISPR-based screening was carried out earlier to isolate alleles of pry-16 (see “Methods”). In that screen we also discovered a mutation (gk3701) in an unrelated gene F56E10.1 (WBGene00018975), now named picd-1 (pry-1 interacting and Cabin1 domain-containing, see “Methods”). The pry-1(gk3681); picd-1(gk3701) double mutant exhibited a significant increase in the protruding-vulva (Pvl) phenotype (77%, compared to 66% in pry-1 mutants alone) and pronounced protrusions that frequently burst in the vulva (Table 1, Fig. 1A,B, Video S1). It should be noted that gk3681 is identical to another pry-1 mutation, gk3682, and both mutant strains were recovered in the same CRISPR screen (see “Methods” and Figure S1). Sequence analysis of picd-1 identified orthologs in other nematode species (Fig. 1C), all of which contain a domain similar to the histone transcription regulator 3 (Hir3)/calcineurin-binding protein (CABIN1) family members (IPR033053, https://www.ebi.ac.uk/interpro/) (Fig. 1C,D). The alignments of PICD-1 with human CABIN1 (isoform a) showed 26% (729/2853) identity and 38% (1080/2853) similarity (EMBOSS stretcher pairwise alignment tool; https://www.ebi.ac.uk/Tools/psa/). Similar sequence conservation was observed in the case of mouse CABIN1 (Fig. 1C). Studies have shown that human CABIN1 is part of the histone H3.3 chaperone complex HUCA (HIRA/UBN1/CABIN1/ASF1a), involved in nucleosome assembly. Similarly, Gene Ontology (GO) analysis (http://www.wormbase.org) revealed that picd-1 is associated with the biological process “DNA replication-independent nucleosome assembly” (GO:0006336) and the cellular component “nucleus” (GO:0005634). Thus, picd-1 is likely to encode a nuclear protein that functions in chromatin assembly and regulation of gene expression. Furthermore, in silico analysis revealed that PICD-1 contains 49 amino acid residues predicted to bind DNA (http://biomine.cs.vcu.edu/servers/DRNApred/#References)18(Table S1).
Phenotype of picd-1 mutants and sequence similarity of PICD-1 with CABIN1 proteins. (A) Representative images of Pvl phenotype in pry-1(gk3682) and pry-1(gk3681); picd-1(gk3701) animals. (B) picd-1 mutation enhances Pvl phenotype of pry-1 mutants. Data represent a cumulative of two replicates (n = 99 for pry-1(mu38), 160 for pry-1(mu38); picd-1(bh40), 118 for pry-1(gk3682) and 156 for pry-1(gk3682); picd-1(gk3701)) and error bars represent the standard deviation (Also see Table 1 and Fig. 3). Statistical analyses for panel (B) were done compared to pry-1 mutants alone using one-way ANOVA with Dunnett’s post hoc test and significant differences are indicated by stars (*): ** (p < 0.01). n.s., not significant. (C) Sequence comparison of C. elegans PICD-1 with mammalian CABIN1 and nematode homologs. Orange colors indicate regions of the proteins where alignments were identified by NCBI BLAST along with similarity and identity scores. Non-colored regions are those without sequence similarity. (D) Sequence alignment dendrogram generated by LIRMM (http://www.phylogeny.fr/simple_phylogeny.cgi) using default parameters.
Mutations in picd-1 do not affect vulval induction but cause morphogenetic defects
In addition to using the gk3701 strain to examine mutant phenotypes, we generated a new allele, bh40, which has multiple in-frame stop codons in exon 1 (see “Methods” and Fig. 2A,B). qPCR analysis showed that bh40 and gk3701 greatly reduced picd-1 transcript levels (Fig. 2C). Interestingly, while the Pvl phenotype of pry-1(mu38) was enhanced by both the alleles (Fig. 1A,B), neither had an obvious impact on the penetrance of the multivulva (Muv) phenotype in pry-1(mu38) animals. In fact, the double mutants showed a slightly less Muv phenotype compared to pry-1(mu38) alone (Table 1, Fig. 1B), which may be due to changes in morphogenetic processes as vulval precursor induction is not affected by any of the picd-1 mutations (Fig. 2D, Table 1). Similar phenotypes were observed following picd-1 RNAi (Figure S2). In agreement with this, picd-1(bh40), but not picd-1(gk3701), exhibited abnormal vulval invagination (Fig. 3A) and the vulval morphology phenotype of pry-1(mu38); picd-1(bh40) double mutant was more severe compared to pry-1(mu38) alone. Furthermore, adult picd-1 mutants were weakly Pvl (Fig. 3B and Table 1).
Analysis of picd-1 alleles and their effect on pry-1 mutant Pvl phenotype. (A) Schematic diagram of the picd-1 open reading frame. The approximate locations of bh40 and gk3701 mutations have been indicated. (B) Sequences for bh40 and gk3701 mutations are shown in gray color. and stop codons are shown in bold (also see “Methods”). (C) Expression levels of picd-1 in pry-1(gk3682) and pry-1(mu38) mutants at the L1 stage compared to wild-type. Data represent the means of two replicates and error bars represent the standard error of means. p values were calculated using Bio-Rad software (one-way ANOVA). (D) Bar graph showing VPC induction score in picd-1 and pry-1 mutants alone, and pry-1; picd-1 double mutants compared to N2. Data represent the means of two replicates. The error bars show the standard deviation (n = 22). Statistical analyses were done using one-way ANOVA with Dunnett’s post hoc test. In panels (C,D), significant differences are indicated by stars (*): ** (p < 0.01). n.s., not significant.
picd-1 regulates vulval morphology. (A) Representative vulval images of wild-type, and picd-1 and pry-1 mutants at the mid-L4 stage. picd-1(bh40) animals exhibited abnormal vulva morphology (20.8 ± 5.8%, p < 0.01, n = 35) compared to N2 animals. Scale bar is 50 µm. (B) Line graph showing the percentage of control and picd-1(bh40) mutants showing Pvl phenotype at 20 °C and 25 °C compared to wild-type controls (Also see Table 1). (C) Line graph showing the percentage of control and picd-1(bh40) animals with Egl phenotype at 20 °C and 25 °C. (B, C) Data represent a cumulative of two replicates (n = 64 for picd-1(bh40) and 58 for N2) and error bars represent the standard deviation. Statistical analyses were done using an unpaired t-test and significant differences are indicated by stars (*): * (p < 0.05), ** (p < 0.01).
Phenotypic analysis of both picd-1 mutant strains did not reveal any obvious defects in movement, feeding, and other characteristics. However, careful examination showed that this gene is involved in the development of the egg-laying system. The picd-1(bh40) worms were weakly egg-laying defective (Egl) (Fig. 3C, Video S2), and their Egl and Pvl phenotypes were enhanced at 25 °C (Fig. 3B,C). No Egl phenotype was observed in the picd-1(gk3701) strain. While more experiments are needed to determine the molecular basis of phenotypic differences between the two alleles, the data collectively demonstrate that picd-1 is required for the development of the reproductive system.
picd-1 is expressed in multiple tissues
To gain further insights into the function of picd-1, we created a stable line carrying a picd-1::GFP transcriptional reporter. The analysis of transgenic animals revealed GFP fluorescence in developing tissues and organs, such as the pharynx, intestine, body wall muscles, hypodermis (seam cells), gonads, and vulva (Fig. 4A). This expression pattern resembled that of pry-1, which was recently described by our group10. As picd-1::GFP animals entered adulthood, fluorescence was localized to the intestine and certain head neurons (Fig. 4A), which persisted throughout the life of the animals. A broad range of picd-1 expression was also supported by previously published RNA-sequencing and microarray studies19,20. Overall, our expression analysis suggests that picd-1 functions in multiple tissues and may play a role in pry-1-mediated developmental and post -developmental processes.
Expression analysis of picd-1. (A) Representative images of animals expressing picd-1::GFP in larvae and adults. Tissues that show fluorescence include pharynx, gonad, hypodermis, intestine, vulva, body wall muscles, and some in the tail region. Scale bars are shown. (B) Expression levels of picd-1 in pry-1 mutants. Data represent the means of two replicates and error bars represent the standard error of means. p values were calculated using Bio-Rad software (one-way ANOVA). In panel (B) significant differences are indicated by stars (*): ** (p < 0.01). (C) Representative images of N2 and pry-1(mu38) adults showing picd-1::GFP expression. Scale bar is 100 µm.
Since picd-1::GFP is expressed in similar tissues as pry-1, and picd-1 mutation enhanced the pry-1 Pvl phenotype, we examined whether pry-1 affects picd-1 expression. The results of qPCR and picd-1::GFP fluorescence showed that picd-1 is downregulated in pry-1 mutants (Fig. 4B,C). The GFP levels were generally reduced throughout the animal (Fig. 4C). These results suggest that picd-1 expression is dependent on pry-1.
picd-1 mutations worsen the phenotypes of pry-1 mutants
Next, we investigated the involvement of picd-1 in other pry-1-mediated developmental and post-developmental processes. picd-1 mutants grew slowly and took longer to reach adulthood than the wild-type and pry-1(mu38) animals (Fig. 5A, Table S2). Furthermore, the growth defect in the pry-1; picd-1 double mutant was significantly worse than in the single mutants (Fig. 5A, Table S2). Among other phenotypes, mutations in picd-1 enhanced seam cell defects of pry-1(mu38) animals (Fig. 5B) that are caused by changes in asymmetric cell division at the L2 stage6,21. Moreover, both picd-1 and pry-1 mutants exhibited defects in alae, which are structures formed by differentiated seam cells6 (Fig. 5C). Interestingly, the P lineage defect in pry-1 mutants that involves P11.p and P12.pa cells was enhanced in pry-1(mu38); picd-1(bh40) but not in pry-1(gk3681); picd-1(gk3701) animals (Table 1, also see “Methods”).
picd-1 regulates developmental timing, seam cell division and alae formation. (A) picd-1 mutants exacerbate the developmental delay of pry-1 mutants. The data shows the average time taken by picd-1(bh40), pry-1(mu38) and pry-1(mu38); picd-1(bh40) double mutants to reach adulthood compared to wild-type animals. The number of animals for two replicates are: n = 60 for N2, pry-1(mu38), and picd-1(bh40); and 95 for pry-1(mu38); picd-1(bh40)) The error bars represent the standard deviation. (B) Bar graph showing the average number of seam cells (two replicates, n = 30) in the wild-type and pry-1(mu38) animals following control (L4440) and picd-1 RNAi. The error bars represent the standard deviation. (C) Representative images showing alae (white arrowheads) in wild-type N2 and picd-1(bh40) animals. Normal alae are marked with an arrowhead and an extra alae in the picd-1 mutant is indicated by a star (*) (41.88 ± 0.22%, n = 43, p < 0.0001). Statistical analyses were done using multiple unpaired t-test and recorded in Table S2. In panel B, star (*) indicates p < 0.05. Scale bar in panel (C) is 25 µm.
In addition, we observed several other developmental abnormalities in the picd-1 mutant animals. The analysis of brood size revealed defects in picd-1(bh40) but not in picd-1(gk3701) animals (Fig. 6A,B). Although the bh40 allele did not affect embryonic viability, it drastically reduced the brood count and enhanced the embryonic lethality of pry-1 mutants (p < 0.001) (Fig. 6A–C). Further analysis revealed that pry-1(gk3681); picd-1(gk3701) and pry-1(mu38); picd-1(bh40) double mutants had abnormal oocytes and gonads, respectively (Fig. 7A,B). More specifically, 46 ± 6% (n = 45, p < 0.01) of pry-1(mu38); picd-1(bh40) animals lacked oocytes in the posterior gonad arm (Figs. 7B). No such phenotype was observed in either of the single mutants.
picd-1 regulates brood size and embryonic viability. Bar graphs showing eggs laid on each day (A), totals number of eggs (B), and percentage of the hatched eggs (C) by N2 and single and double mutant animals. Data represent a cumulative of two replicates (total n = 10 for each genotype shown in panels (A) and (B); and n = 500 for N2, 120 for pry-1(mu38) and 14 for pry-1(mu38); picd-1(bh40) double mutants shown in panel (C) and error bars represent the standard deviation. (A) Statistical analyses were done using two-way ANOVA with Tukey’s multiple comparison test and recorded in Table S2. (B,C) Statistical analyses were done using one-way ANOVA with Dunnett’s multiple comparison test and significant differences are indicated by stars (*): * (p < 0.05), ** (p < 0.01), *** (p < 0.001), and **** (p < 0.0001). n.s., not significant.
picd-1 interacts with pry-1 to regulate oocyte development. (A) pry-1; picd-1 double mutants show abnormal oocytes. Scale bar is 25 µm. (B) Posterior gonad arms of wildtype, picd-1(bh40), pry-1(mu38) and pry-1(mu38); picd-1(bh40) adults. pry-1(mu38); picd-1(bh40) animals frequently lack oocytes (46 ± 6%, n = 45, p < 0.01) in the posterior gonad arm when compared to N2 and pry-1(mu38). Statistical analysis was done using an unpaired t-test. The spermatheca (Sp), embryos (E), and oocytes (Oo) are marked. Vulva opening is marked (#). Anterior is on the left. Scale bar is 100 mm.
picd-1 mutants are sensitive to stress and exhibit a short lifespan
We previously reported that pry-1 plays a role in stress response maintenance7,10. The analysis of heat shock chaperones—hsp-4 (ER-UPR), hsp-6 (MT-UPR), and hsp-16.2 (cytosolic heat shock response, HSR)—showed that all three were upregulated in pry-1 mutant animals (Fig. 8A). Similar experiments in picd-1 mutants showed increased expression of hsp-4, hsp-16.2, and the oxidative stress response gene sod-3 (Fig. 8B). Consistent with these results, both pry-1 and picd-1 mutants showed electrotaxis defects (Fig. 8C and “Methods”), a phenotype observed in animals with abnormalities in stress sensitivity and UPR22.
picd-1 mutants are stress sensitive. (A, B) Expression levels of hsp-4, hsp-6, hsp-16.2, and sod-3 in picd-1(bh40) and pry-1(mu38) young adults. Data represent the means of three replicates and error bars represent the standard error of means. p values were calculated using Bio-Rad software (t-test). (C) Box and whisker plots represent normalized electrotaxis speeds of picd-1(gk3701), picd-1(bh40), pry-1(mu38) and pry-1(mu38); picd-1(bh40) mutants (n = 20). Measurements show 25 to 75th percentiles of data, central horizontal lines represent medians, and vertical lines extend to 10th and 90th percentiles. (D) Bar graphs represent percentage survival following 200 mM paraquat exposure for 4 h (n = 90). (E) Bar graphs represent percentage survival of animals following 25 ng/µl tunicamycin exposure for 4 h. (F) Bar graphs represent percentage survival of animals following 200 mM paraquat exposure for 2 h (n = 120 for N2, 150 for pry-1(mu38) and 169 for pry-1(mu38); picd-1(bh40)). For panels (C–F), data are the cumulative of two replicates and error bars represent the standard deviation. (C, F) Statistical analyses were done using one-way ANOVA with Dunnett’s multiple comparison test. (D, E) Statistical analyses were done using two-way ANOVA with Tukey’s multiple comparison test. In all cases significant differences are indicated by stars (*): * (p < 0.05), ** (p < 0.01), *** (p < 0.001), **** (p < 0.0001). Refer to Table S2 for detailed statistical analyses.
To further elucidate the stress sensitivity of animals lacking picd-1 function, we examined the survivability of the animals following chemical treatments. Both gk3701 and bh40 alleles were sensitive to paraquat and tunicamycin, although the effect was more pronounced following paraquat exposure (Fig. 8D,E). We also tested paraquat sensitivity of pry-1(mu38); picd-1(bh40) double mutants and found no difference compared to pry-1(mu38) alone (Fig. 8F), which could be explained by the significantly reduced expression of picd-1 in pry-1 mutants.
As increased stress sensitivity can affect the lifespan of an animal, and pry-1 mutants are short-lived, we analyzed whether picd-1 plays a role in aging. Neither picd-1(gk3701) nor picd-1(RNAi) enhanced the lifespan defects of animals lacking pry-1 function (Fig. 9A,B, Table 2). Considering that pry-1 mutant animals have a significantly reduced expression of picd-1 than the wild-type animals, it is conceivable that further reduction in picd-1 cannot exacerbate the short-lived phenotype. Alternatively, it is plausible that picd-1 is not involved in lifespan maintenance. To explore this further, we examined the lifespan of picd-1 mutant and RNAi-treated animals in the absence of other mutations. Both gk3701 and bh40 alleles reduced the lifespan of the animals. picd-1(bh40) worms had a significantly reduced lifespan at both 20 °C and 25 °C, and picd-1(gk3701) exhibited a similar phenotype at 25 °C (Fig. 9C,D, Table 2). These results were also supported by the RNAi experiments. The analysis of age-associated biomarkers revealed a progressive age-associated decline in both body bending and pharyngeal pumping rates (Fig. 9E,F). Overall, the data suggest that while picd-1 does not enhance the phenotype of pry-1 mutants, the gene plays an essential role in maintaining the usual lifespan of animals.
picd-1 mutation reduces lifespan and causes age-associated deterioration. (A) picd-1 mutation does not affect the lifespan of pry-1 mutants. (B) picd-1 RNAi reduces the lifespan of control animals but not that of pry-1 mutants. (C,D) Lifespan of picd-1(gk3701) and picd-1(bh40) mutants at 20 °C and 25 °C. See “Materials and methods” section and Table 2 for lifespan data and statistical analyses. (E,F) Bar graphs showing the rates of body bending and pharyngeal pumping of picd-1 mutants compared to wild-type over a period of 5 days. Data represent a cumulative of two replicates (n = 10 animals) and error bars represent the standard deviation. Statistical analyses were done using two-way ANOVA with Tukey’s multiple comparison test. See Table S2 for detailed statistical analyses. (G) Expression analysis of fat-5, fat-6, fat-7, nhr-49, nhr-80 and sbp-1 genes in the picd-1(bh40) mutants compared to wild-type. Data represent the means of two replicates and error bars represent the standard error of means. p values were calculated using Bio-Rad software (one-way ANOVA). (H) Quantification of total lipid using Oil Red O in the wild-type and picd-1(bh40) animals. Data represent a cumulative of two replicates (n > 30 animals) and error bars represent the standard deviation. Statistical analysis was done using an unpaired t-test. In all cases, significant differences are indicated by stars (*): * (p < 0.05), ** (p < 0.01), *** (p < 0.001), and **** (p < 0.0001).
We have previously shown that in addition to affecting lifespan, pry-1 regulates lipid metabolism8,11. This result prompted us to analyze whether picd-1 affects lipid levels and the expression of genes involved in fatty acid synthesis. The analysis of ∆9 desaturases showed that while fat-5 and fat-7 were downregulated, fat-6 was unaffected (Fig. 9G). Among the three transcription factors regulating the expression of ∆9 desaturases, nhr-80 (NHR family) was downregulated, but sbp-1 (SREBP1 homolog) was upregulated (Fig. 9G)23. We also quantified lipids by Oil Red O staining but saw no change in picd-1 mutants (Fig. 9H), possibly due to functional redundancies within the fat24,25 and nhr family of genes23. Hence, we conclude that picd-1 is necessary for the expression of a subset of lipid synthesis genes but is not crucial for regulating lipid levels.
Loss of picd-1 and pry-1 promotes CRTC-1 nuclear localization
Research has shown that calcineurin (a calcium-activated phosphatase) signaling promotes nuclear localization of CRTC-1, leading to a reduction in the lifespan of C. elegans17. Given that human cabin1 negatively regulates calcineurin signaling13,26, we investigated whether picd-1 could affect the subcellular localization of CRTC-1::RFP. The RNAi knockdown of picd-1 caused CRTC-1 to be nuclear localized, consistent with the short lifespan of picd-1 mutants (Figs. 9C,D, 10A,B). Moreover, CRTC-1 responsive genes, dod-24 and asp-1227, were upregulated in the picd-1(bh40) mutants (Fig. 10C).
Reduced or loss of picd-1 function affects CRTC-1 localization and CRTC-1 transcriptional response. (A) picd-1, but not L4440 control, RNAi causes nuclear accumulation of CRTC-1::RFP fluorescence. (B) Quantification of nuclear localization in (A). (C) qPCR analysis of dod-24 and asp-12 in picd-1(bh40) animals shows increased expression. (D–F) Similar experiments performed in pry-1 mutants. For panels (C) and (F), data represent the means of two replicates and error bars represent the standard error of means. (G) CRTC-1::RFP localization analysis in N2 and pry-1(gk3682) mutants following L4440 control RNAi, bar-1 and crtc-1 RNAi treatments. Nuclear fluorescence is absent in the case of crtc-1 RNAi. (H) Quantification of nuclear localization in (G). (B,E,G) Data represent the means of three replicates and error bars represent the standard deviation. At least n > 50 animals were examined in each assay. (I) Lifespan of wild type and pry-1 mutant animals following L4440 control and crtc-1 RNAi (also see Table 2). (J) Bar graphs represent percentage survival of animals following 100 mM paraquat exposure for 2 h. Data represent a cumulative of two replicates (n > 60 animals) and error bars represent the standard deviation. For panels (B,E,G,J), statistical analyses were done using an unpaired t-test. Data for panels (C) and (F) were analyzed using Bio-Rad software (t-test). In all cases, significant differences are indicated by stars (*): * (p < 0.05), ** (p < 0.01), and **** (p < 0.0001). n.s., not significant.
As pry-1 is necessary for picd-1 expression, we examined its effect on the CRTC-1::RFP and found that the fluorescence was nuclear-localized and dod-24 and asp-12 were upregulated in pry-1 mutants (Fig. 10D–F). To further understand the mechanism of PRY-1-PICD-1-mediated CRTC-1 localization, we examined the involvement of the Wnt canonical pathway component BAR-1/β-catenin. The bar-1 RNAi did not affect CRTC-1::RFP nuclear localization in pry-1 mutants (Fig. 10G,H), suggesting that PRY-1 may function in a WNT-independent manner to regulate CRTC-1. This conclusion agrees with our previous findings that pry-1-mediated lifespan maintenance does not depend on bar-110. Next, we examined the involvement of CRTC-1 in PRY-1-mediated lifespan and stress response and found that crtc-1 RNAi performed during the adult stage significantly rescued the short lifespan and stress sensitivity defects of pry-1 mutants (Fig. 10I,J and Table 2). Hence, we propose that PRY-1 inhibits CRTC-1-dependent transcriptional response to delay age-associated processes.
Given that calcineurin and CRTC-1 mediated lifespan regulation involves the C. elegans CREB transcription factor homolog 1 (CRH-1), we wanted to know whether PRY-1 and CRH-1 regulate a common set of target genes. Consistent with our hypothesis, we found a significant overlap in differentially expressed genes between pry-1 and crh-1 mutant transcriptomes (406 common genes, R.F 2.5, p < 5.108e−78)8,27. Furthermore, the number of overlapping sets of genes regulated in an opposite manner between pry-1 and crh-1 mutants were also significant (Figure S3 and Table S3) and were enriched with GO biological processes such as response to stress (18), metabolic processes (18), and cellular processes (40) (FDR < 0.05). In conclusion, these data demonstrate a novel functional relationship between PRY-1 and CRTC-1 in C. elegans.
Discussion
In this study, we identified a new gene, picd-1, in C. elegans that interacts with pry-1 and regulates several larval and adult processes. picd-1 is predicted to encode a nuclear protein containing a conserved Cabin1 domain, which belongs to the HUCA complex in humans12. The HUCA complex is implicated in diverse chromatin regulatory events, where it preferentially deposits a histone variant H3.3. This leads to transcriptional activation by nucleosome destabilization or transcriptional repression through heterochromatinization28. cabin1 is expressed in all human tissues and localized to the nucleoplasm and cytoplasm29,30. Studies in other systems have also uncovered homologous proteins of CABIN1. For example, the yeast Saccharomyces cerevisiae contains Hir1p and Hir2p (both HIRA orthologs) and Hir3, Hpc2, and Asf1p, orthologs of CABIN1, UBN1, and ASF1a, respectively28.
Our study provides the first genetic evidence of a Cabin1 domain-containing protein regulating biological processes in C. elegans. Other complex components in worms include HIRA-1 (HIRA homolog), ASFL-1, and UNC-85 (both ASF1a homologs)31,32,33. However, it remains to be seen if any of these proteins interact with PRY-1. Mutations in picd-1 led to multiple defects such as Pvl, Egl, small brood size, developmental delay, stress sensitivity, and short lifespan. While there are differences in phenotypic severity between two picd-1 alleles, it is important to emphasize that the loss of picd-1 function enhanced various phenotypes of the pry-1 mutant. For example, pry-1; picd-1 double mutant showed a Pvl phenotype and exhibited P11.p cell fate changes. In addition, picd-1 RNAi enhanced seam cell defects in the pry-1 mutants. Interestingly, mutations in picd-1 did not enhance vulval precursor cell induction or Muv phenotype in pry-1 mutants. One possible model to explain genetic interactions between pry-1 and picd-1 is that pry-1 functions via both picd-1 dependent and independent pathways, and picd-1 is regulated by multiple factors.
We analyzed the role of picd-1 in pry-1-mediated non-developmental events, such as egg-laying, embryonic survivability, aging, stress response, and lipid metabolism. Loss of picd-1 function worsened the embryonic lethality of pry-1 mutants. Moreover, pry-1; picd-1 double mutant had a very small brood size due to defects in the gonad arms. These findings suggest that pry-1 and picd-1 interact to regulate embryonic viability and fertility in animals. Similar phenotypes are observed in the mutants of other HIRA complex components. Knockdown of hira-1 leads to embryonic lethality, asfl-1 or unc-85 single mutants have low brood size, and asfl-1; unc-85 double mutant is sterile32,33,34. Together, these data show that pry-1 and picd-1 interact to regulate embryonic viability and fertility in animals. It remains to be seen whether PRY-1 and PICD-1 interact with other HIRA complex components to mediate their function.
Furthermore, we found that picd-1 is required for normal stress response maintenance. picd-1 mutants showed enhanced sensitivity to paraquat and tunicamycin. The mutant animals also exhibited increased levels of UPR markers. Both picd-1 and pry-1 mutants significantly increased hsp-16.2, and hsp-4, suggesting that these genes function together to regulate ER-UPR and HSR. However, more work is needed to determine whether these two genes uniquely affect MT-UPR and oxidative stress and their biological significance.
Mutants that show sensitivity to stress typically have a short lifespan35,36,37. Similar to pry-1 mutants, picd-1(bh40) animals are short-lived and exhibit defects in age-related physiological markers. This result is consistent with the fact that both genes function together to regulate stress response and aging. However, there are functional differences between the two genes. For example, we found that lipid levels were greatly reduced in pry-1 but not in picd-1 mutants. The nhr-80 and fat-7 levels were reduced in picd-1 mutant animals, consistent with the known role of nhr-80 in regulating fat-7 expression24. However, while picd-1 is needed for the expression of fat-5, fat-7 and nhr-80, a lack of its function does not compromise lipid levels in animals. These results are consistent with the above model of picd-1 participating in a subset of pry-1-mediated processes. However, the extent to which the two genes interact and the precise nature of their interactions is unknown.
A possible mechanism of picd-1 function in lifespan maintenance may involve calcineurin. AMPK and calcineurin modulation of CRTCs are conserved in mammals and C. elegans17,38,39,40,41. In C. elegans, AAK-2 and calcineurin regulate CRTC-1 post-translationally in an opposing manner, where activated AAK-2 causes nuclear exclusion of CRTC-1 and extends lifespan. Such a phenotype was also observed after deactivating calcineurin17. Our data showed that loss of picd-1 function resulted in the nuclear localization of CRTC-1 and activated the CRTC-1 target genes. These findings, together with the fact that mammalian cabin1 inhibits calcineurin-mediated signaling13,26,42, allows us to hypothesize that PICD-1 regulates CRTC-1 via downregulation of calcineurin in C. elegans. According to this, loss of picd-1/cabin-1 is expected to increase calcineurin signaling, which may explain the shorter lifespan of picd-1 mutants. Further experiments are needed to investigate the extent to which the regulatory relationship between cabin1 and calcineurin is conserved in C. elegans.
As picd-1 is downregulated in pry-1 mutants, and both genes are needed for the proper subcellular localization of CRTC-1 and its downstream targets, we speculate that PRY-1 and PICD-1 use CRTC-1 to regulate stress response and lifespan of animals. While there is no evidence for the interaction between mammalian Axin and CRTCs, studies have shown that CREB, which associates with CRTCs, is inhibited by Axin-GSK3β signaling43,44. Our work provides the first evidence of genetic interactions between pry-1, picd-1, and crtc-1 in C. elegans, which has uncovered a novel crosstalk between Axin and calcineurin signaling. However, several questions remain unanswered. For instance, the components of pry-1 signaling affecting CRTC-1 nuclear localization are unknown. In preliminary experiments, tax-6 RNAi (calcineurin catalytic subunit) did not affect the pry-1 phenotype; however, more experiments are needed to completely ascertain its requirements. Additionally, whether picd-1 is regulated by pry-1 in a WNT-dependent manner or it is co-regulated by pry-1 and aak-24,10 independently of WNT needs thorough investigation. Moreover, it is unknown whether other HUCA complex components interact with PICD-1 to mediate PRY-1’s role during stress response and lifespan, as well as whether PRY-1 and PICD-1 co-regulate a common set of targets during these processes. Further work is needed to investigate these questions and to gain a deeper understanding of the conserved mechanisms involved in Axin-CABIN1 signaling in eukaryotes.
Materials and methods
Worm strains
Cultures were maintained at 20 °C on standard nematode growth media (NGM) plates seeded with OP50 E. coli bacteria. The strains used in this study are listed below.
N2 (wild-type).
DY220 pry-1(mu38).
VC3710 pry-1(gk3682).
VC3709 pry-1(gk3681); picd-1(gk3701).
DY725 pry-1(mu38); picd-1(bh40).
DY678 bhEx287[pGLC150(picd-1::GFP) + myo-3::wCherry].
DY750 bhEx301[pGLC150(picd-1::GFP) + pGLC 72(dat-1::YFP)].
DY752 pry-1(mu38); bhEx301[pGLC150(picd-1::GFP) + pGLC 72(dat-1::YFP)].
DY698 and DY753 picd-1(bh40).
DY694 picd-1(gk3701).
DY747 pry-1(gk3681).
RG733 wIs78[scm::GFP + ajm-1::GFP].
AGD418 uthIs205[crtc-1::CRTC-1::RFP::unc-54 3’ UTR + rol-6(su1006)].
DY740 pry-1(gk3682); uthIs205[crtc-1::CRTC-1::RFP + rol-6(su1006)].
Mutant alleles and transgenic strains
The gk3701 mutation was discovered in a CRISPR screen that was carried out in Moerman lab to obtain alleles of pry-1 and several other genes of interest6,45,46. Since the original picd-1 mutant strain also contained pry-1(gk3681), it was deconstructed to establish separate strains of picd-1(gk3701) (outcrossed 3x) and pry-1(gk3681) (outcrossed 2x). The presence of CRISPR mutations was confirmed by PCR and sequencing. Molecular changes in pry-1(gk3682) were described earlier by our group6. Both gk3682 and gk3681 carry a CRISPR GFP cassette45 We sequenced gk3681 as part of this study and found that the allele is identical to gk3682 (Table S4). In agreement with this, pry-1(gk3681) animals are phenotypically similar to pry-1(gk3682) (Figure S1 and Table 2).
The picd-1(gk3701) does not contain a CRISPR GFP cassette. Instead, it carries a 5 bp sequence (GGTGA) deletion in the second exon of the gene (flanking 25 nucleotides: GTGAAGAGGATGAGGACAATGGTGA and GGATTCAGAAGAAGAAGATGAAGAA) that causes multiple premature in-frame stop codons.
The other picd-1 allele (bh40) was generated in a separate CRISPR screen using a published protocol47. Here, we replaced the 84 bp in the first exon by a synthetic sequence containing stop codons in different reading frames. See primers in Table S4. bh40 animals were outcrossed 2 × and examined for various phenotypes. Another 2 × outcross of the strain was subsequently carried out (total 4x), which showed similar defects (Table S5).
To generate the picd-1p::GFP transgenic animals (DY678), pGLC150 was injected in N2 background along with myo-3::wCherry or dat-1::YFP marker. pGLC150 was constructed by cloning a 3,885 bp PCR-amplified fragment (using the primers GL1372 and GL1373), spanning the promoter region and a portion of the first exon of the picd-1 gene, into the vector pPD95.81 using the restriction sites SalI and KpnI.
Molecular biology
RNA was extracted from synchronized L3 and day-1 adult animals. Protocols for RNA extraction, cDNA synthesis and qPCR were described earlier7. Briefly, total RNA was extracted using Trizol (Thermo Fisher, USA). The RNA was used to prepare cDNA and, subsequently, perform qPCR using the SensiFast cDNA synthesis kit (Bioline, USA), and SYBR green mix (Bio-Rad, Canada), respectively. Primers are listed in Table S4.
RNAi
RNAi mediated gene silencing was performed using a protocol previously published by our laboratory48. Plates were seeded with E. coli HT115 expressing either dsRNA specific to candidate genes or empty vector (L4440). Gravid hermaphrodites were treated with a mild bleach solution (3:2 ratio of commercial bleach and 4 N NaOH) and eggs were plated. Animals were subjected to RNAi treatment from egg stage unless otherwise stated. Phenotypes were examined at the young adult stage.
Fluorescent and DIC microscopy
Animals were paralyzed in 10 mM Sodium Azide and mounted on glass slides containing 2% agar pads and covered with glass coverslips. Images were captured using a Zeiss Apotome microscope and Zeiss ZEN software. For acquiring live videos of gonad, animals were suspended in M9 without Sodium Azide. Videos were captured by a high-speed camera fitted on a Leica MZ-FLIII fluorescent stereomicroscope49.
Seam cells were identified using scm::GFP and ajm-1::GFP markers, The nuclei of these cells were counted, and adult lateral alae were scored using Nomarski differential interference contrast and epifluorescence optics. Images were acquired using a Hamamatsu Camera mounted on a Nikon Eclipse 80i upright Nomarski fluorescence microscope and NIS Element software (Nikon, USA).
Vulval induction and P cells
Vulval induction and P11.p and P12.p phenotypes were examined in mounted L4 stage animals using a Nomarski microscope. VPCs were considered induced if they gave rise to progeny. Wild-type (N2) animals have three induced VPCs, one each for P5.p, P6.p, and P7.p. Mutants with more than three induced VPCs were termed as ‘over-induced’. Muv and Pvl phenotypes were scored in adults. Vulval morphogenesis is described previously50. At the L4 stage VPC progeny undergo a series of changes that involve invagination of vulva progeny and selective cell fusion to give rise to a characteristic morphology of the vulva. Defects in morphogenetic processes can lead to an abnormal shape of the vulva.
P11.p and P12.pa cells can be readily distinguished under a Nomarski microscope based on their nuclear size and morphology51,52,53. In pry-1 mutants, two P12.pa-like cells are observed and P11.p is missing.
Aging-related analysis
Lifespan experiments of RNAi-treated animals were carried out using a previously described protocol10. Synchronized eggs were allowed to grow on NGM OP50 seeded plates till the late L4 larval stage after which they were transferred to RNAi bacteria seeded plates to perform adult stage-specific knockdown. Cultures were screened daily for dead animals and surviving adults were transferred every other day till the progeny production ceased. Censoring was done for animals that had either escaped, burrowed into the medium, showed a bursting at the vulva, or had progeny hatching inside the uterus54.
Body bending per 1 min and pharyngeal pumping per 30 s were analyzed in young adults over a period of four days7. For this, individual hermaphrodites were placed on OP50 plates and examined under a dissecting microscope. Pharyngeal pumping was assessed by observing the number of pharyngeal contractions. For body bending, animals were stimulated by tapping once on the tail by a platinum wire. Each full sinusoidal motion was counted as one body bend. Only animals that moved actively within 1 min were included in the analysis.
Stress assay and electrotaxis
Oxidative and endoplasmic reticulum mediated stress experiments were performed using 200 mM paraquat (unless specified) (Thermo Fisher Scientific, USA) and 25 ng/µl tunicamycin (Sigma-Aldrich, Canada) respectively. Animals were incubated for 1 h, 2 h, 3 h and 4 h, following a previous published protocol6. The final working concentrations were made in M9. At least 30 animals of each strain were tested in replicates. Means and standard deviations were determined from experiments performed in duplicate. Animals were considered dead if they did not respond to a platinum wire touch and showed no thrashing or swimming movement in M9. Moreover, dead animals usually had an uncurled and straight body shape in comparison to the normal sinusoidal shape of worms.
The electrotaxis assay protocol has been described previously55. Briefly, synchronized worms were introduced into a microfluidic channel and subjected to an electric field of 3 V/cm. Locomotory data was extracted from recorded videos using custom MATLAB-based worm tracking software. Electrotaxis speed data was plotted using box plots.
Oil Red O staining
Neutral lipid staining was done on synchronized day-1 adults using Oil Red O dye (Thermo Fisher Scientific, USA) following a published protocol11. Quantifications were performed using ImageJ software as described earlier56.
Statistical analyses
SigmaPlot 11 was used to plot lifespan data that provided detailed statistical analysis on survival curves. The curves were estimated using the Kaplan–Meier test and differences among groups were assessed using the log-rank test. qPCR data analysis was done using software (Maestro 3.1) that controls the Bio-Rad CFX qPCR machine. The software performs built-statistical analyses of t-test and one-way ANOVA. For all other assays, data from repeat experiments were pooled and analyzed together, and statistical analyses were done using GraphPad Prism 9. p values less than 0.05 were considered statistically significant. Data for all the Figure panels are reported in Table S5.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files). Additional details are available from the corresponding author on reasonable request.
References
Mallick, A., Taylor, S. K. B., Ranawade, A. & Gupta, B. P. Axin family of scaffolding proteins in development: lessons from C. elegans. J. Dev. Biol. 7, 1–23 (2019).
Clevers, H. & Nusse, R. Wnt/β-catenin signaling and disease. Cell 149, 1192–1205 (2012).
Luo, W. & Lin, S. C. Axin: A master scaffold for multiple signaling pathways. Neurosignals 13, 99–113 (2004).
Mallick, A. & Gupta, B. P. AXIN-AMPK signaling : Implications for healthy aging. F1000Research 10, 1–14 (2022).
Sawa, H. & Korswagen, H. C. Wnt signaling in C. elegans *. WormBook https://doi.org/10.1895/wormbook.1 (2013).
Mallick, A., Ranawade, A. & Gupta, B. P. Role of PRY-1/Axin in heterochronic miRNA-mediated seam cell development. BMC Dev. Biol. 19, 1–12 (2019).
Mallick, A. et al. Genetic analysis of Caenorhabditis elegans pry-1 / Axin suppressors identifies genes involved in reproductive structure development , stress responses , and aging. G3 Genes, Genomes, Genet. jkab430, (2021).
Ranawade, A., Mallick, A. & Gupta, B. P. PRY-1/Axin signaling regulates lipid metabolism in Caenorhabditis elegans. PLoS ONE 13, e0206540 (2018).
Mallick, A. et al. The FGFR4 homolog KIN-9 regulates lifespan and stress responses in Caenorhabditis elegans. Front. Aging 3, 1–11 (2022).
Mallick, A., Ranawade, A., van den Berg, W. & Gupta, B. P. Axin-mediated regulation of lifespan and muscle health in C. elegans requires AMPK-FOXO Signaling. Science 23, 101843 (2020).
Mallick, A. & Gupta, B. P. Vitellogenin-2 acts downstream of PRY-1 / Axin to regulate lipids and lifespan in C. elegans. Micropublication Biol. 1, 19–21 (2020).
Rai, T. S. et al. Human CABIN1 is a functional member of the human HIRA/UBN1/ASF1a histone H3.3 chaperone complex. Mol. Cell. Biol. 31, 4107–4118 (2011).
Sun, L. et al. Cabin 1, a negative regulator for calcineurin signaling in T lymphocytes. Immunity 8, 703–711 (1998).
Park, Y. J., Yoo, S. A., Kim, M. & Kim, W. U. The role of calcium–calcineurin–NFAT signaling pathway in health and autoimmune diseases. Front. Immunol. 11, 1–14 (2020).
Park, H. S., Lee, S. C., Cardenas, M. E. & Heitman, J. Calcium-calmodulin-calcineurin signaling: A globally conserved virulence cascade in eukaryotic microbial pathogens. Cell Host Microbe 26, 453–462 (2019).
Bandyopadhyay, J., Lee, J. & Lee, J. Calcineurin, a calcium/calmodulin-dependent protein phosphatase, is involved in movement, fertility, egg laying, and growth in Caenorhabditis elegans. Mol. Biol. Cell 13, 3281–3293 (2002).
Mair, W. et al. Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature 470, 404–408 (2011).
Yan, J. & Kurgan, L. DRNApred, fast sequence-based method that accurately predicts and discriminates DNA-and RNA-binding residues. Nucleic Acids Res. 45, 1–16 (2017).
Grün, D. et al. Conservation of mRNA and protein expression during development of C.elegans. Cell Rep. 6, 565–577 (2014).
Golden, T. R., Hubbard, A., Dando, C., Herren, M. A. & Melov, S. Age-related behaviors have distinct transcriptional profiles in Caenorhabditis elegans. Aging Cell 7, 850–865 (2008).
Gleason, J. E. & Eisenmann, D. M. Wnt signaling controls the stem cell-like asymmetric division of the epithelial seam cells during C elegans larval development. Dev. Biol. 348, 58–66 (2010).
Taylor, S. K. B. et al. C elegans electrotaxis behavior is modulated by heat shock response and unfolded protein response signaling pathways. Sci. Rep. 11, 1–17 (2021).
Watts, J. L. & Ristow, M. Lipid and carbohydrate metabolism in Caenorhabditis elegans. Genetics 207, 413–446 (2017).
Brock, T. J., Browse, J. & Watts, J. L. Genetic regulation of unsaturated fatty acid composition in C elegans. PLoS Genet. 2, 0997–1005 (2006).
Brock, T. J., Browse, J. & Watts, J. L. Fatty acid desaturation and the regulation of adiposity in Caenorhabditis elegans. Genetics 176, 865–875 (2007).
Lai, M. M., Burnett, P. E., Wolosker, H., Blackshaw, S. & Snyder, S. H. Cain, a novel physiologic protein inhibitor of calcineurin. J. Biol. Chem. 273, 18325–18331 (1998).
Burkewitz, K. et al. Neuronal CRTC-1 governs systemic mitochondrial metabolism and lifespan via a catecholamine signal. Cell 160, 842–855 (2015).
Szenker, E., Ray-Gallet, D. & Almouzni, G. The double face of the histone variant H3.3. Cell Res. 21, 421–434 (2011).
Thul, P. J. et al. A subcellular map of the human proteome. Science 356, 2 (2017).
Uhlén, M. et al. Tissue-based map of the human proteome. Science 347, 2 (2015).
Burkhart, K. B., Sando, S. R., Corrionero, A. & Horvitz, H. R. H3.3 nucleosome assembly mutants display a late-onset maternal effect. Curr. Biol. 30, 2343–2352 (2020).
Grigsby, I. F., Rutledge, E. M., Morton, C. A. & Finger, F. P. Functional redundancy of two C. elegans homologs of the histone chaperone Asf1 in germline DNA replication. Dev. Biol. 329, 64–79 (2009).
Grigsby, I. F. & Finger, F. P. UNC-85, a C. elegans homolog of the histone chaperone Asf1, functions in post-embryonic neuroblast replication. Dev. Biol. 319, 100–109 (2008).
Piano, F. et al. Gene clustering based on RNAi phenotypes of ovary-enriched genes in C elegans. Curr. Biol. 12, 1959–1964 (2002).
Morton, E. A. & Lamitina, T. Caenorhabditis elegans HSF-1 is an essential nuclear protein that forms stress granule-like structures following heat shock. Aging Cell 12, 112–120 (2013).
Taylor, R. C. & Dillin, A. XBP-1 Is a cell-nonautonomous regulator of stress resistance and longevity. Cell 153, 1435 (2013).
Libina, N., Berman, J. R. & Kenyon, C. Tissue-specific activities of C elegans DAF-16 in the regulation of lifespan tissues play an important role in establishing the ani-mal’s rate of aging. First, the C elegans genome con-tains more than 35 insulin-like genes expressed in a. Cell 115, 489–502 (2003).
Screaton, R. A. et al. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell 119, 61–74 (2004).
Koo, S. H. et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 437, 1109–1114 (2005).
Komiya, T. et al. Enhanced activity of the CREB co-activator Crtc1 in LKB1 null lung cancer. Oncogene 29, 1672–1680 (2010).
Wang, Y., Vera, L., Fischer, W. H. & Montminy, M. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature 460, 534–537 (2009).
Jang, H., Cho, E. J. & Youn, H. D. A new calcineurin inhibition domain in Cabin1. Biochem. Biophys. Res. Commun. 359, 129–135 (2007).
Gavagan, M., Fagnan, E., Speltz, E. B. & Zalatan, J. G. The scaffold protein axin promotes signaling specificity within the Wnt pathway by suppressing competing kinase reactions. Cell Syst. 10, 515-525.e5 (2020).
Fiol, C. J., Wang, A., Roeske, R. W. & Roach, P. J. Ordered multisite protein phosphorylation. Analysis of glycogen synthase kinase 3 action using model peptide substrates. J. Biol. Chem. 265, 6061–6065 (1990).
Chamberlin, H. M. et al. Evolution of transcriptional repressors impacts caenorhabditis vulval development. Mol. Biol. Evol. 37, 1350–1361 (2020).
Richman, C. et al. C. elegans MANF homolog is necessary for the protection of dopaminergic neurons and ER unfolded protein response. Front. Neurosci. 12, 1–11 (2018).
Vicencio, J., Martínez-Fernández, C., Serrat, X. & Cerón, J. Efficient generation of endogenous fluorescent reporters by nested CRISPR in caenorhabditis elegans. Genetics 211, 1143–1154 (2019).
Seetharaman, A., Cumbo, P., Bojanala, N. & Gupta, B. P. Conserved mechanism of Wnt signaling function in the specification of vulval precursor fates in C. elegans and C. briggsae. Dev. Biol. 346, 128–139 (2010).
Chalich, Y., Mallick, A., Gupta, B. & Jamal Deen, M. Development of a low-cost, user-customizable, high-speed camera. PLoS ONE 15, 1–21 (2020).
Mok, D. Z. L., Sternberg, P. W. & Inoue, T. Morphologically defined sub-stages of C. Elegans vulval development in the fourth larval stage. BMC Dev. Biol. 15, 1–8 (2015).
Eisenmann, D. M. & Kim, S. K. Protruding vulva mutants identify novel loci and wnt signalling factors that function during Caenorhabditis elegans vulva development. Genetics 156, 1097–1116 (2000).
Walston, T. et al. mig-5/Dsh controls cell fate determination and cell migration in C. elegans. Dev. Biol. 298, 485–497 (2006).
Jiang, L. I. & Sternberg, P. W. Interactions of EGF, Wnt and HOM-C genes specify the P12 neuroectoblast fate in C. elegans. Development 125, 2337–2347 (1998).
Amrit, F. R. G., Ratnappan, R., Keith, S. A. & Ghazi, A. T. C. elegans lifespan assay toolkit. Methods 68, 465–475 (2014).
Tong, J., Rezai, P., Salam, S., Selvaganapathy, P. R. & Gupta, B. P. Microfluidic-based electrotaxis for on-demand quantitative analysis of Caenorhabditis elegans’ locomotion. J. Vis. Exp. https://doi.org/10.3791/50226 (2013).
Soukas, A. A., Kane, E. A., Carr, C. E., Melo, J. A. & Ruvkun, G. Rictor/TORC2 regulates fat metabolism, feeding, growth, and life span in Caenorhabditis elegans. Genes Dev. https://doi.org/10.1101/gad.1775409.2004 (2009).
Acknowledgements
We are indebted to Moerman lab for generating both pry-1(gk3681) and picd-1(gk3701) strains. We also thank Drs. Andrew Dillin (University of California, Berkeley) and Brent Derry (University of Toronto) for providing the CRTC-1::RFP strain and crtc-1 RNAi plasmid, respectively. Hannan Minhas assisted with electrotaxis experiments and Wouter van den Berg with the screening of picd-1 CRISPR allele bh40. This work was supported by the NSERC Discovery grant to Bhagwati Gupta and NSERC CGS-D scholarship to Avijit Mallick. Some of the strains were obtained from the Caenorhabditis Genetics Center (CGC), which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440).
Author information
Authors and Affiliations
Contributions
A.M. initially characterized the picd-1 mutants and generated many reagents for the study. A.M., S.M. and S.K.B.T. carried out the experiments. A.M., S.K.B.T., and B.G. analyzed the data. A.M. wrote the first draft of the manuscript and, subsequently, A.M. and S.K.B.T. worked together to revise it. All authors reviewed the final version. B.G. supervised the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mallick, A., Taylor, S.K.B., Mehta, S. et al. Cabin1 domain-containing gene picd-1 interacts with pry-1/Axin to regulate multiple processes in Caenorhabditis elegans. Sci Rep 12, 12029 (2022). https://doi.org/10.1038/s41598-022-15873-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-15873-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.